Ali E.H.1, Alkindi S.2, Mohamed A.O.3, Awadalla k.E.4, Abdlgadir O.5, Adam G.6, Magdi M.7, Ibrahim A.K.5 and Ghebremeskel K.1.
1 Lipidomics and Nutrition Research Centre, School of Human Sciences, London Metropolitan University, UK.
2 Department of Haematology, College of Medicine & Health Sciences, Sultan Qaboos University, Muscat, Oman.
3 Department of Biochemistry, Faculty of Medicine, University of Khartoum, Sudan.
4 Shikan College, El Obeid Sudan.
5 Sudan Sickle Cell Anaemia Centre, El Obeid - SUDAN.
6 Faculty of Education, Al Azhri University, Sudan.
7 Directorate of Planning, Ministry of Health Oman, Muscat, Sultanate of Oman.
Correspondence to: Professor
Salam Alkindi, Department of Haematology, College of Medicine &
Health Sciences, Sultan Qaboos University, P. O. Box 35, Muscat 123,
Oman. Phone: +96824141182; Fax: +96824144887. e-mail:
sskindi@yahoo.com ORCID ID: 0000-0001-6863-5748
Published: January 1, 2023
Received: May 15, 2022
Accepted: December 10, 2022
Mediterr J Hematol Infect Dis 2023, 15(1): e2023002 DOI
10.4084/MJHID.2023.002
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Objective:
Sickle cell trait (SCT) is a congenital condition caused by the
inheritance of a single allele of the abnormal haemoglobin beta gene,
HbS. Carriers of SCT are generally asymptomatic, and they do not
manifest the clinical and haematological abnormalities of sickle cell
anaemia (SCA). However, there is evidence that they display some
symptoms in stressful situations. Pregnancy is a stressful
physiological event, and it is not clear if SCT adversely affects
pregnancy outcomes, particularly in those from developing countries
where people regularly suffer from nutritional insufficiency.
Objective. This study aims to investigate pregnancy outcomes in Sudanese women with SCT.
Subjects and methods.
Pregnant women with (HbAS, n=34) and without (HbAA, n=60) SCT were
recruited during their first trimester at El Obeid Hospital, Kordofan,
Western Sudan. Following appropriate ethical approval and informed
consent from the participants, detailed anthropometric, clinical,
haematological, obstetric, and birth outcome data were registered. In
addition, blood samples were collected at enrolment and at delivery.
Results.
At enrolment in the first trimester, the SCT group did not manifest SCA
symptoms, and there was no difference in the haematological parameters
between the SCT and control groups. However, at delivery, the women
with SCT, compared with the control group, had lower levels of
hemoglobin (Hb, p=0.000), packed cell volume (PCV, p=0.000), mean
corpuscular haemoglobin (MCH, p=0.002) and neutrophil counts (p=0.045)
and higher mean corpuscular volume (MCV, p=0.000) and platelet counts
(p=0.000). Similarly, at delivery, the babies of SCT women had lower
birth weight (p=0.000), lower Hb (p=0.045), PCV (p=0.000), MCH
(p=0.000), and higher neutrophil (p=0.004) and platelet counts
(p=0.000) than the babies of the healthy control group. Additionally,
there were more miscarriages, stillbirths, and admissions to the
Special Care Baby Unit (SCBU) in the SCT group.
Conclusions.
The study revealed that SCT is associated with adverse pregnancy
outcomes, including maternal and neonatal anaemia, low birth weight,
and increased risk of stillbirth, miscarriage, and admission to SCBU.
Therefore, pregnant women with SCT should be given appropriate
pre-conceptual advice and multidisciplinary antenatal and postnatal
care.
|
Introduction
Sickle cell anemia (SCA) is a group of genetic blood disorders characterised
by a mutation involving haemoglobin's beta chain. People who inherit
one sickle cell gene and one normal gene have sickle cell trait (SCT),
whereas those who inherit two abnormal genes have SCA.[1]
Archibald[2]
was the first person to report the presence of the HbS gene in Sudan.
