Acute lymphoblastic leukemia (ALL) is a hematological neoplasm characterized by a clonal lymphoid proliferation in the bone marrow and possible involvement of extramedullary sites. Currently, front-line treatment of adult ALL is based on pediatric-like intensive regimens, often associated with severe and prolonged immunosuppression and a high risk of developing infectious complications.[2] However, in the relapsed/refractory setting, immunotherapeutic agents, particularly blinatumomab and inotuzumab, are promising compounds that have proven effective in inducing hematological remissions and minimal residual disease (MRD) negativity, with less myelosuppression than chemotherapy.
Blinatumomab is a bispecific monoclonal antibody that binds to CD3 and CD19 and activates T cells against leukemic B cells;[3] inotuzumab is a CD22 monoclonal antibody conjugated to calicheamicin, currently approved for the use of relapsed-refractory CD22-positive B-lineage ALL.[4] Despite their clinical efficacy, it is unclear if their use might be detrimental in patients with a concomitant Sars-CoV2 infection. Given the rarity of ALL in the adult population, there is no consensus about incidence, management, and outcome of COVID-19 in patients with hematological malignancies, particularly in patients with ALL.
We describe a 40-year-old male patient with relapsed B-common ALL who developed Sars-CoV2 prior to treatment initiation. In December 2020, the patient with a Klinefelter syndrome and a previous B-common ALL treated with the pediatric-inspired trial NILG ALL10/07 in 2015,[5] off-treatment since 2015, presented pancytopenia, fever, and dyspnea. At Viterbo hospital, peripheral blood morphological examination showed the presence of blasts with a lymphoid habitus. After a negative Sars-CoV2 nasopharyngeal swab, the patient was admitted to our Center in Rome. Laboratory tests confirmed the pancytopenia (Hb 10.3 g/dL, WBC 0.64 x 109/l, Plts 43 x 109/l) and fluorocytometric analysis revealed the presence of 77% CD10+, CD22+, CD45+, CD19+, CD34+, CD123+, CDc79a+, TdT+ blasts, consistent with a diagnosis of relapsed B-common ALL. The molecular profile was negative for the major molecular aberrations (i.e., BCR/ABL1, E2A/PBX1, KMT2A-r).
At relapse, a chest C.T. scan documented a pulmonary embolism, and a Doppler ultrasound showed a saphenous thrombosis of the left leg with a concomitant subcutaneous abscess with cellulitis, extended from the inguinal region to the femoral-popliteal area, which required fondaparinux administration and antibiotic treatment (piperacillin/tazobactam, metronidazole, and daptomycin). In addition, the patient started methylprednisolone, 60 mg/day, but on 1 January 2021, the Sars-CoV2 nasopharyngeal swab proved positive (later confirmed also on bronchoalveolar lavage (BAL), and he was therefore transferred to a COVID-19 ward at our hospital.
