Emrah Kilicaslan1 and Kadir Canoglu2.
1 Department
of Hematology, Sultan 2. Abdülhamid Han Training and Research Hospital,
Health Sciences University, Istanbul, Turkey.
2
Department of Pulmonology, Sultan 2. Abdülhamid Han Training and
Research Hospital, Health Sciences University, Istanbul, Turkey.
Correspondence to:
Kadir Canoglu, Department of Pulmonology, Sultan 2. Abdülhamid Han
Training and Research Hospital, Health Sciences University, Tibbiye
Avenue, Selimiye Street, 34688, Uskudar, Istanbul – Turkey. Tel: (+90)
02165422020. E-mail:
kadircano@gmail.com
Published: January 1, 2023
Received: September 14, 2022
Accepted: December 13, 2022
Mediterr J Hematol Infect Dis 2023, 15(1): e2023003 DOI
10.4084/MJHID.2023.003
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background and objective:
Patients with latent tuberculosis infection (LTBI) receiving
chemotherapy for hematological malignancy (HM) are at high risk of
developing active tuberculosis (TB) infection. The aim of this study is
to show real-life data and results of the T-SPOT test and preventive
isoniazid (INH) therapy in pre-chemotherapy LTBI screening in the HM
patient group.
Methods:
This retrospective study includes 209 HM patients who had T-SPOT test
between 2016 and 2021 in Sultan 2. Abdulhamid Han Training and Research
Hospital in Istanbul, Turkey.
Results:
The prevalence of LTBI was 26.8% in 209 patients (n=56). Preventive INH
therapy was initiated in 82.1% (n=46) of 56 patients with LTBI. 23.9%
(n=11) of the 46 patients who received preventive INH therapy were
unable to complete the treatment. Nine patients died due to malignancy;
one was lost to follow-up, and only one had to stop INH treatment due
to elevated liver enzymes. Elevated liver enzymes occurred in 4 (8.7%)
patients using INH, while gastrointestinal symptoms occurred in 3
(6.5%) patients. Active TB infection emerged in none of the T-SPOT
positive or indeterminate individuals but in one HIV(+) patient in the
T-SPOT negative group. The active TB infection incidence rate was 217
cases/100.000hab/year (95% CI, 29-748).
Conclusions:
INH treatment was generally well tolerated, and very few serious
drug-related side effects were observed. Although LTBI cannot be
demonstrated in patients with HIV(+) HM who are scheduled for
chemotherapy, these patients should be closely monitored for the
development of active TB infection.
|
Introduction
Turkey
has a low incidence of tuberculosis (TB) infection in terms of active
TB infection, with a reported incidence of 14.1/100.000 in the general
population in 2018.[1] Latent tuberculosis infection (LTBI) is a state of cellular immune response to mycobacterial antigens.[2]
The immunosuppression caused by the hematological malignancy (HM) and
the agents used in the treatment may lead to TB reactivation. Thus,
patients with LTBI who are receiving chemotherapy for HM are more
likely to develop active TB infection.[3] However,
there is no worldwide consensus on whether LTBI screening should be
performed before chemotherapy in patients with malignancies other than
allogeneic hematopoietic stem cell transplantation (HCT) recipients.
Two
tests have been developed based on the detection of interferon-gamma
(IFN-G) released from lymphocytes against specific antigens of M. tuberculosis.
These tests, known as The Interferon-Gamma Release Assays (IGRAs), are
the Quantiferon®-TB Gold Plus (QFT) and SPOT®.TB (T-SPOT) tests.
Compared to the TST, IGRAs yield results with at least comparable
sensitivity and better specificity for diagnosing LTBI.[4]
The
aim of this study is to retrospectively evaluate the efficacy and
safety of the T-SPOT test as well as the preventive INH therapy
administered. Thus, the HM patient group aims to share real-life data
and results on the administration of preventive INH therapy based on
T-SPOT alone in screening for LTBI before chemotherapy.
Materials and Methods
Patients and study design.