Subsequently, several studies revealed that the country has a high
prevalence of SCA, with an HbS allele frequency ranging between 0.8% in
the North and over 30% in the Western part of the country.[3-5] The
high HbS allele frequency is due to consanguineous marriages, an influx
of tribes affected by the disease from West Africa, and a history of
endemic malaria.[6-7]
SCA is associated with severe clinical and
haematological manifestations, including recurrent vaso-occlusive
crises, anaemia, neurological, renal, hepatic, growth, and
ophthalmological complications,[1] and poor pregnancy outcomes.[8]
Individuals with SCT generally do not display the haematological and
clinical symptoms of SCA. Indeed, some of them are unaware that they
carry the faulty gene; however, there is evidence that they may exhibit
complications during stressful situations or life events[9-12] or
vigorous physical activities.[13]
Pregnancy is a stressful
physiological event, often associated with emotional changes, anxiety,
and depression.[14-15] Impact of SCT on pregnancy outcome has been
equivocal, with some studies have reported adverse outcomes,[16-27] and
contraception has been suggested,[28] whereas others have not.[29-30],
also in consideration of greater resistance of carriers to malaria in
pregnancy.[29] The reasons for the contradictory findings are not
clear. However, factors such as nutritional status before and during
pregnancy may play a significant role. Although the prevalence of
sickle cell genes in Sudan and other low-income countries like
Sub-Saharan African countries is high,[4,31] published data are scarce
on pregnancy outcomes in women with SCT.
The aim of the study: is to investigate pregnancy outcomes in Sudanese women with SCT.
Subjects and methods
Subjects.
Three hundred sixty-seven (n=367) pregnant women attending their first
antenatal appointment during their first trimester at El Obeid
Hospital, Kordofan, Western Sudan, were screened for sickle cell gene.
Of the 367 women who signed the consent & screened, 34 (n=34) had
SCT (HbAS), and the remaining had normal haemoglobin (HbAA). The 34
women with SCT and the 60 (n=60) HbAA, aged 18-40, who fulfilled the
inclusion /exclusion criteria and were willing to participate in the
study, were selected. The exclusion criteria included those with SCA,
thalassemia, other chronic diseases, a physical disability, restricted
access to food, and malnourished. In addition, those who were living
far from the hospitals were also excluded.
Detailed demographic,
obstetric, medical history, dietary habits, and birth outcome data were
meticulously documented. A blood sample, 5 ml, was collected at
enrolment and delivery (maternal and cord blood). The study was
approved by the Ministry of Health of Sudan, the University of Khartoum
Medical School, and the London Metropolitan University ethical
committees.
Methods. Demography. In this prospective observational study, a questionnaire was
specifically developed to extract participants' obstetric, medical and
haematological history data from hospital records.
Anthropometry. Weight and height were assessed using standard measurement methods.
Haematological variables including haemoglobin concentration (Hb), PCV, MCV, mean MCH, and white
cell (WBC) and platelet (PLTS) counts were collected. HbS was
quantified using a capillary electrophoreses machine (Minicab Sebia
flex piercing, Lisses, France).
Statistical analysis.
The data are expressed as mean ± standard deviation (sd) or
percentages, and the level of statistical significance is set at
p<0.05. Quantitative data were tested for normality and homogeneity
of variance and subsequently analysed with an independent t-test
(parametric data) or Mann–Whitney U test (non-parametric data).
Socio-demographic, clinical, and laboratory characteristics data with
cells' frequency of five or more were assessed with a chi-square test
on the contingency platform. Chi-square, Yate's Correction of
Continuity, and Fisher's exact test were used when the observed cell
count was less than five (n=5), under the assumption of independence of
rows and columns and conditional on the marginal totals. SPSS
Statistics for Windows, version 26 (IBM SPSS Ltd., Woking, Surrey, UK)
was used to analyse the data.
Results
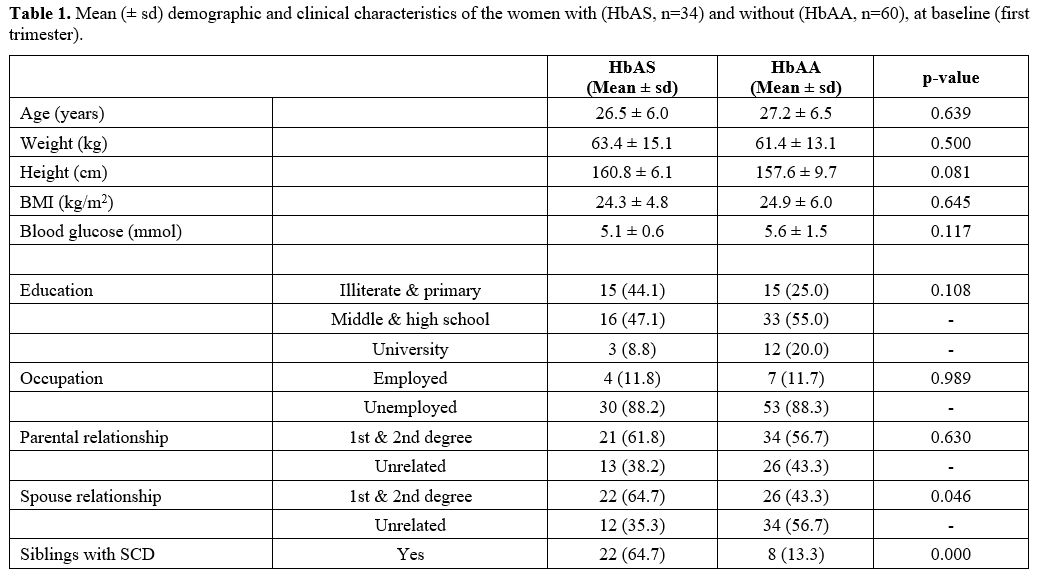
Demographic and clinical characteristics. Table 1 presents
the demographic and clinical characteristics of the women with SCT
(HbAS) and normal haemoglobin (HbAA). There was no difference in age,
weight, height, or body mass index at baseline between the two groups
(p>0.05).
