Gheyath K. Nasarallah1,2₼, Aisha D. Fakhroo3₼, Taushif Khan4, Farhan S. Cyprian5, Fatima Al Ali4, Manar M.A. Ata4, Sara Taleb6, Hadeel T. Zedan1,2, Duaa W. Al-Sadeq1,5, Fathima H. Amanullah1, Ali A. Hssain7, Ali H. Eid5, Laith J. Abu-Raddad8, Abdullatif Al-Khal7, Asmaa A. Al Thani2,3, Nico Marr9 and Hadi M. Yassine2,3*.
1 Biomedical Research Center, Qatar University, Doha, Qatar.
2 Department of Biomedical Sciences, College of Health Sciences-QU health, Qatar University, Doha, Qatar.
3 Research and Development Department, Barzan Holdings, Doha, Qatar.
4 Research Branch, Sidra Medicine, Doha, Qatar.
5 Basic Medical Science Department, College of Medicine-QU Health, Qatar University, Doha, Qatar.
6 Genomics and Precision Medicine, Hamad Bin Khalifa University, Doha, Qatar.
7 Hamad Medical Corporation, Doha, Qatar.
8 Infectious Disease Epidemiology Group, Weill Cornell Medicine-Qatar, Cornell University, Doha, Qatar.
9 College of Health and Life Sciences, Hamad Bin Khalifa University, Doha, Qatar.
₼ Both authors equally contributed.
Correspondence to:
Hadi M. Yassine, M.Sc., Ph.D. Université Joseph KI-ZERBO,
Laboratoire de Biologie Moléculaire et de Génétique (LABIOGENE), P.O.
Box 7021, Ouagadougou 03, Burkina Faso, Burkina Faso.Tel.: +974 4403 6819. E-mails:
hyassine@qu.edu.qa
Published: November 1, 2022
Received: August 3, 2022
Accepted: October 13, 2022
Mediterr J Hematol Infect Dis 2022, 14(1): e2022076 DOI
10.4084/MJHID.2022.076
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background and Objectives:
The heterogeneity of the coronavirus disease of 2019 (COVID-19) lies
within its diverse symptoms and severity, ranging from mild to lethal.
Acute respiratory distress syndrome (ARDS) is a leading cause of
mortality in COVID-19 patients, characterized by a hyper cytokine
storm. Autoimmunity is proposed to occur as a result of COVID-19, given
the high similarity of the immune responses observed in COVID-19 and
autoimmune diseases. Here, we investigate the level of autoimmune
antibodies in COVID-19 patients with different severities.
Results:
Initial screening for antinuclear antibodies (ANA) IgG using ELISA
revealed that 1.58% (2/126) and 4% (5/126) of intensive care unit (ICU)
COVID-19 cases expressed strong and moderate ANA levels, respectively.
An additional sample was positive with immunofluorescence assays (IFA)
screening. However, all the non-ICU cases (n=273) were ANA negative
using both assays. Samples positive for ANA were further confirmed with
large-scale autoantibody screening by phage
immunoprecipitation-sequencing (PhIP-Seq). The majority of the
ANA-positive samples showed "speckled" ANA pattern by microscopy and
revealed autoantibody specificities that targeted proteins involved in
intracellular signal transduction, metabolism, apoptotic processes, and
cell death by PhIP-Seq; further denoting reactivity to nuclear and
cytoplasmic antigens.
Conclusion:
Our results further support the notion of routine screening for
autoimmune responses in COVID-19 patients, which might help improve
disease prognosis and patient management. Further, results provide
compelling evidence that ANA-positive individuals should be excluded
from being donors for convalescent plasma therapy in the context of
COVID-19.
|
Introduction
The
severity of COVID-19 is diverse, with a wide range of symptoms
characterized from mild to lethal. A robust immune response is usually
initiated upon viral infections, involving both the innate and adaptive
immune systems to irradicate the virus.[1] Typically,
viruses evade these immune responses through one of the following
mechanisms; (1) Molecular Mimicry, (2) bystander activation, and (3)
epitope spreading.[1,2] In COVID-19 patients, several
immunological impacts have been observed, including hyper-immune
response, abnormal cytokine/chemokine production, T cells
hyperactivation, increased monocytes, macrophages, and neutrophils
count.[1,3] The cytokine storm (i.e.,
abnormal cytokine secretion) is associated with fatality in COVID-19
patients, who usually experience a hyper-pro-inflammatory immune
response leading to ARDS.[1,3] These COVID-19 immune responses are quite similar to those observed in autoinflammatory and autoimmune conditions.[1,4]
It is known that infectious diseases trigger autoimmunity, specifically
through molecular mimicry, and several viruses have been associated
with autoimmune diseases.[1,5,6] For
example, enteric viruses have been associated with type 1 diabetes, and
herpesviruses infections have led to the development of several
autoimmune disorders, including multiple sclerosis, systemic lupus
erythematosus, and rheumatoid arthritis.[1,6,7,8] In addition, mice infected with murine coronavirus developed immune-mediated encephalomyelitis.[9]
Further, rhinovirus and coronavirus were shown to be the highest
frequently detected pathogens in patients with psoriasis flares
following respiratory tract infections.[10] Severe
COVID-19 patients have been suspected of developing autoimmunity.
Several reports have suggested a link between SARS-CoV-2 infection and
Kawasaki-like disease, acute inflammation of the blood vessels
affecting children.[5,11] Moreover, patients with severe COVID-19 pneumonia reported neutralizing IgG autoantibodies against type I IFNs.[12]
In addition, other autoantibodies, such as anti-platelet autoantibodies
(APA), were reported in COVID-19 patients, leading to immune
thrombocytopenia.[13] Accordingly, critically ill
COVID-19 patients may experience elevated levels of other autoimmune
antibodies, including antinuclear antibodies (ANA).[14,15]
High levels of ANA have been previously associated with several
autoimmune disorders, such as lupus erythematosus (SLE) and rheumatoid
arthritis (RA).[16] This study investigates whether
COVID-19 severe outcome could be associated with autoimmunity. We
measured ANA levels in blood sera samples collected from COVID-19
patients with different clinical severities (i.e., ICU "Severe" vs.
Non-ICU "Mild or Asymptomatic"). We report a higher frequency of ANA in
severe COVID-19 cases, suggesting a possible contribution of COVID-19
to autoimmunity and exacerbated disease outcome.
Method
Sample Collection and Ethical Compliance.
This study was approved by the IRB committees of Hamad Medical
Corporation (MRC-01-20-145) and Qatar University (QU-IRB 1289-EA/20).