This retrospective study included 209 patients over 18 diagnosed with
HM and having a T-SPOT test for LTBI screening between January 1, 2016,
and December 31, 2021, in Sultan 2. Abdulhamid Han Training and
Research Hospital in Istanbul, Turkey. This hospital is a tertiary
public hospital and a hematology reference center. The T-SPOT test is
not performed in our hospital. Instead, patients have the T-SPOT test
performed in private laboratories by paying a fee. Those with active TB
infection at the time of diagnosis of malignancy, those who did not
have a T-SPOT test, those who had TB infection in the past, and those
whose health records could not be accessed were excluded from the
study. This study was approved by the Ethics Committee of Istanbul
Medeniyet University (Decision No: 2022/0027). Due to the study's
retrospective nature, written informed consent forms were not obtained
from the patients.
Data collection.
Age, gender, previous TB history, presence of viral hepatitis and human
immunodeficiency virus (HIV), HM type, whether chemotherapy was
administered, whether HCT was performed, the type of HCT performed
(autologous/allogeneic), comorbid diseases, whether preventive INH
therapy was administered, mortality status and follow-up times were
recorded. All patients were followed up monthly in the first two months
for drug toxicity that may develop due to INH treatment and then for
the treatment with laboratory and clinical controls every two months.
During follow-up, elevated liver enzymes due to INH were defined as
transaminases five-fold higher than the upper limit of normal (ULN) or
three-fold higher in the presence of symptoms. In this case, INH
treatment was discontinued. In patients who developed transaminase
elevations that did not reach critical levels, INH treatment was not
discontinued, and these patients were closely monitored. In patients
with severe transaminase elevation due to HM involvement of the liver,
transaminase levels were expected to decrease with chemotherapy to
start INH at the time of diagnosis. If the patient's liver enzymes were
more than 3-fold higher than the ULN, INH therapy was initiated when
they were < 3-fold the ULN after chemotherapy.
T-SPOT test analysis.
T-SPOT.TB® (Oxford, Immunotec, UK) kit was used for T-SPOT test
analysis. The test was performed with 5 cc blood samples placed in
tubes containing lithium heparin. When the sample was processed in the
laboratory, it was placed in a sterile plastic container, 150 µl of
T-Cell Xtend (Oxford Immunotec International) was added, and after
waiting for a while at room temperature, mononuclear cells were
extracted from the serum using a series of centrifugation processes.
The collected suspension was transferred to an antigen-coated 4-well
plate. In these wells, positive and negative controls, Panel A and
Panel B, were analyzed. The prepared plate was incubated for 16-20
hours in a 5% CO2 oven at 37°C. The spots were examined after
incubation, numerous pieces of washings, the addition of conjugate, and
substrate. T-SPOT.TB positive was considered as eight or more spots,
negativity as ≤ 4 spots, and values in the between as borderline.
Patients
were divided into three groups: positive, borderline, and negative,
according to T-SPOT test results. T-SPOT-positive patients were
evaluated by the pulmonology clinic for active TB infection. The
patients' lung imaging (lung radiography or lung computed tomography
(CT)) and respiratory symptoms (cough, hemoptysis, fever, and/or
chills) were thoroughly evaluated. Preventive INH therapy was not
administered to T-SPOT test-positive patients who did not receive
chemotherapy, underwent low-intensity chemotherapy, declined INH
treatment, and had negative or borderline T-SPOT test results. Since
there is no clear consensus on indicating LTBI treatment in patients
with HM, as a general approach in our clinic, we did not give
preventive INH to patients who received low-intensity chemotherapy and
did not receive chemotherapy.
Statistical analysis.
The prevalence of LTBI was determined by dividing the number of
patients with a positive T-SPOT test by the total number of patients.