 |
- Table 1. Mean (± sd)
demographic and clinical characteristics of the women with (HbAS, n=34)
and without (HbAA, n=60), at baseline (first trimester).
|
Although SCT women compared with the healthy
control group, had lower levels of educational achievements –
illiterate and primary (44.1 vs 25.0%; P=0.108), middle and high school
(47.1 vs 55.0%; p>0.05) and university (8.8 vs 20.0%; p=0.000) (Table 1); however, the two groups had comparable employment and household income (p=.989) (Table 2).
Although it did not reach a level of statistical
significance, women with SCT were less likely to own their own
house (41.2 vs 60.0%;
p=.210) and more likely to live with their relatives (38.2 vs 25.0%;
p>0.05) or in rented accommodation (20.6 vs 15.0%; p>0.05).
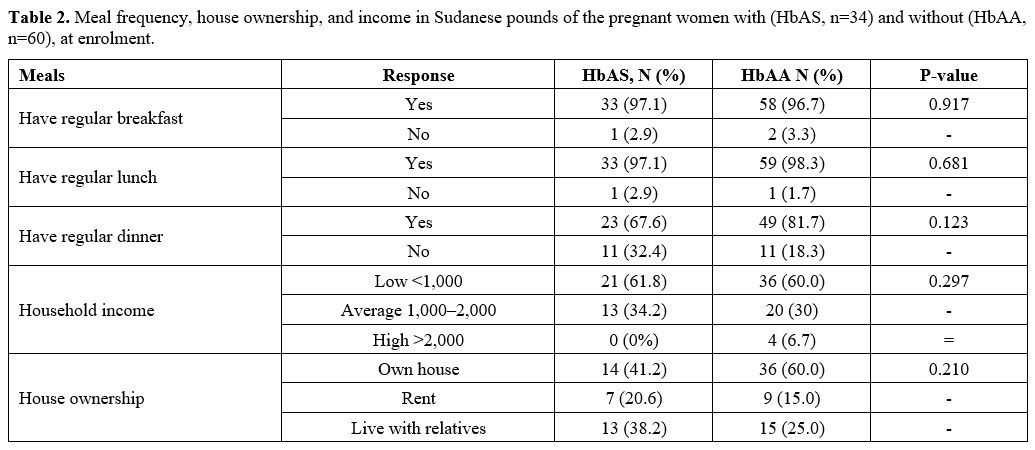 |
- Table 2. Meal
frequency, house ownership, and income in Sudanese pounds of the
pregnant women with (HbAS, n=34) and without (HbAA, n=60), at enrolment.
|
The
study reveals that consanguineous marriage is still common in Western
Sudan, particularly for those with a genetic disorder who are
economically disadvantaged. Compared with the control group, there was
a higher level of marriage to first and second-degree relatives in the
women with SCT (64.7 vs 43.3%; p=.046) and their respective parents
(61.8 vs 56.7%; p= .000).
Haematological parameters.
At enrolment in the first trimester, the SCT group did not manifest SCA
symptoms, and there was no difference in the haematological parameters
between the SCT and control groups (Table 3). At delivery (Table 4), women with SCT compared with the control group had lower levels of
Hb (p=0.000), PCV (p=0.000), MCH (p=0.002), and neutrophil counts
(p=0.045), and higher MCV (p=0.000) and platelet counts (p=0.000).
Similarly, at delivery, the babies of SCT women had lower Hb (p=
0.045), PCV (p=0.000), MCH (p=0.000), and higher neutrophil (p=0.004)
and platelet (p=0.000) counts (Table 5).
