Nicola Sgherza1, Paola Curci1, Rita Rizzi1,2, Angela Maria Vittoria Larocca3, Luigi Vimercati4, Silvio Tafuri4, Maria Chironna4 and Pellegrino Musto1,2.
1 Hematology and Bone Marrow Transplantation Unit, AOUC Policlinico, Bari, Italy.
2 Department of Precision and Translational Medicine with Ionian Area, “Aldo Moro” University School of Medicine, Bari, Italy.
3 Hygiene Unit, AOUC, Policlinico Bari, Bari, Italy.
4 Interdisciplinary Department of Medicine, “Aldo Moro” University School of Medicine, Bari, Italy.
Correspondence to:
Prof. Pellegrino Musto, Hematology and Bone Marrow Transplantation
Unit, AOUC Policlinico and Department of Precision and Translational
Medicine with Ionian Area, “Aldo Moro” University School of Medicine,
Bari, Italy. E-mail:
pellegrino.musto@uniba.it
Published: January 1, 2023
Received: October 24, 2022
Accepted: December 21, 2022
Mediterr J Hematol Infect Dis 2023, 15(1): e2023011 DOI
10.4084/MJHID.2023.011
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
To the editor
Monoclonal
gammopathy of undetermined significance (MGUS) is a pre-malignant
plasma cell disorder reported in approximately 3-4% of individuals aged
> 50 years, characterized by a low risk (about 1% per year) of
progression into “overt” myeloma or other lymphoproliferative diseases.
It is usually asymptomatic, but a higher risk of deep venous thrombosis
and infections,[1,2] as well as immune dysregulation, have been reported.[3]
About this last point, considering that vaccination against SARS-CoV-2
is the main strategy to prevent adverse outcome of COVID-19 (declared a
pandemic by the World Health Organization in March 2020), the
evaluation of humoral response to COVID-19 vaccines has gained
increasing interest as valuable surrogate of vaccine effectiveness. In
this field, several papers investigated antibody response to
anti-SARS-CoV-2 vaccination in patients with hematological diseases; by
contrast, few data are available about patients with MGUS. Terpos et
al.[4] reported no significant differences in terms of
“neutralizing antibody response” between MGUS patients and healthy
controls (HCs); in particular, in this analysis, 21 of 25 patients
(84%) achieved clinically relevant antibody response after two vaccine
doses. Abella et al.[5] confirmed these data,
reporting no differences in the vaccine-induced humoral responses
between uninfected MGUS subjects (n=15) and HCs after two doses. Storti
et al.[6] evaluated humoral and cellular response
after two doses of anti-SARS-CoV-2 vaccine in 40 patients with
monoclonal gammopathies at different stages of disease, including 6
patients with MGUS, reported as “responders”. Konishi et al.[7]
evaluated antibody titer in 13 MGUS patients after three doses of
anti-SARS-CoV-2 vaccine, comparing it with that of HCs after two doses
and no differences were described. Further studies concerning plasma
cell dyscrasia and including patients with MGUS have been published,
but they report generalized data without clearly distinguishing between
MGUS and other conditions[8,9] While most of the
studies investigated humoral response after two vaccine doses, our
present, real-life observational study, aimed to evaluate the rate of
response and the titers of anti-spike IgG antibodies after a “booster”
(third) dose. Secondary outcomes included comparisons of anti-spike IgG
titers between MGUS patients and age and sex-matched healthcare
workers, who were enrolled in the study as HCs.
Data of MGUS patients were extracted from medical records; further
information was taken from “Infections Regional Information System
(IRIS)”, a regional (Puglia, Italy) platform by which authorized
medical health workers can view the results of the nasopharyngeal swabs
for SARS-CoV-2 performed, along with other information.
Quantitative determination of anti-spike IgG antibodies was performed
using a commercially available Abbott immunoassay, at least two weeks
after the “booster” dose. Results were reported as arbitrary units
AU/mL. Informed consent was obtained prior to the collection of data
and specimens. Statistical analyses were carried out using GraphPad
Prism version 8.3.0 (GraphPad Software Inc., San Diego, CA, USA).
Twenty COVID-19-naïve and fully vaccinated MGUS patients followed at
Hematology Unit - AOU Policlinico di Bari (Italy) were enrolled in this
study. Mean age was 63.15 years (range 39-86). Characteristics of MGUS
patients are reported in Table 1A.
The most frequent MGUS-isotype was IgG (70%), followed by IgA (20%) and
IgM (10%). Most of patients (95%) were at low or low-intermediate risk,
according to Mayo Clinic prognostic model.
