Presentation of patients with APL is often characterized by coagulopathy.[1]
At diagnosis, a percentage close to 76% of APL patients have some clinical and/or laboratory features of coagulopathy, from skin or soft tissue bleedings to intracranial hemorrhage.[2-3] While physicians pay attention to bleeding-related complications in APL, it is also important to note that it is not uncommon to develop thrombotic events, particularly in patients on treatment.[4]
Lately, the introduction of new drugs such as all-trans retinoic acid (ATRA) and arsenic trioxide (ATO) allowed for reducing complications: bleeding events were predominant rather than severe thrombotic events (29% vs. 12%).[5]
The major cause of death during induction was due to hemorrhage;[6-7] even if with the administration of ATRA or ATO, overall mortality due to bleeding complications has been halved from 14% of the pre-ATRA era.[5,8]
APL is characterized by a coagulopathy which is unique among leukemias. At presentation, most patients showed abnormalities in their coagulation profile, such as prolonged prothrombin time (PT), partial thromboplastin time (PTT), and thrombin time (TT), but it happens to observe normal values. Typical laboratory features of coagulation factors consumption are represented by fibrin degradation products and D-dimer increase, and fibrinogen level decrease.[9] Thrombocytopenia was common and often severe.[4,10]
The two main mechanisms behind the coagulopathy of APL are tissue factor (TF)-induced disseminated intravascular coagulation (DIC) and primary hyperfibrinolysis.[10]
In the pre-ATRA era, chemotherapy exacerbated bleeding manifestations.[5]
Even if the introduction of ATRA seemed to reduce the risk of bleeding-related complications because the balance of prothrombotic factors shifts toward thrombosis, the risk of thrombosis did not appear to reduce.[2] Clinical features improved after starting treatment; in particular, their improvement was faster when ATRA was added to chemotherapy, but it may take several days. In fact, laboratory parameters and clinical manifestations were often discordant.[11-16]
The effect on the hemorrhagic risk of ATO addition on induction therapy was less studied. Incorporating ATO into induction therapy should reduce hemorrhagic complications; ATO, in combination with ATRA, may reverse coagulopathy even more promptly than ATRA alone.[17] Heparin prophylaxis during treatment with ATO was not recommended, except for the treatment of thrombotic events.[6]
Coagulation laboratory alterations are typically reversed following treatment with ATRA plus ATO: it seemed that coagulopathy generally abates by 5–7 days, and the abnormal coagulation and fibrinolytic profiles returned to normal after 14 days or longer.[5,8,18]
In this scenario of patients treated with ATO plus ATRA, since little data about coagulation patterns are available, our study aimed to evaluate coagulation-related parameters in low/intermediate risk APL patients at presentation and investigate mechanisms of APL coagulopathy by measuring changes in these values prior to and during ATO plus ATRA treatment.
From our data analysis, we investigated coagulative patterns through our real-life monocentric experience.
We included each patient affected by APL, treated according to the ATO plus ATRA regimen, which was part of the standard treatment for APL. We included patients at low and intermediate risk according to Sanz’ risk score; high-risk patients were excluded by treating physicians as per standard practice.[19]
Each patient was treated with intravenous ATO (0.15 mg/kg/die) and oral ATRA (25 mg per square meter of body-surface area) as first-line treatment. Platelets transfusion was administered with a target of platelet count over 30×109/L or 50×109/L in the presence of bleeding. The administration of fresh frozen plasma (FFP) was performed in patients with a prolonged PT value>3 ec compared to normal and/or a fibrinogen level less than 150 mg/dl.[8]
The DIC score for all patients in this study was calculated using the overt DIC criteria of the International Society on Thrombosis and Hemostasis (ISTH).[20]
The study was approved by our institutional ethics committee and conducted in accordance with the Declaration of Helsinki.