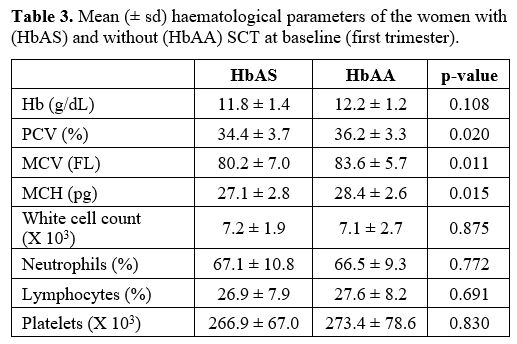 |
Table 3. Mean (± sd) haematological parameters of the women with (HbAS) and without (HbAA) SCT at baseline (first trimester). |
 |
Table 4. Mean (± sd) haematological parameters of the women with (HbAS) and without (HbAA) SCT at delivery.
|
 |
Table 5. Mean (± sd) haematological parameters of the babies of women with (HbAS) and without (HbAA) SCT at delivery.
|
Outcome of pregnancy. Birth outcome data are shown in Table 6.
The mean birth weight of the babies born to the HbAS mothers was lower
than that of the HbAA women's babies (p=0.000). However, there was no
difference in head circumference between the babies of the two groups
(p>0.05).
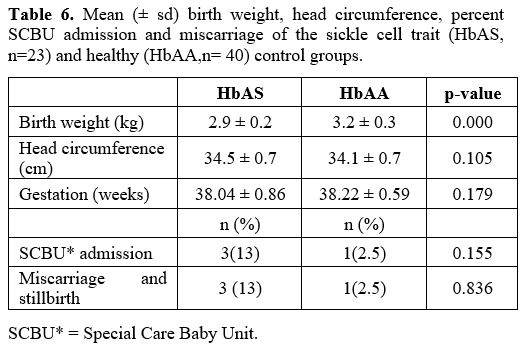 |
- Table 6. Mean (± sd)
birth weight, head circumference, percent SCBU admission and
miscarriage of the sickle cell trait (HbAS, n=23) and healthy (HbAA,n=
40) control groups.
|
Three babies needed admission to the Special Care
Baby Unit in the SCT group Vs one in control (13 vs 2.5%, p>0.05).
The reason for admissions included neonatal jaundice, low APGAR score,
and hypoglycemia at birth. Similarly, the levels of miscarriage or
stillbirth were higher in the SCT group (13 vs 2.5%; p>0.05) than in
the control group pregnancies.
Discussion
This
study investigated maternal and foetal outcomes in pregnancies
complicated by SCT in Western Sudan. This region of the country is
unique in that it has a high prevalence of SCA that overlaps equally
with widespread poverty, malnutrition, illiteracy, and consanguineous
marriages.
Similarly consistent with the previous studies,[4,31]
the majority of the women with and without SCTs are married to their
first or second cousins. However, the number is considerably higher
(64.7 vs 43.3%) in the SCT group. In addition, a significant number of
the women in the current study had low educational backgrounds
(illiterate and primary school level).
Our cohort's HbAS and HbAA pregnant women had equal employment
opportunities and comparable earnings, however women were not college
educated or/and did not own their homes. Indeed, families affected by
SCA may not be able to send their children to a university because of
the financial burden associated with higher education. Although the
numbers of women with SCT in our cohort were small, the findings were
consistent with other observations, indicating that women with HbAS
suffer from unemployment, lack of health insurance, and marriage
discrimination.[4] Other studies which compared SCTs and healthy controls did not find a difference in socioeconomic status.[28]
The current study
revealed that siblings of the HbAS group were more likely to be
affected by SCA than their HbAA counterparts (65 vs 13%). A similar
observation has been reported in the same region by Munsoor and Alabid.[31]
In this region of Sudan, it appears that the tribal habit of marriage
among relatives propagates the sickle cell gene. Consanguineous
marriage is a factor that supports the spread of the disease.
At baseline, first trimester, the women with and without SCT had
comparable weight, height, body mass index, and blood glucose levels.
Perhaps this was to be expected, as the two groups had similar meal
frequencies (most ate breakfast, lunch, and dinner regularly) and
consumed similar types and amounts of foods.
Although the two groups of women had comparable levels of Hb at
baseline (first trimester), the SCT group had significantly lower
percentages of PCV, MCV, and MCH, probably indicating iron deficiency.