Informed consent was obtained from all patients per the approved
protocol, and informed consent was obtained from next of kin in case of
death. The samples' numbers in table 1
are only identifiable to the researchers and are not related to the
national nor the medical IDs of the patients. Sera samples were
collected from COVID-19 patients at different clinical stages and
classified into two groups; (1) mild/asymptomatic (non-ICU, n=273), (2)
Severe/Critical (ICU, n=126). Out of the 126 ICU patients, only 80 had
sera extracted at different time points, assuming that ICU admission
was on Day 1. For those with repetitive samples, at least two-time
points were tested, and positive results were reported. All methods
were performed following the relevant guidelines and regulations.
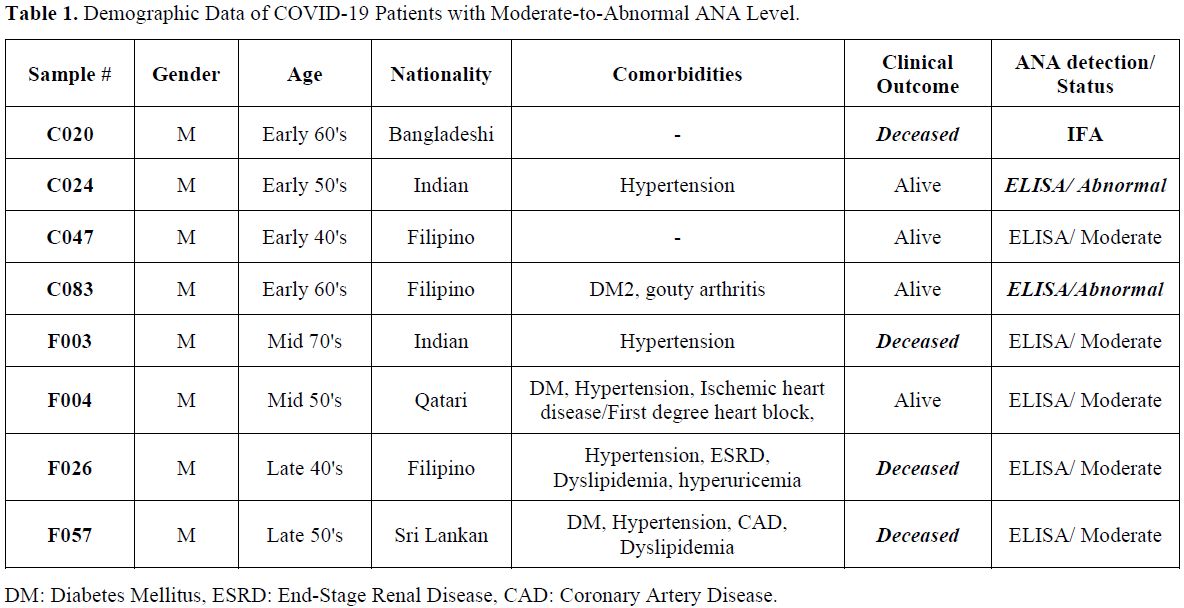 |
Table
1. Demographic Data of COVID-19 Patients with Moderate-to-Abnormal ANA Level. |
ANA IgG ELISA assay.
All samples [mild/asymptomatic (non-ICU, n=273) & Severe/Critical
(ICU, n=126)] were initially screened by ELISA. Sera samples were
diluted 1:101 in sample diluent (DILSPE), and ELISA was performed using
an ANA Screening IgG kit (DIA.PRO, Italy) according to the
manufacturer's standards. The cutoff value was calculated as negative
control (OD450nm) + 0.250. Samples with cut of value (S/Co) <0.8,
S/Co (0.8-1.1), S/Co>1.1 were considered negative "normal",
equivocal "moderate" and positive "abnormal" respectively. Note that
samples that tested positive and equivocal were repeated in triplicates
with mean and standard deviation calculated.
ANA HEp-2 IFA Assay.
All ICU patients' sera (n=126) and an equivalent number of randomly
selected non-ICU sera (n=121) were subjected to Indirect Fluorescent
Antibody (IFA). A technique was performed using the AccuDiagTM
ANA HEp-2 IFA kit (Diagnostic Automation/Cortez Diagnostics, US)
according to the manufacturer's standards. A positive reaction is
indicated by the presence of any pattern of nuclear apple-green
staining observed at a 1:40 dilution based on a 1+ to 4+ scale of
staining intensity (+1 weak ➔ +4 strong). Samples were regarded as
positive if they tested positive with either ELISA or IFA test. These
samples were then subjected to PhIP-Seq analysis.
Phage Immunoprecipitation-Sequencing (PhIP-Seq) and Peptide Enrichment Analysis. PhIP-Seq and peptide enrichment analysis were performed as previously described[17]
using the T7 Human ORF 90mer library, a phage display library
expressing 90-aa protein fragments tiling through the human proteome
with a 45-aa overlap.[18] The T7 Human ORF 90mer
Library was obtained from S. Elledge (Brigham and Women's Hospital and
Harvard University Medical School, Boston, MA, USA). In brief, we
imputed −log10(P) values as described previously[17]
by fitting a zero-inflated generalized Poisson model to the
distribution of reading counts obtained from the tested samples
following immunoprecipitation; then we regressed the parameters for
each peptide sequence based on the read counts obtained from an input
library sample (i.e., prior to immunoprecipitation). These −log10(P)
values were considered peptide enrichment scores and reflected a
quantitative measure for the presence of autoantibody specificity in a
given sample. A peptide enrichment score of ≥ 2.3 was considered
statistically significant. We also removed peptides from the downstream
analysis enriched in mock-IP samples, which served as negative
controls. We only considered peptides significantly enriched in at
least two test samples. We then computed log odds ratios (LOD) for all
retained peptides (n = 328) to identify autoantibody specificities that
were differentially enriched between ICU patients and asymptomatic
COVID-19 cases. Peptides with a |LOD| ≥ ln(1.5) were considered
differentially enriched (n = 79).
Gene Set Enrichment Analysis.
Of the 79 differentially enriched peptides, 62 were derived from coding
sequences with a defined gene annotation (Entrez) and were considered
for gene enrichment analysis. We used the Molecular Signatures Database
(MSigDB) for this analysis as previously described.[19] Out of the 62 queried genes, 42 were found to be significantly enriched (P-value < 10-5 and FDR q-value < 0.05) in one of the 20 gene sets.
Detection of Shared Linear B Cell Epitopes.