Active TB cumulative incidence was determined by dividing the number of
newly diagnosed active TB cases by the total number of patients during
follow-up. The person-year method was used to calculate the incidence
rate, and the Poisson regression model was used to calculate the
confidence interval (CI). Parametric tests were used without performing
the normality test due to the compatibility of the Central Limit
Theorem. In the data analysis, the mean and standard deviation of the
continuous variables and the minimum and maximum values of the features
were used to define the categorical variables, including the frequency
and percentage values. One Way ANOVA test statistic was used to compare
the mean of three independent groups. Tukey statistic was evaluated as
a Post Hoc test if a difference was detected with ANOVA. Chi-square
test statistics were used to evaluate the relationship between two
categorical variables. Statistical analysis between groups was made
according to their T-SPOT results. Median follow-up time is given as
median (Q1-Q3) and min-max. In the data evaluation, www.e-picos.com, NY,
New York software, and MedCalc Statistical Software version 16.4.3
statistical package software were used.
Results
The study included two hundred-nine patients with HM (Figure 1). The clinical and sociodemographic characteristics of the 209 patients participating in the study are shown in Table 1. The mean age was 58 (18-85), with a female/male distribution of 81/128.
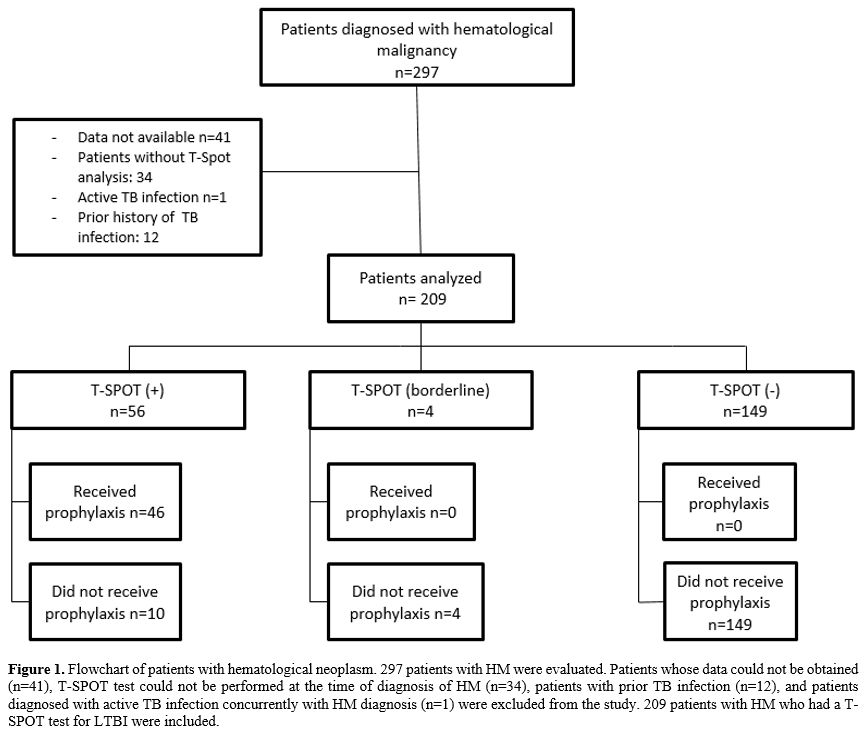 |
Figure 1. Flowchart of
patients with hematological neoplasm. 297 patients with HM were
evaluated. Patients whose data could not be obtained (n=41), T-SPOT
test could not be performed at the time of diagnosis of HM (n=34),
patients with prior TB infection (n=12), and patients diagnosed with
active TB infection concurrently with HM diagnosis (n=1) were excluded
from the study. 209 patients with HM who had a T-SPOT test for LTBI
were included. |
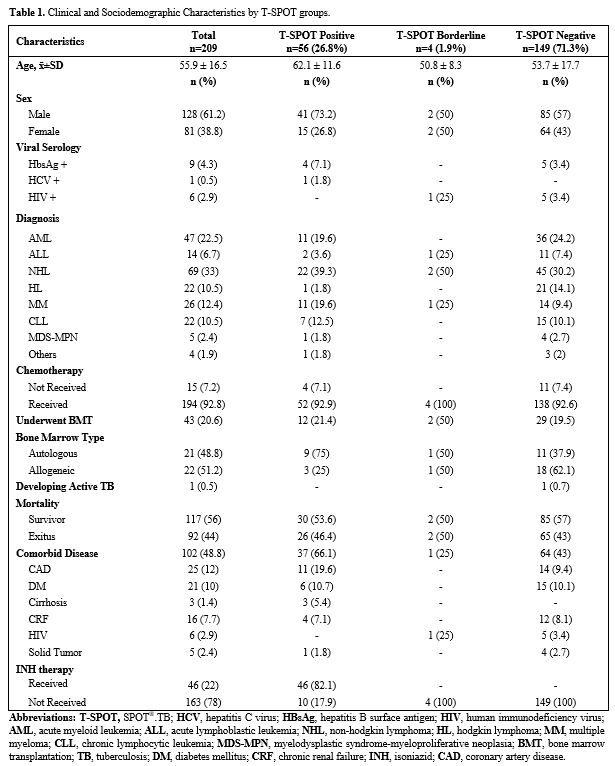 |
Table 1. Clinical and sociodemographic characteristics by T-SPOT groups.