The patient did not receive COVID-19 vaccination since no vaccination was available at the time of relapse.
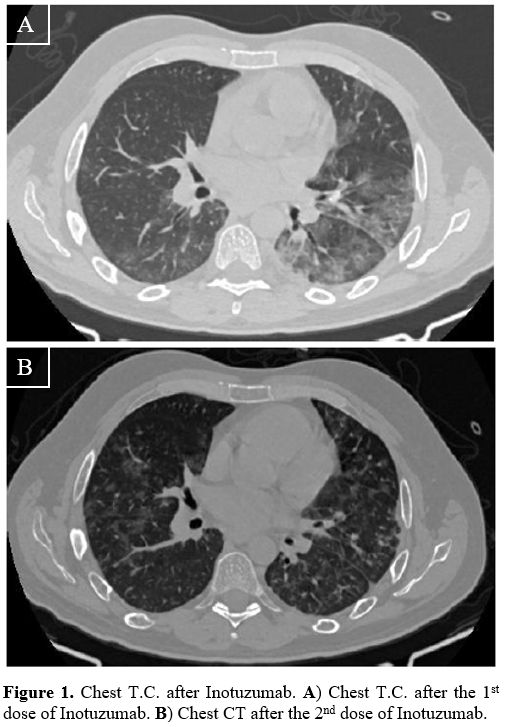
Our ALL strategy contemplated 2 cycles of inotuzumab (0.8 mg/m2 D1, 0.5 mg/m2 D8 and D15) and an allogeneic transplant (allo-SCT), depending on donor availability. Since the patient was asymptomatic for COVID-19 infection and the C.T. scan was negative, on 5 January 2021, the first dose of inotuzumab was administered, followed by the anti-viral drug remdesivir as prophylaxis for ten days (loading dose 250 mg/day, then 100 mg/day) starting on day +1 after inotuzumab. However, on day 9, the clinical conditions worsened, with an acute respiratory syndrome requiring oxygen support; chest C.T. showed bilateral multilobar ground-glass opacities (Figure 1A). By day 15, after eight days of neutropenia, an improvement in the radiological findings and respiratory symptoms was documented. Since a bone marrow aspirate showed a partial response (30% of blasts), on 22 January (day 17), the second dose of inotuzumab (0.5 mg/m2) was administered. As already observed after the first dose, the respiratory syndrome worsened (Figure 1B) but again improved with oxygen and steroids support without non-invasive ventilation; fondaparinux was already ongoing for the pre-existing thrombosis. This condition lasted until day +50 when it gradually improved. On day +49, we documented the presence of an antibody response to Sars-Cov2 (33.6 U/ml, cut-off >1U/ml), but the Sars-Cov2 nasopharyngeal swab was persistently positive (Figure 2); thus, convalescent plasma (CP, 200 ml/day continuous infusion for three days) was administered, without complications.
 |
Figure
1. Chest T.C. after Inotuzumab. A) Chest T.C. after the 1st dose of Inotuzumab. B) Chest CT after the 2nd dose of Inotuzumab. |
On day +67, a bone marrow aspirate revealed a complete hematological remission with a minimal residual disease of 1% by flow cytometry, and on day +75, after 20 days from the C.P. infusion, the patient had his first Sars-CoV2 PCR negative result (Figure 2).
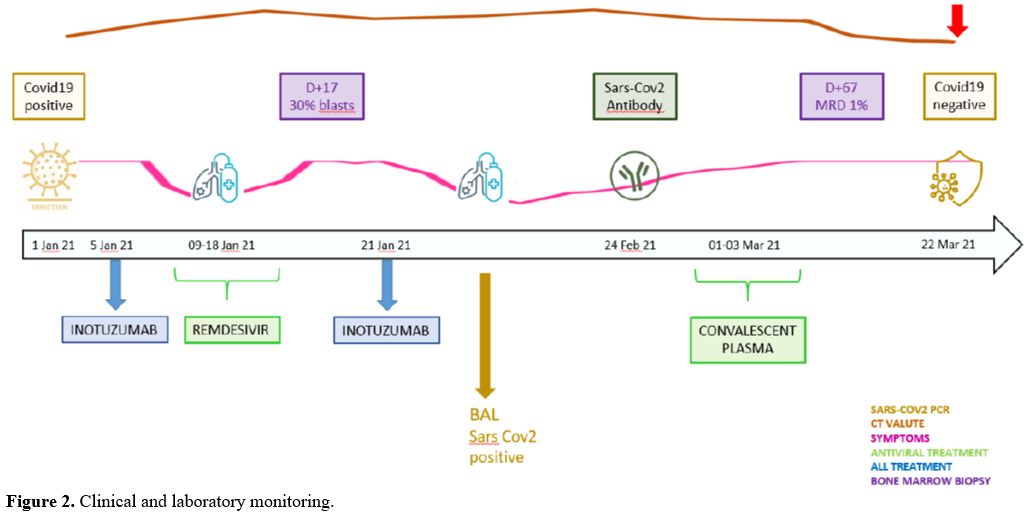 |
Figure 2. Clinical and laboratory monitoring. |
On day +84, the patient was dismissed in hematological remission, with a 1% persistence of leukemic cells by flow cytometry, a negative swab for Sars-Cov2, and complete recovery of COVID19-related pneumonia. Meanwhile, there was a slight worsening of the lower left limb cellulitis, despite the continuous administration of antimicrobic treatment. In addition, MRI was suggestive of osteomyelitis; thus, the patient was kept on oral rifampicin and levofloxacin for an additional month.