A Hb value of less than 11 g/dl during the first and third trimesters
is frequently seen, especially in this area of Sudan, with a high
prevalence of hunger, malnutrition, and iron deficiency.[32–35]
Consistent with previous reports,[11,18–19]
at delivery, this study found significantly lower Hb, PCV percentages,
and MCH concentrations in women with SCT compared to their healthy
counterparts (HbAA).
Following the recommendation of the WHO,[36] iron and iron-folate tablets are made available free of charge to pregnant women in Sudan.[37]
Compliance with iron, folic acid supplementation is related to maternal
education level, appropriate antenatal education and care, knowledge
about anaemia and iron-folic acid supplements, and regular antenatal
care visits.[38–41]
The values of haematological parameters of the babies born to women
with SCT and healthy controls closely mirror those of their mothers at
delivery, with the Hb, PCV, and MCH levels of the former being
significantly lower than those of the latter group, in agreement with
earlier studies.[42–43]
Therefore, it is evident that maternal SCT had adverse effects on the
haematological parameters of their babies. The Hb, PCV, MCV, and MCH
values of the babies were significantly lower than those of the
neonates of the healthy control group, and the normal cord blood
reference ranges reported from Sudan.[44]
Furthermore, babies
born to SCT mothers had lower birth weight and a higher chance of
admission to the special care baby unit; mothers had more miscarriages
and a higher chance of stillbirth than the HbAA group.[25]
The reason for admissions to SCABU included neonatal jaundice, low
APGAR score, and hypoglycemia at birth. These findings are consistent
with other studies, which reported anaemia and neonatal/foetal
mortality,[18,20] low birth weight,[20–21] prematurity and pre-eclampsia,[23] intrauterine foetal hypoxia[19] and placental infarction and calcification[20] in pregnancies complicated by SCT.
It is not evident why SCT pregnancy is associated with adverse birth
outcomes. Nevertheless, several studies have underscored that iron
deficiency and anaemia are risk factors for adverse pregnancy outcomes
viz preterm delivery, prematurity, low birth
weight;[34,45–46]
placental pathologic changes – infarction and calcification – may also
play a role, including its association with increased stillbirth.[20,47]
This study has several limitations, including the relatively small size
of SCT patients. Also, due to logistic and financial reasons,
reticulocyte count and iron studies could not be determined. In
addition, the placentae were not evaluated for pathologic changes
(placental infarction or calcification) and postpartum follow-up was
not conducted. All of these make affirmative conclusions about causes
of stillbirth and other adverse outcomes difficult.
Conclusions
The
study revealed that SCT is associated with adverse pregnancy outcomes,
including maternal and neonatal anaemia, low birth weight, and
increased risk of stillbirth. Therefore, women with SCT who embark on
pregnancy should be given appropriate pre-conceptual advice and
multidisciplinary antenatal and postnatal care.
Acknowledgements
The
authors are grateful to the mothers for graciously consenting to
participate in the study, Drs S. Maki and H. Abbakar for conducting
obstetric assessments, Sister L. S. Adoom for her work in the delivery
room and documentation of birth outcome data, and Mrs F. Eltahir for
helping with recruitment and collection of demographic data. Also, we
are thankful to Dr O. E. Hassan and Miss K. E. Hassan for facilitating
blood samples collection, storage and transport to London and Drs, S.
I. Hussein and M. Elshiekh for their valuable advice and guidance
throughout the duration of the study.
Authors'
contributions
GK
conceived the idea, designed and initiated the study and edited the
manuscript, AEH. conducted the fieldwork (recruitment, collection of
blood samples, data analysis and manuscript drafting), AG helped with
the recruitment and collection of demographic data, AS, and MAO,
provided valuable advice on SCD and haematology and helped with data
evaluation and critical editions of the manuscript, MM, helped with
statistical data analysis, AO, and IAK helped with laboratory analysis
and AKE facilitated the follow-up with the participants and attended
the deliveries.
References
- Ware RE, de Montalembert M, Tshilolo L Abboud MR,
sickle cell disease. Lancet. 2017 Jul 15;390(10091):311-323. doi:
10.1016/S0140-6736(17)30193-9. Epub 2017 Feb 1 https://doi.org/10.1016/S0140-6736(17)30193-9 PMid:28159390
- Archibald RG. A case of sickle cell anaemia in Sudan. Trans R Soc Trop Med Hyg 1926; 19: 389-39. https://doi.org/10.1016/S0035-9203(26)90485-9
- Adam MA, Adam NK, Mohamed BA. Prevalence of sickle
cell disease and sickle cell trait among children admitted to Al Fashir
Teaching Hospital North Darfur State, Sudan. BMC Research Notes 2019;
12. Available at: https://bmcresnotes. https://doi.org/10.1186/s13104-019-4682-5 PMid:31619285 PMCid:PMC6796395
- Daak AA, Elsamani E, Ali EH, et al. Sickle cell
disease in western Sudan: genetic epidemiology and predictors of
knowledge attitude and practices. Trop Med Int Health 2016; 21:642-653.