|
T-SPOT
test results were positive in 56 of 209 patients (26.8%), borderline in
4 (1.9%), and negative in 149 (71.3%). The mean age of those with
positive, borderline, and negative T-SPOT tests was 62.1±11.6,
50.8±8.3, and 53.7±17.7, respectively. The mean age of T-SPOT-positive
patients was higher than that of negative patients (p=0.001).
Sixty-nine
patients (33%) were diagnosed with non-Hodgkin lymphoma, 47 (22.5%)
patients with acute myeloid leukemia, 26 (12.4%) patients with multiple
myeloma, 22 (10.5%) patients with Hodgkin lymphoma, 22 (10.5%) patients
with chronic lymphocytic leukemia, 14 (6.7%) patients with acute
lymphoblastic leukemia, 5 (2.4%) patients with myelodysplastic
syndrome-myeloproliferative neoplasia (MDS/MPN) and 4 (1.9%) patients
with other HM.
The median follow-up period was 740 days (range
2-2119) for the entire population, 732 days (2-2119) for those with
negative T-SPOT test, 808 days (10-2093) for those with positive T-SPOT
test, 523 days (99-680) for those with borderline T-SPOT test, and 785
days (26-2093) for 46 patients with positive T-SPOT test and whose INH
was started. During this period, 94 (45%) of 209 patients died due to
malignancy. It was found that 26 (46.4%) patients with positive T-SPOT
test were dead. No death from TB infection or toxic effect of INH
therapy was observed. While 194 (92.8%) of 209 patients received
chemotherapy, chemotherapy was not administered to 15 (7.2%) patients.
4 (7.1%) of 56 patients with positive T-SPOT test did not receive
chemotherapy. HCT was performed in 43 (20.6%) of 209 patients, of which
21 (48.8%) autologous HCT and 22 (52.2%) allogeneic HCT were performed.
Comorbidities were found in 102 (48.8%) of the patients. Most
frequently, coronary artery disease was found in 25 patients (12.2%),
followed by diabetes mellitus in 21 (10%) patients, chronic renal
failure in 16 (7.7%) patients, viral hepatitis in 10 (4.8%) patients,
HIV positivity in 6 (2.9%) patients, solid tumor in 5 (2.4%) patients.
The
prevalence of LTBI was 26.8% in the patients included in the study.
Preventive INH therapy was initiated in 46 (82.1%) of 56 patients with
LTBI, while preventive INH therapy was not initiated in 10 patients.
Two (20%) of these patients did not receive preventive INH therapy
because they did not accept prophylactic treatment, 4 (40%) were
followed without chemotherapy, and 4 (40%) received low-intensity
chemotherapy. The patients were scheduled to receive preventive INH
therapy at a 300 mg/day dose for nine months. Only 1 out of 46 patients
had to stop their INH treatment due to adverse effects. However, 1
(2.2%) patient was lost to follow-up, and 9 (19.6%) patients died due
to malignancy before the preventive therapy period was completed.
The
patients were followed up for side effects related to INH. Elevated
liver enzymes developed in 4 (8.7%) patients, and gastrointestinal
symptoms developed in 3 (6.5%) patients. Except for one patient whose
preventive INH therapy was discontinued due to liver enzymes five-fold
higher than the ULN without any symptom, no indication for interruption
or complete discontinuation of treatment occurred in the other patients.