He returned to Sicily, where he received the second cycle of inotuzumab and two doses of I.T. methotrexate, achieving MRD negativity (by flow and IG/TR). The sars-COv2 vaccine was not administered to the patient since the patient was going to be allografted shortly after and also because, as already mentioned, he did indeed developed antibodies. Therefore, after a conditioning regimen with TBI and cyclophosphamide, on 9 July 2021, the patient received a MUD allo-SCT. He presented a mild vein occlusive disease (VOD), treated with defibrotide. Stem cell engraftment was on day 17. The patient is currently in good clinical condition, with a normal blood count and without graft signs.
To date, few cases are reporting the management of ALL during COVID-19 infection. Treating ALL patients with a concomitant COVID 19 infection is a challenge,[6], since a therapeutic delay might negatively impact prognosis. Different reasons drove our therapeutic choice: the presence of a novel and poorly known viral disease, the initial patient’s management in a non-hematology department, and his wish to continue treatment in Sicily. Considering these aspects, we deemed that inotuzumab ozogamicin was the most appropriate choice since it is more manageable than blinatumomab. Indeed, inotuzumab administration does not require a continuous infusion, which may interfere with remdevisir infusion.
Furthermore, at variance from blinatumomab, whose most frequent side effect is represented by the cytokine release syndrome,[7] which can confound and worsen the clinical picture of COVID-19, inotuzumab is in this respect is more manageable; lastly, inozutumab is a more potent compound in treating ALL with a high burden of disease. Finally, inotuzumab is more myelosuppressive than blinatumomab but less than systemic chemotherapy. This effect may have led to an exacerbation of the respiratory symptoms related to COVID19 pneumonia after each dose.
The optimal COVID-19 pneumonia treatment remains unclear and particularly delicate in onco-hematologic patients. Remdesivir is a nucleotide analog prodrug that inhibits viral RNA replication. Recent guidelines recommend remdesivir in the presence of a severe infection. A multicenter randomized trial of remdesivir versus placebo for COVID-19+ patients demonstrated that patients treated with remdesivir have a shorter median time to recovery than the placebo group (10 vs. 15 days) and a lower mortality rate (6.7% vs. 11.9%).[8] However, the optimal duration of treatment with remdesivir is not well-established. In our COVID19 center, remdesivir is used for five days in immunocompetent patients. Supported by other studies,[8] we opted to use remdesivir for ten days in our patient - given the primary hematologic neoplasm - as a bridge between the first and second dose of inotuzumab. This choice appeared appropriate since its use improved the respiratory distress that occurred after the administration of inotuzumab.
Nevertheless, the patient remained persistently positive in the nasopharyngeal swab for Sars Cov2. A turning point was the use of C.P., a passive immunization method previously applied to other viral infections.[9] The neutral antibodies (NAbs) against Sars-Cov2 inhibit the entry and viral amplification and activate different pathways (complement activation, antibody-dependent cellular cytotoxicity, and phagocytosis).[10]
It was reported that C.P. might reduce mortality and improve symptoms in the elderly with COVID-19,[11] though with disappointing results in the non-onco-hematologic population.[12] Data on the use of C.P. in COVID-19 patients are increasing, but the experience in immunocompromised patients is very limited. Immunocompromised patients are unable to develop an appropriate humoral immune response to SARS-CoV-2 and are ideal candidates for passive immunization. Tremblay and colleagues[13] studied 24 patients treated with C.P. with cancer, including 14 with hematological malignancies: of the latter, eight were discharged, one was mechanically ventilated, and five died. Heuso et al.[14] treated 17 patients with CP (15 with hematological diseases). All but one patient showed a clinical improvement, and 9/17 documented a virus clearance. Britains and collaborators[15] studied five onco-hematologic patients with COVID-19 treated with C.P. All patients developed NAbs, and all but one witnessed a clinical improvement. However, there is not yet a consensus[16] regarding the dose and timing; results of studies with a larger number of patients are awaited (NCT04393727).
Unexpectedly, our patient developed antibodies against Sars-Cov2 before C.P. administration: despite the administration of inotuzumab weakening the patient’s immune system, he possibly presented a subset of lymphocytes capable of producing antibodies.
Our experience demonstrates the complexity in the care of ALL patients and Sars-Cov2 infection. At present, the patient has recovered from COVID-19 and is at five months from allogeneic transplant in complete molecular remission and good clinical conditions, clearly showing that treatment must be continued, if feasible, and that a multidisciplinary approach is required for the optimal management of these cases.