https://doi.org/10.1111/tmi.12689 PMid:27028397
- Sabahelzain MM, Hamamy H. The ethnic distribution of sickle cell disease in Sudan. Pan Afr Med J. 2014 May 3;18:13. https://doi.org/10.11604/pamj.2014.18.13.3280 PMid:25360197 PMCid:PMC4213521
- Saha N. · Hamad R.E. Mohamed S.c Inbreeding Effects
on Reproductive Outcome in a Sudanese Population. Hum Hered
1990;40:208-212. https://doi.org/10.1159/000153932 PMid:2379925
- Luzzatto, L. Sickle Cell Anaemia and Malaria,
Mediterranean Journal of Hematology and Infectious Diseases, 2012,
4(1), p. e2012065. https://doi.org/10.4084/mjhid.2012.065 PMid:23170194 PMCid:PMC3499995
- Oakley LL, Mitchell S, von Rege I, Hadebe R, Howard
J, Robinson SE, Oteng-Ntim E. Perinatal outcomes in women with sickle
cell disease: a matched cohort study from London, UK. Br J Haematol.
2021 Dec 8. https://doi.org/10.1111/bjh.17983 PMid:34881428
- Yeral M , Boğa C. Is Sickle Cell Trait Really Innocent?. Turk J Haematol. 2021 Jun 1;38(2):159-160. https://doi.org/10.4274/tjh.galenos.2020.2020.0344 PMid:33053967 PMCid:PMC8171209
- Abdulsalam, A. A., Bashour, H. N., Monem, F. S,
Hamadeh FM, Pregnancy outcome among Palestinian refugee women with
sickle cell trait in Damascus, Syria. Saudi Med J. 2003
Sep;24(9):986-90.
- Adam, I., Ibrahim, Y., Elhardello, O. (2018). Prevalence, types and determinants of anemia among pregnant women in
Sudan: A systematic review and meta-analysis, BMC Hematol, vol. 18,
no. 31. https://doi.org/10.1186/s12878-018-0124-1 PMid:30455961 PMCid:PMC6225563
- Adam, M. A., Adam, N. K., Mohamed, B. A. (2019)
'Prevalence of sickle cell disease and sickle cell trait among children
admitted to Al Fashir Teaching Hospital North Darfur State, Sudan', BMC
Research Notes, vol. 12, p. 659. https://doi.org/10.1186/s13104-019-4682-5 PMid:31619285 PMCid:PMC6796395
- Adeyemi, A. B., Adediran, I. A., Kuti, O., Owolabi,
A. T., Durosimi, M. A. (2009) 'Outcome of pregnancy in a population of
Nigerian women with sickle cell trait', J Obstet Gynaecol, vol. 26, no.
2, pp. 133-137. https://doi.org/10.1080/01443610500443428 PMid:16483970
- Naik RP, Haywood C Jr. Sickle cell trait
diagnosis: clinical and social implications. Hematology Am Soc Hematol
Educ Program. 2015;2015(1):160-7. https://doi.org/10.1182/asheducation-2015.1.160 PMid:26637716 PMCid:PMC4697437
- de Weerth C, Buitelaar JK. Physiological stress
reactivity in human pregnancy - a review. Neurosci Biobehav Rev. 2005
Apr;29(2):295-312. https://doi.org/10.1016/j.neubiorev.2004.10.005 PMid:15811500
- Bjelica A, Cetkovic N, Trninic-Pjevic A,
Mladenovic-Segedi L. The phenomenon of pregnancy - a psychological
view. Ginekol Pol. 2018;89(2):102-106. https://doi.org/10.5603/GP.a2018.0017 PMid:29512815
- O'Hara C, Singer DE, Niebuhr DW. The Risk of
Pregnancy Related Hypertension Disorder Associated with Sickle Cell
Trait in U.S. Service Women. Mil Med. 2020 Feb 12;185(1-2):e183-e190. https://doi.org/10.1093/milmed/usz143 PMid:31247087 PMCid:PMC7413597
- Ugboma HAA, George IO. Sickle Cell Disease in
Pregnancy: Maternal and Fetal Outcome in Port Harcourt, Nigeria. J Adv
Med Medical Res. 2015; 7(1): 40-44. https://doi.org/10.9734/BJMMR/2015/11602 PMid:26111969
- Hamdi IM, Kamakshi S K, Ghani EA. Pregnancy outcome in women with sickle cell trait. Saudi Med J. 2002 Dec;23(12):1455-7.