Three
of the 209 HM patients had liver enzymes more than 3-fold higher than
the ULN at the onset of the disease and before INH, which was started
after chemotherapy when the transaminase values of the three patients
were less than 3-fold the ULN. After INH treatment, no enzyme elevation
was observed in these patients.
Only one of the 209 HM patients
developed an active TB infection during the follow-up period. The
cumulative incidence of active TB infection was 0.48%, yielding an
incidence rate of 217 cases/100.000hab/year (95% CI, 29-748). None of
the T-SPOT-positive patients developed active TB infection.
Interestingly, the patient who developed active TB infection was found
in the T-SPOT-negative group. This patient received R-CODOX-M &
R-IVAC (rituximab, doxorubicin, vincristine, cyclophosphamide,
cytarabine, methotrexate & rituximab, cytarabine, etoposide,
ifosfamide, methotrexate) chemotherapy protocol for a total of 4 months
with the diagnosis of plasmablastic lymphoma and HIV(+). The patient's
CD4 count was 110 cells/mm3.
Persistent fever, weight loss, and sweating developed approximately one
month after chemotherapy was finished and five months after the T-SPOT
test. PET/CT revealed pulmonary nodular lesions and intra-abdominal
lymphadenomegaly with FDG uptake. In addition, granulomatous
inflammation with caseification necrosis was observed in the tru-cut
biopsy performed from the lung and intra-abdominal lymph nodes, and
acid-fast stained bacilli were observed in the biopsy tissue.
Discussion
The
prevalence of LTBI at the time of diagnosis in HM patients was found to
be 26.8% in our study. To the best of our knowledge, this study is the
first in our country to screen for LTBI using only the T-SPOT test in
patients with HM. In our country, different results were encountered
when examining the frequencies of LTBI with T-SPOT in non-HM patients.
T-SPOT was found positive in 28 (20%) of 141 patients with a mean age
of 33, who were sent to them from different branches for a T-SPOT test
in a university hospital microbiology laboratory.[5] Binay et al.[6] found a T-SPOT positive rate of 22% in their study of 100 HIV-infected patients. In a study by Senturk et al.,[7]
the prevalence of LTBI was 13.8% due to the T-SPOT test performed
before anti-TNF treatment in 109 patients with rheumatic disease.
Immunocompromised
patients have been shown to have a nine-fold higher risk of developing
active TB infection from LTBI compared to the general population.[8] Ganzel et al.[9]
found that the MDS/MPN (148.8/100.000 patients) and lymphoma
(154.1/100.000 patients) groups had the highest cumulative incidence of
active TB infection after a cancer diagnosis. Niu et al.[10]
found that 66 of 4712 HM patients developed active TB infection with a
prevalence of 1.40%. The prevalence of active TB in HM patients was
higher compared to the general population. In our study, the cumulative
incidence of active TB in HM patients was found to be 0.48%, with a
population rate of 478/100.000. The cumulative incidence of active TB
in our HM patient group was substantially higher than in the general
population of our country. Thus, all HM patients were considered to be
at high risk of developing TB infection.
INH is the most studied
and proven medicine in the treatment of LTBI. LTBI treatment with INH
for nine months provides 90% protection and appears to be the optimal
duration.[11] Elevated liver enzymes caused by INH therapy have been determined in various ways in the literature. Osorio-López et al.[12]
planned INH as LTBI treatment for 93 patients with HM for nine months,
and they observed 15.1% of drug-related adverse effects. 4.3% of the
patients had to discontinue the treatment due to side effects related
to INH, and 3.2% (n=3) of them were due to elevated liver enzymes.