To test for shared linear B cell epitopes between the identified
autoantigens and SARS-CoV-2 antigens, we built a pairwise distance
matrix that captured the maximum size of linear sequence identity of
amino acids between the 79 differentially enriched human 90 aa peptides
and 17 reference sequence of SARS-CoV2 proteins in UniProtKB (https://covid-19.uniprot.org; used for analysis not to deposit the data) as previously described.[20] A linear sequence identity of 7 amino acids or more was considered a shared linear B cell epitope.
Results
ANA
antibodies were screened in non-ICU (n=273) and ICU (n=126) COVID-19
patients using ANA IgG ELISA. All of non-ICU patients (n=273) tested
negative; (S/Co<0.8) with an average of 0.286 (+/- 0.073). On the
other hand, 7/126 ICU patients (~5.6%) reported a S/Co value of
>0.8, showing moderate to high ANA levels (Figure 1a).
Four percent of the ICU patients (5/126) tested equivocal (S/Co:
0.8-1.1, moderate ANA level), whereas 1.58% (2/126) tested positive
(S/Co>1.1, abnormal ANA level) (Figure 1b).
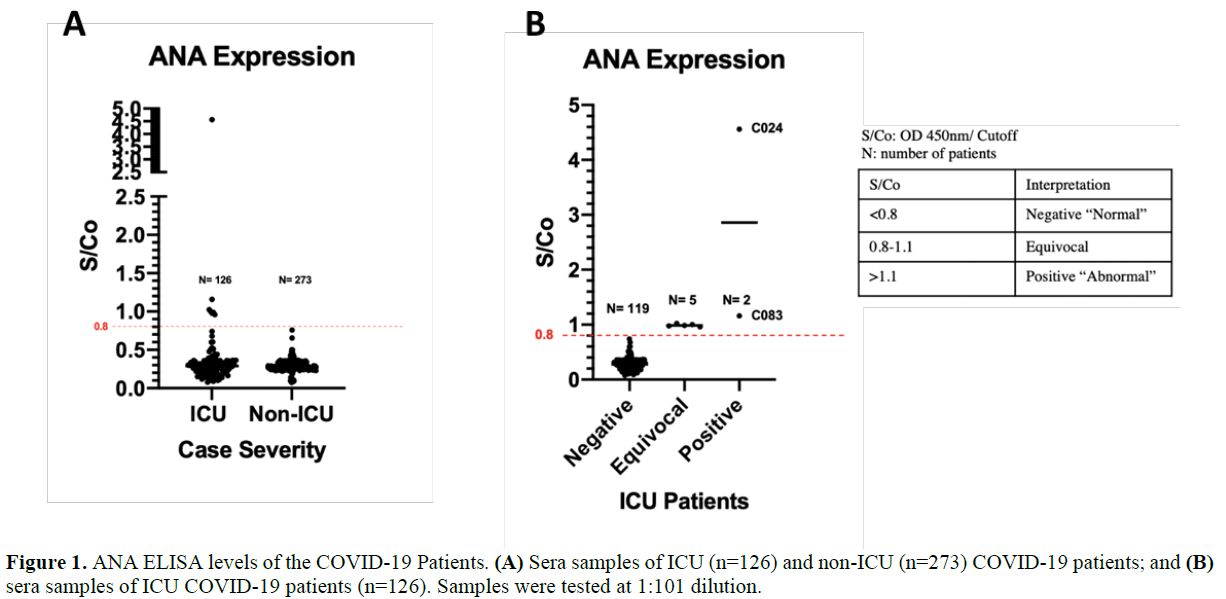 |
Figure
1. ANA ELISA levels of the COVID-19 Patients. (A) Sera samples of ICU (n=126) and non-ICU (n=273) COVID-19 patients; and (B) sera samples of ICU COVID-19 patients (n=126). Samples were tested at 1:101 dilution. |
The positive samples, C024 and C083, had a S/Co value of 4.561 and 1.159, respectively (Figure 1b). To confirm the presence of ANA, IFA was performed using HEp-2 cells on all ICU and an equivalent number of non-ICU samples (Figure 2).
An additional sample (C020) tested strongly positive for IFA. The
majority of the samples (C024, F026, F004, and C047) showed a
"speckled" ANA pattern (Figure 2). In addition to nuclear ANA level, cytoplasmic ANA level was observed in samples F003, F004, C020, F057, and C083 (Figure 2). Samples F057 and C083 showed a "punctate nuclear envelope" ANA pattern (Figure 2). All patients who tested equivocal/positive for ANA are males within the age range of 41-75 years (Average= 55 years) (Table 1).
In relation, 71.4% of the ANA-positive patients (5/7) are co-diagnosed
with hypertension, and the mortality rate was 42.9% (3/7), specifically
the samples F003, F026, and F057 that showed moderate ANA Level (Table 1).
Diabetes was also common among ANA-positive patients, accounting for
42.8% of patients (3/7), including patient C083, which exhibited
abnormal ANA levels. Finally, we performed a large-scale autoantibody
screen of the ANA-positive (C024, C083) and five equivocal sera samples
using phage-immunoprecipitation sequencing.[9,12]
Randomly selected ANA-negative samples obtained from ICU patients with
COVID-19 (n = 7) and from asymptomatic COVID-19 cases (n = 15) were
assayed for comparison. Principal component analysis of the peptide
enrichment scores confirmed that most ANA-positive and equivocal
samples of ICU cases clustered separately from the asymptomatic
COVID-19 cases, except for the day 9 sample of C024 and the sample
collected from C83 on day 17 after ICU admission (Figure 3A).
We identified 79 autoantibody specificities that were differentially
enriched in ICU patients in comparison to the asymptomatic COVID-19
cases (Supplementary Table S1),
allowing a clear separation of critical versus asymptomatic COVID-19
cases by hierarchical clustering (except for one asymptomatic case) (Figure 3B).
Interestingly, our unbiased screen revealed autoantibody specificities
against several known autoantigens present in AAgAtlas 1.0, a human
autoantigen database,[21] including nuclear proteins
such as the DEAD-Box Helicase 42 (DDX42) and Mtr4 Exosome RNA Helicase
(MTREX), as well as proteins involved in immune defenses and cellular
signaling, such as Immunoglobulin Superfamily DCC Subclass Member 1
(DCC) and Ras Suppressor Protein 1 (RSU1). To functionally characterize
the self-antigens that were being targeted in critically ill COVID-19
patients, we performed a gene set enrichment analysis using the
Molecular Signatures Database (MSigDB)[11] and
including all of the 79 putative autoantigens for which we had found
autoantibodies to be differentially enriched among the tested ICU cases
when compared to asymptomatic COVID-19 cases. This analysis confirmed
that autoantibodies in ICU patients primary targeted intracellular
proteins involved in intracellular signal transduction, metabolism,
apoptotic processes, and cell death. Autoimmune responses were
primarily observed in samples with moderate and high ANA levels,
particularly in the samples of patient C024 with the highest ANA
measurements, which appeared to increase over time (Figure 3C and Supplementary Table S2).