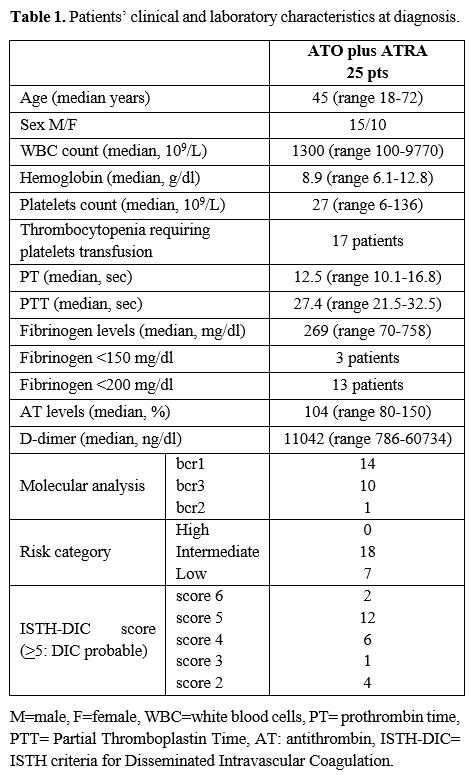
Twenty-five patients admitted to our Department of Hematology between January 2009 and June 2021 were included in the study: fifteen male and ten female patients with a median age of 45 years (range 18-72), mostly intermediate risk (18 patients, 72%). Characteristics of the patients at diagnosis are shown as median values in Table 1.
 |
|
PT and PTT median values were in the normal range. Elevated D-dimer levels were seen in all patients. The overall incidence of thrombocytopenia requiring platelets transfusion was 68% (17 out of 25) of patients. Only three patients had fibrinogen <150mg/dl; however, fibrinogen level<200 mg/dl was seen in 13 patients (in 8 patients from the diagnosis and in 5 patients after a median of 4 days from the ATRA introduction). When calculating the ISTH-DIC score, 14 patients had an overt DIC (defined as a score ≥5) at presentation, and 11 patients showed a condition of subclinical DIC.
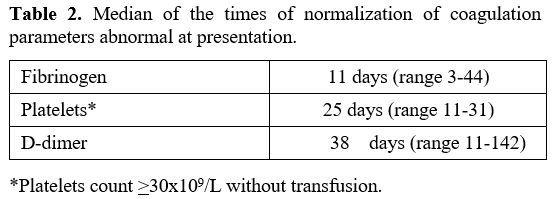
We followed the dynamic changes in the hemostatic parameters during induction therapy, as summarized in Table 2.
 |
|
The first parameter to normalize was fibrinogen, after a median time of 11 days (range 3-44 days) after the beginning of the therapy; only five patients received FFP, and 17 patients required platelet transfusion reaching the count >30x109/L without transfusion after a median of 25 days (range 11-31). A median of 8 units (range 3-23) was the need for platelet transfusion. No major hemorrhagic events were registered during ATO plus ATRA treatment.
D-dimer levels normalized after a median of 38 days without any clinical evidence of complications except for two thrombotic events (one deep vein thrombosis and one superficial vein thrombosis of the leg after 34 days from ATRA introduction) properly managed with low molecular weight heparin treatment.
All patients were discharged after ATO plus ATRA induction and achieved complete hematologic remission in a median time of 36 days (range 25–47 days). In addition, all patients obtained molecular remission after a median time of 3 months (range 1-6), and all but one patient, dying of progressive disease, are alive and in molecular response at a median follow-up of 43 months (range 1-145).
Coagulopathy in APL is of utmost importance even because previous studies did not aim to measure the coagulation parameters and analyze them as potential predictors.
Retrospective analysis pointed out predictive risk factors for bleeding and thrombosis, but a major limitation of these studies was the inherent bias linked to the effect caused by treatment. For example, the administration of FFP in APL patients with reduced fibrinogen levels makes difficult the evaluation of it as a bleeding risk factor, and in general, the blood product administration makes difficult to stratify patients on bleeding or thrombotic risk.[12] As far as low and intermediate-risk patients are concerned, bleeding symptoms disappeared in most patients rapidly with the beginning of ATO plus ATRA treatment.
A direct comparison between our study and others[13-14] is difficult due to the different populations and treatments considered; our analysis included APL patients in low and intermediate risk who did not necessitate chemotherapy; moreover, we did not use the oral formulation of arsenic.
Our coagulation pattern data are in line with previously published data in terms of characteristics at baseline and dynamic changes in hemostatic variables. Our data are the product of a real-life experience in a single center in Western Europe, but with the administration of intravenous ATO and the collection of a complete coagulation pattern.
In conclusion, parameters often did not return to normal in the first month of treatment, suggesting that the coagulopathy did not fully recover and persisted. Therefore, prolonged monitoring of coagulation and fibrinolysis parameters was still required. Also, increased D-dimer levels, which reflect the product of fibrinolysis of cross-linked fibrin, suggested the long-standing activation of coagulation.
The evidence of alterations in blood clotting tests seems not to correspond to clinically significant thrombotic or hemorrhagic complications. ATO plus ATRA regimen shows a series of advantages compared to chemotherapy, allowing to treat patients and reducing possible complications, also in the setting of coagulopathy.