Sánchez-García et al.[13] observed elevated liver
enzymes in 18 (85%) of 21 HM patients due to INH therapy. INH treatment
was discontinued in 3 (14%) patients. They mentioned that the higher
liver enzymes detected more than the literature could be due to the
patients' high mean age. Our study observed side effects related to IHN
in 7 (15.2%) patients. INH treatment had to be discontinued in only one
patient (2.2%) out of 46 patients due to elevated liver enzymes. Of the
46 patients planned for INH treatment, 35 completed their treatment for
nine months. The main reason for not completing preventive INH therapy
was early mortality due to malignancy. The lower liver enzyme elevation
in our study could be the attention we paid to drug interactions in the
selection of chemotherapy in patients scheduled for INH therapy.
T-SPOT
test results were borderline in 4 patients (1.9%) in our study
population. This rate is roughly comparable to the rates reported in
the literature. For example, Rego et al.[14] reported
a borderline result rate of 1.8% in 645.947 T-SPOT tests. In our study,
these patients were not administered preventive INH therapy, and none
developed active TB infection.
The risk of reactivation is
greatest within the first two years of Mycobacterium tuberculosis
exposure and also reflects LTBI reactivation.[15] The
median follow-up period for 46 T-SPOT-positive patients who started INH
was more than two years (median 785 (26-2093) days). None of these
patients developed active TB infection during the follow-up period. One
hundred forty-nine patients who were T-SPOT negative and did not
receive preventive INH therapy were followed for a median of 732 days.
Only one of these patients developed an active TB infection. The
patient who developed active TB infection was diagnosed with active TB
infection approximately two months after the chemotherapy ended. The
sensitivity of the T-SPOT test in diagnosing LTBI is higher compared to
QFT and TST (approximately 90, 80, and 80 percent, respectively).[16] Shangguan et al.[17]
investigated risk factors for false-negative T-SPOT results in 833
patients with active TB infection. They found that advanced age, female
gender, and HIV coinfection were independent risk factors associated
with false-negative T-SPOT.TB results. The sensitivity of the T-SPOT.TB
test was found to be 33.3% in HIV-infected active TB patients, and they
showed that HIV-positive patients had a 6-fold higher risk of
false-negative T-SPOT results compared to negatives. Active TB
infection is an opportunistic infection in HIV(+) patients. The risk of
developing active TB infection in HIV-infected people is 20-37 times
higher than in non-HIV-infected people.[18]
Preventive therapy is recommended in these patients in the presence of
LTBI. Co-administration of HM and chemotherapy in HIV(+) patients
without LTBI, it is thought that it would be prudent to monitor these
patients for active TB closely.
Fever, lymphadenomegaly, cough,
sweating, loss of appetite, weight loss, and malaise are the most
prominent symptoms of active TB infection.[19] Since
the symptoms of the two diseases may overlap, the diagnosis of active
TB infection may be missed or delayed. Immunosuppression by HM and
chemotherapeutic agents may alleviate the symptoms of TB. As a result,
a delayed or missed active TB infection has negative effects on
mortality in this high-risk population for active TB infection.[20] Silva et al.[21]
observed the development of active TB infection at a rate of 2.6% in HM
patients during their follow-up, and they found the TB-related
mortality rate to be 62.5% in these patients. Our study observed no
death from TB infection in 209 patients diagnosed with HM. Therefore,
performed LTBI screening and administered preventive INH therapy are
considered beneficial.
The mean age of those with positive T-SPOT
was significantly higher than those with negative results. The higher
rate of LTBI observed in the older age group may be due to increased
cumulative exposure to TB bacillus.[22]
Our
study had some limitations. First, it was performed retrospectively in
a single-center tertiary hospital. Second, we did not include the
epidemiologic factors for TB infection in this study, such as
occupation (diary workers), socioeconomic status, history of TB
exposure, consumption of unpasteurized milk products, and cattle
exposure. All these variables might affect the T-SPOT results and also
allow the classification of the patients according to their risk, and
this could eventually help to identify patients at higher risk of LTBI
even with a negative T-SPOT. Third, as this study was conducted in a
country with a low TB burden, its results may not apply to countries
with moderate-to-high ones.