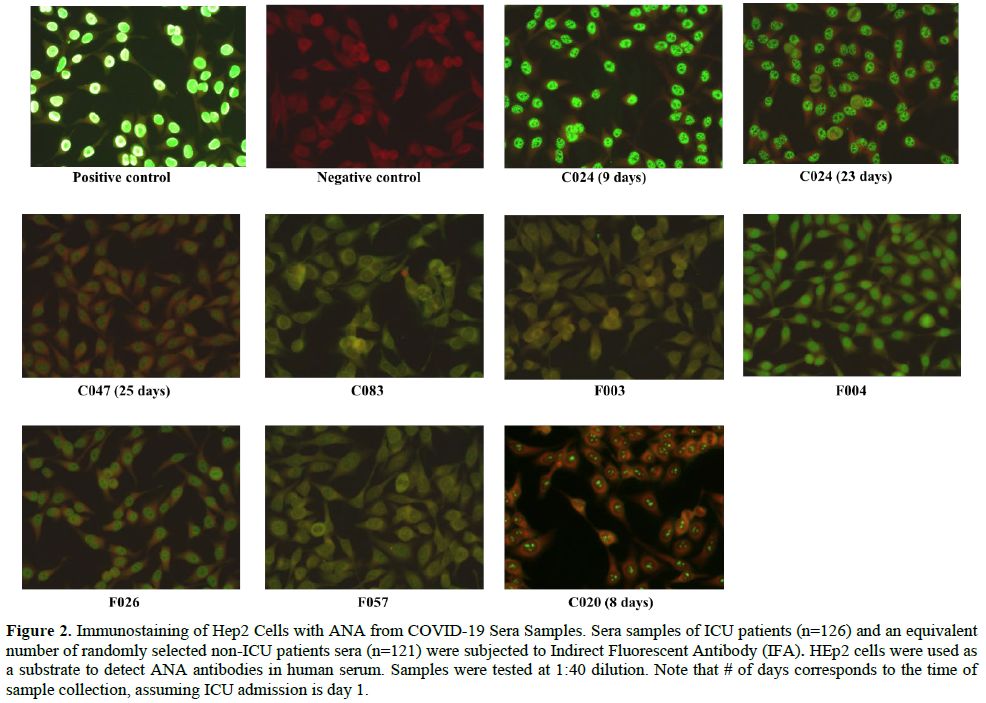 |
Figure 2. Immunostaining
of Hep2 Cells with ANA from COVID-19 Sera Samples. Sera samples of ICU
patients (n=126) and an equivalent number of randomly selected non-ICU
patients sera (n=121) were subjected to Indirect Fluorescent Antibody
(IFA). HEp2 cells were used as a substrate to detect ANA antibodies in
human serum. Samples were tested at 1:40 dilution. Note that # of days
corresponds to the time of sample collection, assuming ICU admission is
day 1. |
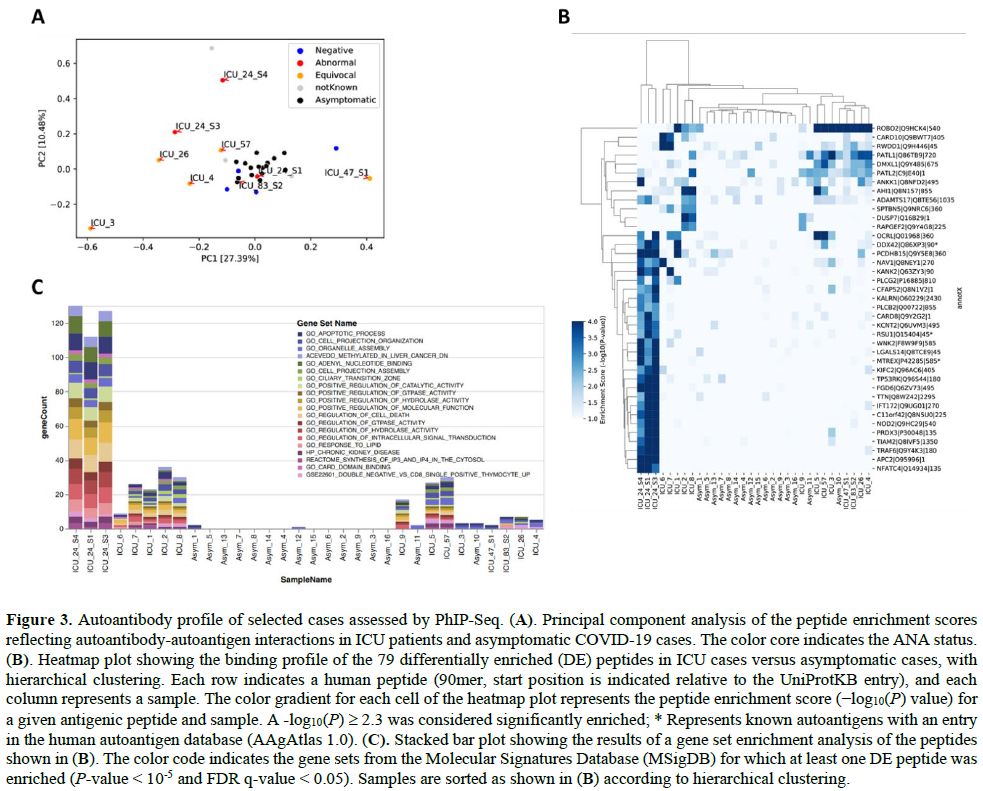 |
Figure 3. Autoantibody profile of selected cases assessed by PhIP-Seq. (A).
Principal component analysis of the peptide enrichment scores
reflecting autoantibody-autoantigen interactions in ICU patients and
asymptomatic COVID-19 cases. The color core indicates the ANA status. (B).
Heatmap plot showing the binding profile of the 79 differentially
enriched (DE) peptides in ICU cases versus asymptomatic cases, with
hierarchical clustering. Each row indicates a human peptide (90mer,
start position is indicated relative to the UniProtKB entry), and each
column represents a sample. The color gradient for each cell of the
heatmap plot represents the peptide enrichment score (−log10(P) value) for a given antigenic peptide and sample. A -log10(P)
≥ 2.3 was considered significantly enriched; * Represents known
autoantigens with an entry in the human autoantigen database (AAgAtlas
1.0). (C). Stacked bar plot showing the results of a gene set enrichment analysis of the peptides shown in (B).