- Manzar. S Maternal sickle cell trait and fetal hypoxia. Am J Perinatol. 2000;17(7):367-70. https://doi.org/10.1055/s-2000-13444 PMid:12141523
- Taylor MY, Wyatt-Ashmead J, Gray J, Bofill JA,
Martin R, Morrison JC. Pregnancy loss after first-trimester viability
in women with sickle cell trait: time for a reappraisal?. Am J Obstet
Gynecol. 2006 Jun;194(6):1604-8. https://doi.org/10.1016/j.ajog.2006.02.027 PMid:16635469
- Hoff C, Wertelecki W, Dutt J, Hernandez R, Reyes
E, Sharp M. Sickle cell trait, maternal age and pregnancy outcome in
primiparous women. Hum Biol. 1983 Dec;55(4):763-70.
- Rathod, K. B., Jaiswal, K. N., Shrivastava, A. C.,
Shrikhande, A. V. (2007) 'Study of the placenta in sickle cell
disorders', Indian J Pathol Microbiol. 2007 Oct;50(4):698-701.
- Wilson, S., Ellsworth, P., Key, N. S. Pregnancy in
sickle cell trait: What we do and don't know', Br J Haematol. 2020
Aug;190 (3):328-335. https://doi.org/10.1111/bjh.16518 PMid:32064587 PMCid:PMC7415474
- Canelón SP, Butts S, Boland MR, Evaluation of
Stillbirth Among Pregnant People with Sickle Cell Trait. JAMA Netw
Open. 2021 Nov 1;4(11): e2134274. https://doi.org/10.1001/jamanetworkopen.2021.34274 PMid:34817585 PMCid:PMC8613600
- Jain D., Atmapoojya P., Colah R.,Lodha P.Sickle
cell disease and pregnancy. Mediterr J Hematol Infect Dis 2019, 11(1):
e2019040, https://doi.org/10.4084/mjhid.2019.040 PMid:31308916 PMCid:PMC6613624
- Wellenstein WL, Sullivan S, Darbinian J, Weintraub
MLR, Greenberg M, Adverse Pregnancy Outcomes in Women with Sickle Cell
Trait. AJP Rep. 2019 Oct;9(4):e346-e352. https://doi.org/10.1055/s-0039-1695743 PMid:31723455 PMCid:PMC6847694
- de Sanctis V., Soliman A.T., Daar S., Canatan D.,
Di Maio S., Kattamis C.Current issues and options for hormonal
contraception in adolescents and young adult women with sickle cell
disease: an update for health care professionals.Mediterr J Hematol
Infect Dis 2020, 12(1): e2020032 https://doi.org/10.4084/mjhid.2020.032 PMid:32395221 PMCid:PMC7202337
- Adeyemi AB, Adediran IA, Kuti O, Owolabi AT,
Durosimi MA. Outcome of pregnancy in a population of Nigerian women
with sickle cell trait. J Obstet Gynaecol. 2006 Feb;26(2):133-7. https://doi.org/10.1080/01443610500443428 PMid:16483970
- Baill IC, Witter FR. Sickle trait and its
association with birthweight and urinary tract infections in pregnancy.
Int J Gynaecol Obstet. 1990 Sep;33(1):19-21. https://doi.org/10.1016/0020-7292(90)90649-6 PMid:1974527
- Munsoor, M. M., Alabid, A. SCT among relatives of sickle cell patients in Western Sudan. Canad. J. Med, 2011; 2 (2): 20-25.
- Kadry S, Sleem C, Samad RA. Hemoglobin levels in pregnant women and its outcomes. Biom Biostat Int J. 2018;7(4):326-336.