Conclusions
Although
LTBI is difficult to demonstrate in patients living with HIV and HM who
are scheduled for chemotherapy, these patients should be closely
monitored for the development of active TB infection. INH treatment was
generally well tolerated. Serious drug-related side effects were
observed very little. There was no interaction with the
chemotherapeutics used. Due to advances in cancer treatment, patients
with HM have a longer life expectancy in an immunocompromised state,
which increases the susceptibility to TB. Thus, it is thought that the
risk of TB infection will remain on the agenda in patients with HM.
Authorship Statement
All
authors meet the ICMJE authorship criteria. Emrah Kilicaslan and Kadir
Canoglu contributed to the study concept and design, as well as data
acquisition, interpretation and analysis, writing and critical revision
of the final manuscript.
Ethics Committee Approval
This study was approved by the Ethics Committee of Istanbul Medeniyet University Hospital (decision no: 2022/0027).
References
- Turkish Republic Ministry of Health. Tuberculosis
Control Report 2020 in Turkey. Available at:
https://hsgm.saglik.gov.tr/tr/tuberküloz-haberler/turkiye-de-verem-savasi.html.
Accessed March 01, 2022.
- Carranza
C, Pedraza-Sanchez S, de Oyarzabal-Mendez E, Torres M. Diagnosis for
Latent Tuberculosis Infection: New Alternatives. Front Immunol.
2020;11:2006. https://doi.org/10.3389/ fimmu.2020.02006 PMid:33013856 PMCid:PMC7511583
- Cheng MP, Abou Chakra CN, Yansouni
CP, Cnossen S, Shrier I, Menzies D, Greenaway C. Risk of Active
Tuberculosis in Patients with Cancer: A Systematic Review and
Meta-Analysis. Clin Infect Dis. 2017;64:635-644. https://doi.org/
10.1093/cid/ciw838 PMid:27986665
- Trajman
A, Steffen RE, Menzies D. Interferon-Gamma Release Assays versus
Tuberculin Skin Testing for the Diagnosis of Latent Tuberculosis
Infection: An Overview of the Evidence. Pulm Med. 2013;2013:601737. https://doi.org/10.1155/2013/601737 PMid:23476763 PMCid:PMC3582085
- Tanriverdi Cayci Y, Korkmaz F,
Birinci A. Retrospective evaluation of T-Spot. TB test results that
sent to our tuberculosis laboratory. Ortadogu Med J. 2017;9:24-27. https://doi.org/10.21601/ortadogutipdergisi.293217
- Binay
UD, Fincanci M, Fersan E, Karakecili F. Comparison of Tuberculin Skin
Test (TST) and T-SPOT.TB Tests for Diagnosis of Latent Tuberculosis
Infection (LTBI) in HIV-infected Patients. Mikrobiyol Bul.
2019;53:388-400. https://doi.org/10.5578/mb.68601
PMid:31709936
- Sargin
G, Sentürk T, Ceylan E, Telli M, Cildag S, Dogan H. TST, QuantiFERON-TB
Gold test and T-SPOT.TB test for detecting latent tuberculosis
infection in patients with rheumatic disease prior to anti-TNF therapy.
Tuberk Toraks. 2018;66:136-143. https://doi.org/10.5578/tt.66444
PMid:30246657
- Malone
JL, Ijaz K, Lambert L, Rosencrans L, Phillips L, Tomlinson V, Arbise M,
Moolenaar RL, Dworkin MS, Simoes EJ. Investigation of
healthcare-associated transmission of Mycobacterium tuberculosis among
patients with malignancies at three hospitals and at a residential
facility. Cancer. 2004;101:2713-2721. https://doi.org/10.1002/
cncr.20698 PMid:15547933
- Ganzel
C, Silverman B, Chemtob D, Ben Shoham A, Wiener-Well Y. The risk of
tuberculosis in cancer patients is greatest in lymphoma and
myelodysplastic syndrome/myeloproliferative neoplasm: a large
population-based cohort study. Leuk Lymphoma. 2019;60:720-725.
https://doi.org/10.1080/ 10428194.2018.1499904 PMid:30188229
- Niu
T, Li J, Jiang M, Yang Y, Liu T. Clinical Research On Hematological
Malignancies Complicated With Active Tuberculosis: A Single Center
Experience In China. Blood. 2013;122:5592. https://doi.org/10.1182/blood.V122.21.5592.5592
- Snider
DE Jr, Caras GJ, Koplan JP. Preventive therapy with isoniazid.