The color code indicates the gene sets from the Molecular Signatures
Database (MSigDB) for which at least one DE peptide was enriched (P-value < 10-5 and FDR q-value < 0.05). Samples are sorted as shown in (B) according to hierarchical clustering.
|
Discussion
COVID-19
disease progression may pass through up to four different phases. The
first phase is characterized by an initial viral infection phase that
is usually mild or asymptomatic in approximately 80% of patients. The
host-virus interactions then delineate the progression of the disease.
Some patients progress to a second phase, characterized by a
hyper-immune response (i.e., cytokine storm). A state of
hypercoagulability occurs in the third phase.[1] In
combination, they may lead to organ damage in the fourth phase, which
is usually mediated by the host's innate immune system.[1,5,14]
In this present study, we assessed the generation of antinuclear
autoimmune antibodies (ANA) in critically ill COVID-19 patients to
better understand the disease prognosis and pave the way for the
possible use of immunomodulatory drugs for the treatment of these
patients. It is worth noting that several immunomodulatory drugs have
been proven to be effective in relieving COVID-19 symptoms, such as
tocilizumab.[1]
Interestingly, ANAs were
exclusively observed in ICU COVID-19 patients (8/126, 6.34%), which
suggests a potential correlation between COVID-19 severity and ANA
production. In other words, SARS-CoV-2 infection may have triggered the
production of ANA autoantibodies leading to possible cases of
autoimmunity in severely ill COVID-19 patients. However, the mechanism
is yet to be studied. Only one patient (C24) had a high level of ANA as
tested by ELISA. We tested this patient's samples at different time
points, and all were highly positive (Figure 2). It was inapplicable to follow up with this patient to see the ANA titer levels after he was discharged from the hospital.
Immunological
dysregulation, including the production of autoimmune antibodies, has
been previously described in COVID patients.[12,22,23]
In one study, Pascolini et al. reported the presence of anti-platelet
autoantibodies (APA) in three COVID-19 patients suffering from
immune-mediated thrombocytopenia. Such antibodies were not detected in
our samples when using the PhIP-Seq assay. In general, viral infections
trigger autoimmunity through one of the following mechanisms, (1)
molecular mimicry, (2) bystander activation, and (3) epitope spreading.[2,5,6]
In molecular mimicry, viruses display antigens structurally similar to
self-antigens activating a cross-reactive immune response against both
self and non-self-antigens. During bystander activation, a non-specific
hyper anti-viral immune response characterized by a pro-inflammatory
environment causes the release of self-antigens from damaged tissues,
which are then presented by antigen-presenting cells (APC), triggering
autoreactive T cells and autoimmunity. One example is HIV, which mimics
the human T-cell receptor (TCR) to a great extent where autoantibodies
are produced.[24] In support of this mechanism, our
large-scale autoantibody screen of selected patients by PhIP-Seq
revealed several known and novel autoantigens among ICU cases,
particularly those with moderate and high ANA responses. An in-depth
analysis of these autoantibody specificities confirmed that these
autoimmune responses were primarily directed against intracellular
proteins and, therefore, likely as a consequence of extensive tissue
damage during disease progression. Of note, the patient with the
highest ANA serum levels and most robust autoantibody responses as
assessed by PhIP-Seq (a 53-year-old male with Indian nationality) had a
clinical history of hypertension but was otherwise previously healthy.
Similarly,
epitope spreading is characterized by the release of more self-antigens
activating autoreactive T cells that eventually spread to other
autoreactive T cells (i.e., the diversification of epitope
specificity). A recent study identified cross-reactive epitopes between
SARS-CoV-2 and human molecular chaperones.[25]
Bioinformatics analysis showed that a family of heat shock proteins
(Hsp70) shared antigenic epitopes with SARS-CoV-2, capable of inducing
autoimmunity against endothelial cells through the process of molecular
mimicry.[25] Thus, a similar mechanism may apply to
ANA production, where cross-reactive epitopes between SARS-CoV-2 and
nuclear antigens may exist. However, none of the autoantigens we
identified in this study shared linear B cell epitopes with any of the
SARS-CoV-2 protein reference sequences (Supplementary Figure S1).
In
terms of comorbidities, hypertension was common among patients who
showed ANA levels, specifically in deceased patients (F003, F026, and
F057). Thus, this may suggest a potential link between hypertension and
ANA level. However, a more extensive cohort study is needed to validate
this hypothesis. Autoimmune diseases such as lupus and RA have
increased risk for hypertension and cardiovascular disease.[26]
Therefore, we speculate that hypertension may act as a risk factor
promoting a pro-inflammatory environment, possibly leading to
autoimmunity through bystander activation. Although these samples
showed moderate ANA levels, it is inconclusive because extra time
points of the samples are needed to check ANA level change over time.
The second most common comorbidity was diabetes, specifically patient
C083, who experienced both type 2 diabetes and abnormal ANA level. Type
2 diabetes is suspected to be an autoimmune condition given the
presence of circulating autoantibodies against β cells;[27] thus, this may contribute to abnormal ANA levels (i.e., risk factor).
Furthermore,
ANA level was confirmed using IFA, where HEp-2 cells were immunostained
with ANA expressed in sera samples. ANA level comes in different
patterns depending on the antigens to which ANA binds. According to the
results, most positive/equivocal samples showed speckled patterns
suggesting potential antigens such as n-RNP, Sm, and SSB/La.[28]
Interestingly, only one patient had a detectable ANA level with a
speckled pattern. Further, one sample (C020) showed a nucleolar
pattern. Previous studies have shown a correlation between ANA
nucleolar pattern and systemic sclerosis.[29]
According to the study, a nucleolar pattern of ANA was associated with
pulmonary fibrosis (i.e., lung scarring) (P<0.01), suggesting a
critical organ involvement with a decreased chance of survival in
systemic sclerosis patients.
Regarding COVID-19, a similar
association was observed between the presence of the ANA reactivity
with nucleolar pattern and severe COVID-19 disease.[14,15] Further, in a small study by Chang et al.,[30]
autoantibodies were detected in moderate and critical cases of
COVID-19. The study involved 47 PCR-confirmed COVID-19 hospitalized
patients. The total ANA positive rate was 21.3%, which is higher than
our study but lower than other studies, as reported in their
discussion. Interestingly, similar to our findings, ANA titers were
mostly weak (Median 1:40), showing 50% nucleolar and 30% speckled
staining. While 9.1% (1/11) of their patients with autoantibodies and
8.3% (3/36) of patients without autoantibodies died, almost 50% of
ANA-positive patients in our study died. It is worth noting that ANA
positivity in our study was confirmed with large-scale autoantibody
screening by phage immunoprecipitation-sequencing (PhIP-Seq), which
revealed autoantibody specificities that predominantly targeted
proteins involved in intracellular signal transduction, metabolism,
apoptotic processes, and cell death by PhIP-Seq.
