In this setting, a recent study[7] evaluated immune responses following SARS-CoV-2 vaccines, BNT162b2 COVID-19 mRNA or ChAdOx1 nCoV-19, in 66 patients with hemoglobinopathies (TDT=51; sickle cell disease=15). Twenty-three out of 32 (71.80%) and 33/34 (97.05%) patients developed a significant antibody response after one and two doses, respectively. Another study[8] investigated the efficacy and safety of the Sinopharm vaccine for SARS-CoV-2 in 434 Iranian patients with hemoglobinopathies (β-thalassemia major=303, β-thalassemia intermedia=118, sickle-thalassemia=13). Only 55% of subjects developed antibodies against COVID-19, suggesting a reduced protective effect of this type of vaccine. Although informative, these studies are limited by the lack of a control group.
We prospectively compared the serological responses and possible side effects after anti-SARS-CoV-2, BNT162b2 mRNA COVID-19 vaccine in 65 TDT patients with beta-thalassemia major (selected and prioritized for vaccination as per indications of the Italian Ministry of Health), with those of 63 age and sex-matched healthcare workers, who were enrolled in the study as healthy controls (HCs). All patients were regularly followed at Hematology and Bone Marrow Transplantation Unit-AOUC Policlinico and Immuno-Hematology, Transfusion Medicine Service ASL, "Di Venere" Hospital, Bari, Italy. Characteristics of TDT patients and HCs are reported in Table 1. The primary objectives of this real-life, case-control, observational, prospective study, based on routine clinical and laboratory data, were the rate of response and the titers of anti-spike IgG antibodies after fully (two doses) vaccination in TDT patients. Secondary outcomes included comparisons of anti-spike IgG titers between TDT patients and HCs and the identification of possible factors influencing the response. Comparisons between groups were performed using Mann–Whitney test. Statistical analyses were carried out using GraphPad Prism version 8.3.0 (GraphPad Software Inc., San Diego, CA, USA). All patients provided written informed consent, and the study was conducted according to Italian laws concerning non-interventional studies and the protection of workers exposed to occupational risks.
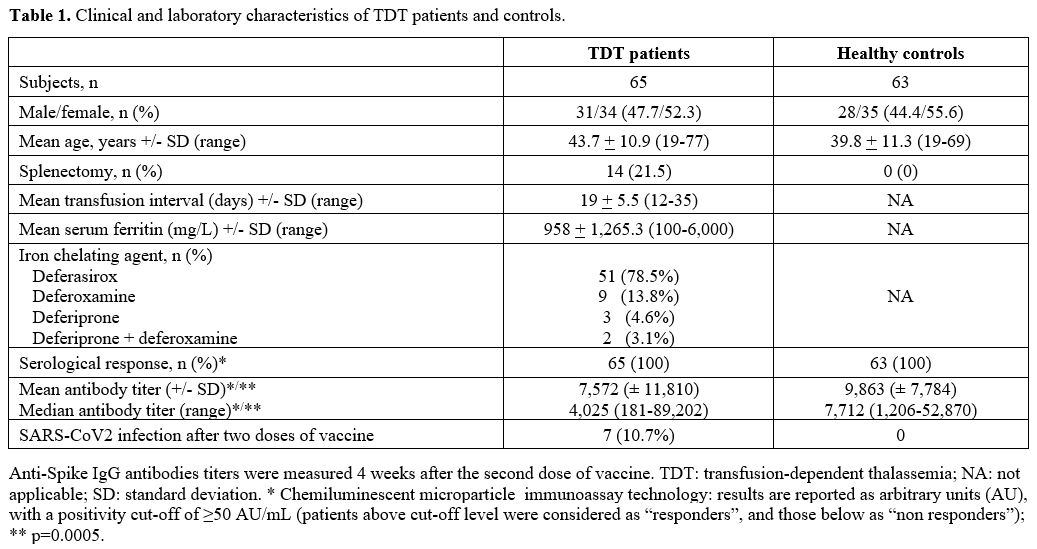 |
Table 1. Clinical and laboratory characteristics of TDT patients and controls. |
All TDT patients and HCs received two vaccine doses on days 1 and 21 between Apr 1 and May 15, 2021. Previous SARS-CoV-2 infection and lack of consent to the study were the only two exclusion criteria. All participants underwent serology tests, measuring their response to the COVID-19 vaccine four weeks after the second vaccine dose and, for TDT patients, after a median time from the last transfusion of 11 days (range 7-16). In addition, quantitative determination of anti-spike immunoglobulin G (IgG) antibodies was performed with the commercially available Abbott immunoassay. Results were reported as arbitrary units (AU), with a positivity cut-off of ≥ 50 AU/mL; patients above the upper cut-off level were considered as "responders", and those below this threshold as "non-responders", according to the indication of the manufacturer.
All HCs and TDT patients (100%) achieved a titer greater than 50 AU/mL (thus, they were all considered as “responders”). However, antibody titers were significantly lower and heterogeneous (p=0.0005) in the TDT patients (mean 7,572 ± 11,810; median 4,025, range 181-89,202) compared to HC group (mean 9,863 AU/mL ± 7,784; median 7,712, range 1,206-52,870) (Figure 1).