Cost-effectiveness of different durations of therapy. JAMA.
1986;255:1579-1583. https://doi.org/10.1001/jama.255.12.1579 PMid:3081740
- Osorio-López
EA, Vilar-Compte D, García-Tirado J, Martin-Onraet A. Prevalence of
latent tuberculosis in patients with hematological neoplasms in a
cancer referral hospital in Mexico City. BMC Infect Dis. 2021;21:1-7.
https://doi.org/10.1186/s12879-021-06236-y PMid:34059022
PMCid:PMC8168316
- Sánchez-García
EM, Gamallo R, Blanco-Moure A, Viejo MA, Amador L, Anibarro L. Toxicity
and adherence to treatment for latent tuberculosis infection in
patients with hematologic malignancies. Infection. 2013;41(5):903-907. doi: 10.1007/s15010-013-0489-9.
https://doi.org/10.1007/s15010-013-0489-9 PMid:23737388
- Rego
K, Pereira K, MacDougall J, Cruikshank W. Utility of the T-SPOT®.TB
test's borderline category to increase test resolution for results
around the cut-off point. Tuberculosis (Edinb) 2018;108:178-185. https://doi.org/10.1016/j.tube.2017.12.005 PMid:29523321
- Cheng
MP, Kusztos AE, Bold TD, Ho VT, Glotzbecker BE, Hsieh C, Baker MA,
Baden LR, Hammond SP, Marty FM. Risk of Latent Tuberculosis
Reactivation After Hematopoietic cell Transplantation. Clin Infect Dis.
2019;69:869-872. https://doi.org/10.1093/cid/ciz048 PMid:30689792 PMCid:PMC6938207
- Pai
M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for
the diagnosis of latent tuberculosis infection: an update. Ann Intern
Med 2008;149:177-184. https://doi.org/10.7326/0003-4819-149-3-200808050-00241 PMid:18593687 PMCid:PMC2951987
- Shangguan
Y, Fang H, Wang S, Ji Z, Shi P, Feng X, Xu K. Risk factors for negative
T-SPOT.TB assay results in patients with confirmed active tuberculosis:
A retrospective study. J Infect Dev Ctries. 2020;14:1288-1295. https://doi.org/10.3855/jidc.12063 PMid:33296342
- Beshaw
MA, Balcha SA, Lakew AM. Effect of Isoniazid Prophylaxis Therapy on the
Prevention of Tuberculosis Incidence and Associated Factors Among HIV
Infected Individuals in Northwest Ethiopia: Retrospective Cohort Study.
HIV AIDS (Auckl). 2021;13:617-629. https://doi.org/10.2147/HIV.S301355 PMid:34135640 PMCid:PMC8197569
- Mayock RL, MacGregor RR. Diagnosis, prevention and early therapy of tuberculosis. Dis Mon. 1976;22:1-60. https://doi.org/10.1016/s0011-5029(76) 80006-5 PMid:817877
- Kaplan
MH, Armstrong D, Rosen P. Tuberculosis complicating neoplastic disease.
A review of 201 cases. Cancer. 1974;33:850-858. https://doi.org/10.1002/1097-0142(197403)33:3<850::AID-CNCR2820330334>3.0.CO;2-H PMid:4592905
- Silva
FA, Matos JO, de Q Mello FC, Nucci M. Risk factors for and attributable
mortality from tuberculosis in patients with hematologic malignances.
Haematologica. 2005;90:1110-1115.
- Kizza
FN, List J, Nkwata AK, Okwera A, Ezeamama AE, Whalen CC, Sekandi JN.
Prevalence of latent tuberculosis infection and associated risk factors
in an urban African setting. BMC Infect Dis. 2015;15:165. https://doi.org/10.1186/s12879-015-0904-1 PMid:25879423 PMCid:PMC4392742
[TOP]