Unlike other
studies, we did not detect neutralizing anti-cytokine (IFN) antibodies
in the PhIP-Seq assay, as previously reported.[12,31] Their study[12]
identified individuals with high titers of neutralizing autoantibodies
against type I IFN-α2 and IFN-ω in about 10% of patients with severe
COVID-19 pneumonia. We selected only ANA-positive samples for further
screening with PhIP-Seq, which could be the reason for the negative
outcome. Further, with our PhIP-Seq screen, we have a limited ability
to detect autoantibodies that target conformational epitopes (due to
the smaller size of peptides, 90 aa in length, that are being used for
phage display) which may have limited the sensitivity.
Screening
randomly selected pre-pandemic samples (n=2655) from Qatar Blood Bank
donors (unpublished results) revealed ten samples with abnormal ANA
levels (~0.38%). Among these positive ANA samples, 5 (50%) were
positive for documented viral infections (Supplemental Table 3).
Four samples (A, B, C, and D) tested positive for B19 Virus IgG, two
samples (A and E) for WNV (West Nile Virus) IgG, and one sample (A) for
Dengue virus. B19 virus has been associated with several autoimmune
diseases, such as rheumatoid arthritis, systemic lupus,
antiphospholipid syndrome, systemic sclerosis, and vasculitides.[32]
It has also been shown to induce cross-reactive autoantibodies
utilizing molecular mimicry between parvovirus VP1 protein and host
proteins (e.g., human cytokeratin and transcription factor GATA 1).[32]
Likewise, WNV infection has been reported to promote autoimmune
conditions, including myasthenia gravis (MG), through the process of
molecular mimicry.[33] In addition, several studies
have shown cross-reactivity between antibodies directed against dengue
virus nonstructural protein 1 (NS1) and human platelets/endothelial
cells damaging them.[34] As observed, molecular
mimicry and autoimmunity are common among these viruses, suggesting a
similar mechanism taking place during SARS-CoV-2 infection.
Conclusions
This
study sheds light on a potential relationship between COVID-19 and
autoimmunity, particularly ANA production. Nevertheless, some
limitations are associated with this study. First, the sample size is
relatively small, and a larger-scale study may be needed. Second, some
of the sera samples were only taken at one-time points, which makes it
harder to explore ANA change over time. Third, people are admitted to
ICU mostly seven days after infection, so the time points of the ICU
samples are calculated from their admission to the ICU rather than the
beginning of the infection. Thus, the acute vs. convalescent phase
inconsistency of the ICU and non-ICU samples may affect the accuracy of
the results. Still, this study provides a closer insight into the
immunological progression of the disease and its prognosis. Therefore,
we propose including the screening for autoimmune antibodies as a
routine test for COVID-19 patients.
Acknowledgment
The authors would like to thank all the nurses and staff who facilitated the sample collection.
Statement of Ethics
This
study was approved by IRB committees of Hamad Medical Corporation
(MRC-01-20-145), Sidra Medicine (IRB Protocol #: 1511001953), and Qatar
University (QU-IRB 1289-EA/20). In addition, we have received written
informed consent from the participants.
Funding Sources
This study was supported by funds from QNRF, grant # NPRP11S-1212-170092.
Authors' contribution
HMY and GKN designed the study. ADF, TK, FA, MMA,
HTZ, DWA, and FHA ran the experiments. FSC, ST, and AHH collected samples. AHE,
LAR, AA, AAA, and NM helped in logistics and supervision. All authors read and
approved the manuscript.
Data Availability Statement
All
data are provided in this manuscript either in the main text or in the
supplemental files. Inquiries about additional information can be
requested directly from the corresponding author.
References
- Rodríguez, Y., Novelli, L., Rojas, M., De Santis,
M., Acosta-Ampudia, Y., Monsalve, D. M., ... & Gershwin, M. E.
(2020). Autoinflammatory and autoimmune conditions at the crossroad of
COVID-19. Journal of autoimmunity, 114, 102506. https://doi.org/10.1016/j.jaut.2020.102506 PMid:32563547 PMCid:PMC7296326
- Fujinami
RS, von Herrath MG, Christen U, Whitton JL. Molecular mimicry,
bystander activation, or viral persistence: infections and autoimmune
disease. Clin Microbiol Rev. 2006 Jan;19(1):80-94. https://doi.org/10.1128/CMR.19.1.80-94.2006 PMid:16418524 PMCid:PMC1360274
- Pierce
CA, Preston-Hurlburt P, Dai Y, Aschner CB, Cheshenko N, Galen B,
Garforth SJ, Herrera NG, Jangra RK, Morano NC, Orner E, Sy S, Chandran
K, Dziura J, Almo SC, Ring A, Keller MJ, Herold KC, Herold BC. Immune
responses to SARS-CoV-2 infection in hospitalized pediatric and adult
patients. Sci Transl Med. 2020 Oct 7;12(564):eabd5487. https://doi.org/10.1126/scitranslmed.abd5487 PMid:32958614 PMCid:PMC7658796
- Metzemaekers
M, Cambier S, Blanter M, Vandooren J, de Carvalho AC,
Malengier-Devlies B, Vanderbeke L, Jacobs C, Coenen S, Martens E,
Pörtner N, Vanbrabant L, Van Mol P, Van Herck Y, Van Aerde N, Hermans
G, Gunst J, Borin A, Toledo N Pereira B, Dos Sp Gomes AB, Primon Muraro
S, Fabiano de Souza G, S Farias A, Proenca-Modena JL, R Vinolo MA;
CONTAGIOUS Consortium, Marques PE, Wouters C, Wauters E, Struyf S,
Matthys P, Opdenakker G, Marques RE, Wauters J, Gouwy M, Proost
P.Kinetics of peripheral blood neutrophils in severe coronavirus
disease 2019. Clin Transl Immunology. 2021 Apr 29;10(4):e1271. https://doi.org/10.1002/cti2.1271 PMid:33968405 PMCid:PMC8082714
- Galeotti,
C., & Bayry, J. (2020). Autoimmune and inflammatory diseases
following COVID-19. Nature Reviews Rheumatology, 1-2. https://doi.org/10.1038/s41584-020-0448-7 PMid:32499548 PMCid:PMC7271827
- Smatti,
M. K., Cyprian, F. S., Nasrallah, G. K., Al Thani, A. A., Almishal, R.
O., & Yassine, H. M. (2019). Viruses and autoimmunity: a review on
the potential interaction and molecular mechanisms. Viruses, 11(8),
762. https://doi.org/10.3390/v11080762 PMid:31430946 PMCid:PMC6723519
- Jun
HS, Yoon JW. The role of viruses in type I diabetes: two distinct
cellular and molecular pathogenic mechanisms of virus-induced diabetes
in animals. Diabetologia. 2001 Mar;44(3):271-85. https://doi.org/10.1007/s001250051614 PMid:11317656
-
Vaughan JH, Nguyen MD, Valbracht JR, Patrick K, Rhodes GH. Epstein-Barr
virus-induced autoimmune responses. II. Immunoglobulin G autoantibodies
to mimicking and nonmimicking epitopes. Presence in autoimmune disease.