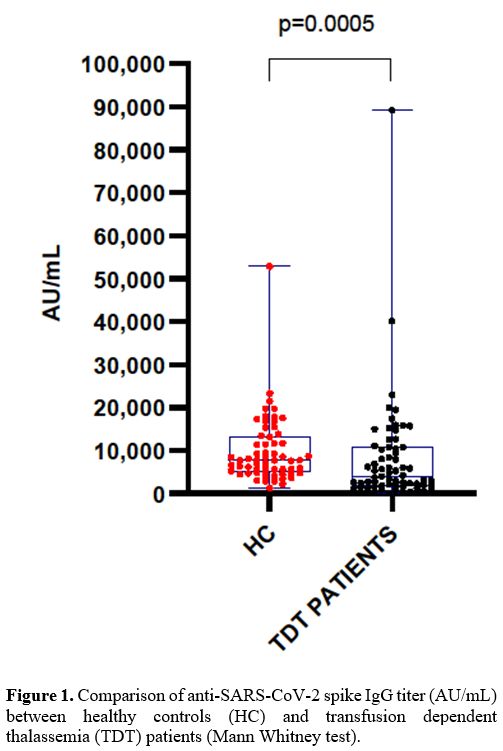 |
Figure 1.Comparison of anti-SARS-CoV-2 spike IgG titer (AU/mL) between healthy controls (HC) and transfusion dependent thalassemia (TDT) patients (Mann Whitney test). |
A possible explanation of this difference could derive from immunomodulation carried out by different potential mediators (platelet-derived factors, white blood cell-derived substances, components of hemolytic contents, and extracellular vesicles) contained in red blood cell (RBC) products periodically received by TDT patients. However, although many potential mediators have been identified, the mechanisms for "RBC transfusion-related immunomodulation", are not yet fully characterized.[9]
We did not find any correlation between some patient's parameters (age, sex, genotype, transfusion interval, serum ferritin level, splenectomy, use of deferasirox compared to other iron-chelating agents, number of units of RBC transfused/year) and anti-spike IgG antibodies titers (data not shown). As previously reported, the antibody response was also not influenced by the AB0 blood group.[10]
With a median follow-up of 209 days (range, 199-223) after the second dose of the anti-SARS-CoV-2 vaccine, 7 cases of SARS-CoV-2 infection occurred among TDT patients. They were all asymptomatic or with mild symptoms (asthenia, nasal congestion, moderate fever). No symptomatic subjects were registered among HCs. It has to be specified that asymptomatic cases were "discovered" among TDT patients since our policy requests a nasopharyngeal swab for SARS-CoV-2 within 48/72 hours before every transfusion session; similarly, asymptomatic cases were likely excluded among HCs as a nasopharyngeal swab for SARS-CoV-2 is routinely performed every 30 days for health surveillance. Two interesting additional cases among TDT patients followed at our Center and not included in this study deserve to be reported. The first one was a 19-year-old woman who achieved a titer of 19 AU/mL (non-responder) four weeks after only a single dose of vaccine, as she had previously contracted SARS-CoV-2 infection. This patient received a second dose several months later; notwithstanding, on December 2021, she developed a severe COVID-19 with respiratory distress, which required hospitalization. No data about the serological response after the second dose was available in this patient. The second patient was a 42-year-old woman who contracted SARS-CoV-2 infection 21 days after the first dose of vaccine while waiting for the second dose. Four weeks later, a titer of 40,100 AU/mL was detected, suggesting a robust response, likely due to the effect of the first dose combined with the viral contact. Regarding safety profile, no relevant side effects were recorded among TDT patients.
To the best of our knowledge, this is the first case-control study of serological response after two doses of anti-SARS-CoV-2 vaccine in this specific population of non-neoplastic individuals. Overall, our findings support the efficacy and safety of a full course of the BNT162b2 mRNA COVID-19 vaccine in TDT patients. Our study, however, has several limitations. First, a few patients were included; certainly, a larger series of subjects, preferably within a multicenter study, is needed to achieve greater generalizability of our findings. Second, the efficacy of the vaccine to prevent SARS-CoV-2 infection or clinically significant COVID-19 in the long term remains unclear due to the short duration of the observation period; longer follow-up is therefore needed, and results after a booster (third) dose are also eagerly awaited. Last, this study evaluated only the serological response in anti-spike IgG antibodies (neutralizing IgG antibodies against nucleocapsid and receptor-binding domain were not analyzed). Clear-cut relationships between these antibodies and protection against the virus have not been unequivocally established. As observed in other contexts, neutralizing antibodies, memory B-cell development, and T-cell immune response after vaccination could play an even more important role in protecting against SARS-CoV-2 infection.
In conclusion, in our experience, two doses of BNT162b2 anti-SARS-CoV-2 mRNA vaccination were well tolerated and induced a serological response in all patients with TDT, though quite heterogeneous and at a lower level of magnitude with respect to HCs. Vaccination did not completely protect from SARS-CoV-2 infection, but the clinical outcome of COVID-19 in these patients was favorable, and the resolution was rapid in all cases. Therefore, we strongly recommend complete anti-SARS-CoV-2 vaccination in these subjects. Notwithstanding, as TDT patients remain particularly vulnerable to severe effects of SARS-CoV-2 infections, their continuous monitoring, regardless of vaccination status, is advisable. Vaccinated TDT patients should also continue to practice strict COVID-19 ongoing protective measures, including masks, social distancing, and screening, as well as prioritize vaccination for family members and caregivers, particularly in the light of the current spread of new SARS-CoV-2 variants of concern.