- Solomon, Y., Sema, A., Menberu, T. Adherence and
associated factors to iron and folic acid supplementation among
pregnant women attending antenatal care in public hospitals of Dire
Dawa, Eastern Ethiopia. Eur J Midwifery. 2021 Aug 25;5:35. https://doi.org/10.18332/ejm/138595 PMid:34514359 PMCid:PMC8386124
- Tarekegn, M., Wubshet, M., Atenafu, A., Derso, T.,
Woretaw, A. Antenatal care and mothers' education improved iron-folic
acid adherence at Denbiya district health centers, Northwest Ethiopia:
using pills count method. Arch Public Health. 2019 Jun 25;77:30. https://doi.org/10.1186/s13690-019-0356-y PMid:31285822 PMCid:PMC6591834
- Gebremariam, A. D., Tiruneh, S. A., Abate, B. A.,
Engidaw, M. T., Asnakew, D. T. Adherence to iron with folic acid
supplementation and its associated factors among pregnant women
attending antenatal care follow up at Debre Tabor General Hospital,
Ethiopia, 2017. PLoS One. 2019 Jan 7;14(1):e0210086. https://doi.org/10.1371/journal.pone.0210086 PMid:30615646 PMCid:PMC6322725
- Peña-Rosas JP, De-Regil LM, Gomez Malave H,
Flores-Urrutia MC, Dowswell T. Intermittent oral iron supplementation
during pregnancy. Cochrane Database Syst Rev. 2015 Oct
19;2015(10):CD009997. https://doi.org/10.1002/14651858.CD009997.pub2
- Abdullahi H, Gasim GI, Saeed A, Imam AM, Adam I.
Antenatal iron and folic acid supplementation use by pregnant women in
Khartoum, Sudan. BMC Res Notes. 2014 Aug 7;7:498. https://doi.org/10.1186/1756-0500-7-498 PMid:25099760 PMCid:PMC4132242
- Dapper DV, Didia BC. Haemorheological parameters
of umbilical cord blood of Nigerian newborns: correlations with
maternal parameters. West Afr J Med. Jul-Sep 2006;25(3):226-30. https://doi.org/10.4314/wajm.v25i3.28283 PMid:17191424
- Sanni OB, Chambers T, Li JH , Rowe S , Woodman AG
, Ospina MB, Bourque SL. A systematic review and meta-analysis of the
correlation between maternal and neonatal iron status and haematologic
indices. EClinical Medicine. 2020 Oct 8;27:100555. https://doi.org/10.1016/j.eclinm.2020.100555 PMid:33205030 PMCid:PMC7648126
- Angelo A, Derbie G, Demtse A, Tsegaye A, Umbilical
cord blood hematological parameters reference interval for newborns
from Addis Ababa, Ethiopia. BMC Pediatr. 2021 Jun 11 ;21 (1):275. https://doi.org/10.1186/s12887-021-02722-z PMid:34116664 PMCid:PMC8194248
- Snook J, Bhala N, Beales ILP, Cannings D, Kightley
C, Logan RPH, Pritchard DM, Sidhu R, Surgenor S,Thomas W, Verma AM,
Goddard AF, British Society of Gastroenterology guidelines for the
management of iron deficiency anaemia in adults Society of
Gastroenterology guidelines for the management of iron deficiency
anaemia in adults. Gut 2021;0:1-22. https://doi.org/10.1136/gutjnl-2021-325210 PMid:34497146 PMCid:PMC8515119
- Baill, I. C., Witter, F. R. Sickle trait and its
association with birthweight and urinary tract infections in pregnancy.
Int J Gynaecol Obstet. 1990 Sep;33(1):19-21. https://doi.org/10.1016/0020-7292(90)90649-6 PMid:1974527
- Barfield, W. D., Barradas, D. T., Manning, S. E.,
Kotelchuck, M., Shapiro-Mendoza, C. K.. Sickle cell disease and
pregnancy outcomes: women of African descent. Am J Prev Med. 2010
Apr;38(4 Suppl):S542-9. https://doi.org/10.1016/j.amepre.2009.12.020 PMid:20331956
- Elgari, M., Waggiallah, H. A. Cord blood
hematological profile of Sudanese neonates at birth in Khartoum State.
National Journal of Integrated Research in Medicine, 2014; 5(4):22-25.
eISSN: 0975-9840 pISSN: 2230 - 9969
- Dapper D V, Didia B C. Haemorheological parameters
of umbilical cord blood of Nigerian newborns: Correlations with
maternal parameters. West Afr J Med. Jul-Sep 2006 ;25(3):226-30. https://doi.org/10.4314/wajm.v25i3.28283 PMid:17191424
- De Weerth C, Buitelaar J. K. Physiological stress
reactivity in human pregnancy - A review. Neurosci Biobehav Rev. 2005
Apr;29(2):295-312. https://doi.org/10.1016/j.neubiorev.2004.10.005 PMid:15811500
- El-Hazmi MAF, Al-Hazmi AM, Warsy AS. Sickle cell
disease in Middle East Arab countries. Indian J Med Res. 2011
Nov;134(5):597-610. https://doi.org/10.4103/0971-5916.90984 PMid:22199098 PMCid:PMC3249957
[TOP]