Vaughan JH, Nguyen MD, Valbracht JR, Patrick K, Rhodes GH. J Clin
Invest. 1995 Mar;95(3):1316-27. https://doi.org/10.1172/JCI117782 PMid:7533789 PMCid:PMC441471
- Pewe,
L., & Perlman, S. (2002). Cutting edge: CD8 T cell-mediated
demyelination is IFN-γ dependent in mice infected with a neurotropic
coronavirus. The Journal of Immunology, 168(4), 1547-1551. https://doi.org/10.4049/jimmunol.168.4.1547 PMid:11823480
- Sbidian,
E., Madrange, M., Viguier, M., Salmona, M., Duchatelet, S., Hovnanian,
A., ... & Bachelez, H. (2019). Respiratory virus infection triggers
acute psoriasis flares across different clinical subtypes and genetic
backgrounds. British Journal of Dermatology, 181(6), 1304-1306. https://doi.org/10.1111/bjd.18203 PMid:31150103 PMCid:PMC7161746
- Verdoni,
L., Mazza, A., Gervasoni, A., Martelli, L., Ruggeri, M., Ciuffreda, M.,
... & D'Antiga, L. (2020). An outbreak of severe Kawasaki-like
disease at the Italian epicentre of the SARS-CoV-2 epidemic: an
observational cohort study. The Lancet. https://doi.org/10.1016/S0140-6736(20)31103-X
- Bastard,
P., Rosen, L. B., Zhang, Q., Michailidis, E., Hoffmann, H. H., Zhang,
Y., ... & Manry, J. (2020). Autoantibodies against type I IFNs in
patients with life-threatening COVID-19. Science, 370(6515). https://doi.org/10.1126/science.abd4585 PMid:32972996 PMCid:PMC7857397
- Pascolini,
S., Granito, A., Muratori, L., Lenzi, M., & Muratori, P. (2021).
Coronavirus disease associated immune thrombocytopenia: causation or
correlation? Journal of Microbiology, Immunology, and Infection, 54(3),
531. https://doi.org/10.1016/j.jmii.2020.08.006 PMid:32859531 PMCid:PMC7434403
- Gagiannis
D, Steinestel J, Hackenbroch C, Schreiner B, Hannemann M,
Bloch W, Umathum VG, Gebauer N, Rother C, Stahl M, Witte HM, Steinestel
K. Clinical, Serological, and Histopathological Similarities Between
Severe COVID-19 and Acute Exacerbation of Connective Tissue
Disease-Associated Interstitial Lung Disease (CTD-ILD). Front Immunol.
2020 Oct 2;11:587517. https://doi.org/10.3389/fimmu.2020.587517 PMid:33123171 PMCid:PMC7566417
- Pascolini
S, Vannini A, Deleonardi G, Ciordinik M, Sensoli A, Carletti
I, Veronesi L, Ricci C, Pronesti A, Mazzanti L, Grondona A, Silvestri
T, Zanuso S, Mazzolini M, Lalanne C, Quarneti C, Fusconi M, Giostra F,
Granito A, Muratori L, Lenzi M, Muratori P. COVID-19 and Immunological
Dysregulation: Can Autoantibodies be Useful? Clin Transl Sci. 2021
Mar;14(2):502-508. https://doi.org/10.1111/cts.12908 PMid:32989903 PMCid:PMC7536986
- Grygiel-Górniak,
B., Rogacka, N., & Puszczewicz, M. (2018). Antinuclear antibodies
in healthy people and non-rheumatic diseases-diagnostic and clinical
implications. Reumatologia, 56(4), 243. https://doi.org/10.5114/reum.2018.77976 PMid:30237629 PMCid:PMC6142026
- Mohan
D, Wansley DL, Sie BM, Noon MS, Baer AN, Laserson U, Larman HB.
Publisher Correction: PhIP-Seq characterization of serum antibodies
using oligonucleotide-encoded peptidomes. Nat Protoc. 2019
Aug;14(8):2596. Erratum for: Nat Protoc. 2018 Sep;13(9):1958-1978. https://doi.org/10.1038/s41596-018-0025-6 PMid:30190553 PMCid:PMC6568263
- Xu
GJ, Shah AA, Li MZ, Xu Q, Rosen A, Casciola-Rosen L, Elledge SJ.
Systematic autoantigen analysis identifies a distinct subtype of
scleroderma with coincident cancer. Proc Natl Acad Sci U S A. 2016 Nov
22;113(47):E7526-E7534. https://doi.org/10.1073/pnas.1615990113 PMid:27821747 PMCid:PMC5127349
- Liberzon
A, Birger C, Thorvaldsdóttir H, Ghandi M, Mesirov JP, Tamayo P. The
Molecular Signatures Database (MSigDB) hallmark gene set collection.
Cell Syst. 2015 Dec 23;1(6):417-425. https://doi.org/10.1016/j.cels.2015.12.004 PMid:26771021 PMCid:PMC4707969
- Khan
T, Rahman M, Al Ali F, Huang SS, Ata M, Zhang Q, Bastard P, Liu Z,
Jouanguy E, Beziat V, Cobat A, Nasrallah GK, Yassine HM, Smatti MK,
Saeed A, Vandernoot I, Goffard JC, Smits G, Migeotte I, Haerynck F,
Meyts I, Abel L, Casanova JL, Hasan MR, Marr N. Distinct antibody
repertoires against endemic human coronaviruses in children and adults.
JCI Insight. 2021 Jan 26:144499. https://doi.org/10.1172/jci.insight.144499 PMid:33497357 PMCid:PMC7934927
- Wang
D, Yang L, Zhang P, LaBaer J, Hermjakob H, Li D, Yu X. AAgAtlas 1.0: a
human autoantigen database. Nucleic Acids Res. 2017 Jan
4;45(D1):D769-D776. https://doi.org/10.1093/nar/gkw946 PMid:27924021 PMCid:PMC5210642
- Pascolini,
S., Vannini, A., Deleonardi, G., Ciordinik, M., Sensoli, A., Carletti,
I., ... & Muratori, P. (2021). COVID‐19 and immunological
dysregulation: can autoantibodies be useful? Clinical and Translational
Science, 14(2), 502-508. https://doi.org/10.1111/cts.12908 PMid:32989903 PMCid:PMC7536986
- Bastard
P, Gervais A, Le Voyer T, Rosain J, Philippot Q, et al. Autoantibodies
neutralizing type I IFNs are present in ~4% of uninfected individuals
over 70 years old and account for ~20% of COVID-19 deaths. Bastard P,
Gervais A, Le Voyer T, Rosain J, Philippot Q, et al. Sci Immunol. 2021
Aug 19;6(62):eabl4340.
- Root-Bernstein,
R. (2017). Human immunodeficiency virus proteins mimic human T cell
receptors inducing cross-reactive antibodies. International journal of
molecular sciences, 18(10), 2091. https://doi.org/10.3390/ijms18102091 PMid:28972547 PMCid:PMC5666773
- Gammazza,
A. M., Légaré, S., Bosco, G. L., Fucarino, A., Angileri, F., de
Macario, E. C., ... & Cappello, F. (2020). Human molecular
chaperones share with SARS-CoV-2 antigenic epitopes potentially capable
of eliciting autoimmunity against endothelial cells: possible role of
molecular mimicry in COVID-19. Cell Stress and Chaperones, 25(5),
737-741. https://doi.org/10.1007/s12192-020-01148-3 PMid:32754823 PMCid:PMC7402394
- Wolf, V. L., & Ryan, M. J. (2019). Autoimmune disease-associated hypertension. Current hypertension reports, 21(1), 10. https://doi.org/10.1007/s11906-019-0914-2 PMid:30712132 PMCid:PMC6394456
- Itariu,
B. K., & Stulnig, T. M. (2014). Autoimmune aspects of type 2
diabetes mellitus-a mini-review. Gerontology, 60(3), 189-196. https://doi.org/10.1159/000356747 PMid:24457898
- ANA patterns. ICAP International consensus on ANA patterns. retrieved January 13, 2021, from: https://www.anapatterns.org/nuclear_patterns.php
- Hesselstrand,
R., Scheja, A., Shen, G. Q., Wiik, A., & Åkesson, A. (2003). The
association of antinuclear antibodies with organ involvement and
survival in systemic sclerosis. Rheumatology, 42(4), 534-540. https://doi.org/10.1093/rheumatology/keg170 PMid:12649400
- Chang SH, Minn D, & Kim YK. Autoantibodies in moderate and critical cases of COVID-19. Clin Transl Sci. 2021;14:1625-1626. https://doi.org/10.1111/cts.13036 PMid:33934534 PMCid:PMC8239866
- Bastard
P, Rosen LB, Zhang Q, Michailidis E, Hoffmann HH, et al. Autoantibodies
against type I IFNs in patients with life-threatening COVID-19.
Science. 2020 Oct 23;370(6515):eabd4585. https://doi.org/10.1126/science.abd4585 PMID: 32972996; PMCID: PMC7857397.
- Lunardi,
C., Tinazzi, E., Bason, C., Dolcino, M., Corrocher, R., & Puccetti,
A. (2008). Human parvovirus B19 infection and autoimmunity.
Autoimmunity reviews, 8(2), 116-120. https://doi.org/10.1016/j.autrev.2008.07.005 PMid:18700174
- Hawkes,
M. A., Hocker, S. E., & Leis, A. A. (2018). West Nile virus induces
a post-infectious pro-inflammatory state that explains transformation
of stable ocular myasthenia gravis to myasthenic crises. Journal of the
neurological sciences, 395, 1-3. https://doi.org/10.1016/j.jns.2018.09.015 PMid:30267806
- Lin,
Y. S., Yeh, T. M., Lin, C. F., Wan, S. W., Chuang, Y. C., Hsu, T. K.,
... & Lei, H. Y. (2011). Molecular mimicry between virus and host
and its implications for dengue disease pathogenesis. Experimental
Biology and Medicine, 236(5), 515-523. https://doi.org/10.1258/ebm.2011.010339 PMid:21502191
Supplementary Files
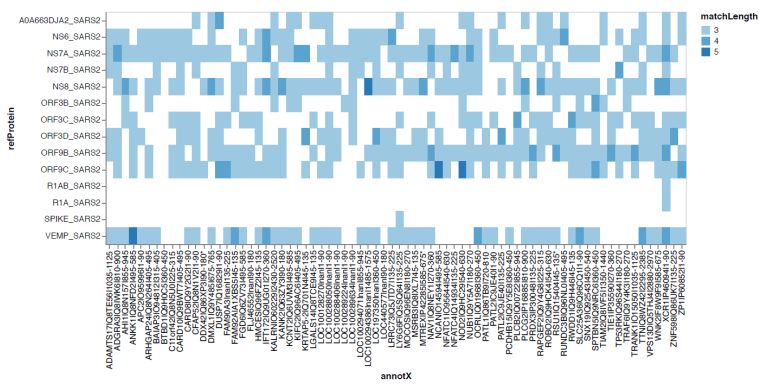 |
Supplementary Figure S1 |
 |
Supplementary Table 1 |
 |
Supplementary Table 2 |
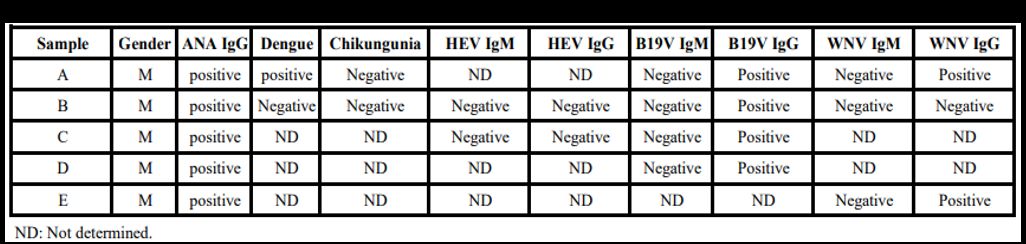 |
Supplementary Table 3. Screening of positive ANA prepandemıc samples (i.e., prior Covid-19) against different viruses
|
[TOP]