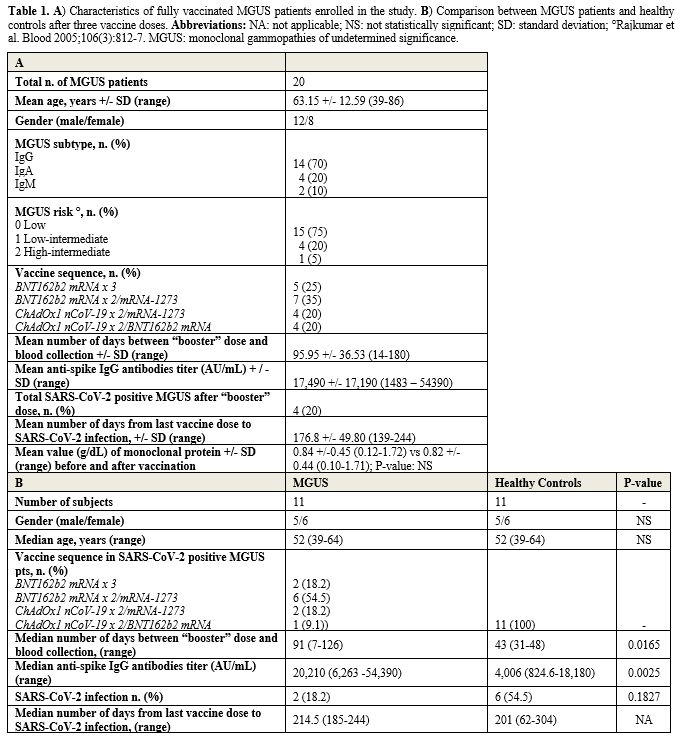 |
- Table 1. A)
Characteristics of fully vaccinated MGUS patients enrolled in the
study. B) Comparison between MGUS patients and healthy controls after
three vaccine doses. Abbreviations: NA: not applicable; NS: not
statistically significant; SD: standard deviation; °Rajkumar et al.
Blood 2005;106(3):812-7. MGUS: monoclonal gammopathies of undetermined
significance.
|
After
a mean number of 96 days (range 14-180) from third vaccine dose, all
MGUS patients (100%) achieved a titer greater than 50 AU/mL; thus, they
were all considered as “responders”. Notably, in contrast with
previously reported data,[10] administration of the
first two doses of ChAdOx1 was not associated to lower antibody titer
compared to that after two BNT162b2 doses. We did not find any
correlation between gender (p-value=0.1768), MGUS-subtype
(p-value=0.1956), MGUS risk-stratification (p-value=0.1647), sequence
of vaccine doses (p-value=0.4144), days from third vaccine dose to
blood collection (p-value=0.3347) and titer of anti-spike IgG
antibodies. Age > 63 years (vs < 63) was instead associated with
a significant lower antibody titer (p-value=0.0122). Despite immune
stimulation, monoclonal protein remained stable after vaccination in
all MGUS patients analyzed (Table 1A).
Four MGUS patients (20%) experienced a breakthrough infection,
asymptomatic or with mild symptoms, after a mean number of 177 days
(range 139-244) from the “booster” dose. In these patients the median
titer of anti-spike IgG antibodies of 4,741 AU/mL (range: 3,218 –
20,210) before infection was not significantly different (p-value=
0.2376) from that of uninfected MGUS patients (12,870 AU/mL; range:
1,483-54,390). These cases of infection were reported between May and
August 2022 and attributable realistically to Omicron BA.2, BA.4 and
BA.5 variant of SARS-CoV-2.
Then we compared serological response of MGUS patients with those of
age and sex matched healthcare workers, enrolled in the study as HCs.
It was possible only for 11 patients; the remaining nine ones were
older than HCs and therefore not comparable with healthcare workers.
All HCs had received three doses of BNT162b2 mRNA vaccine. Quite
unexpectedly, the median titer of anti-spike IgG antibodies was
significant higher in MGUS patients than in HCs (p-value=0.0025) (Table 1B).
This might be due to longer time frame between “booster” dose and blood
collection in MGUS patients than HCs (91 vs 43 days; p-value: 0.0165).
Indeed, it is known that antibody titer progressively increases after
vaccine-dose and gradually declines over the ensuing months. Regarding
the number of cases of breakthrough infection, it was higher (but not
significantly; p-value= 0.1827) in HCs than MGUS patients, probably due
to the major infectious risk of healthcare workers.
Though still preliminary, to the best of our knowledge, this is the
first report of serological response after three doses of
anti-SARS-CoV-2 vaccines in MGUS patients. Obviously, the study has
several limitations, such as the limited number of patients enrolled,
the lack of information about serological response after second dose
(before third dose), the lack of information regarding
neutralizing IgG antibodies against nucleocapsid and receptor-binding
domain cellular (this study evaluated only anti-spike IgG antibodies),
the different timing of blood collection (among MGUS patients and
between MGUS patients and HCs), the lack of data on antigen-specific B-
and T-cell responses information. Notwithstanding, our study highlights
some relevant points. First, humoral immune response is not attenuated
in MGUS patients after three doses of anti-SARS-CoV-2 vaccine,
confirming data after two doses; notably, all patients (100%) achieved
clinically relevant antibody response, improving reported data after
two vaccine doses (84%).[4] This aligns with reported
data that MGUS patients did not show an increased incidence of
SARS-CoV-2 infection compared to the general population and that MGUS
did not appear to represent a risk for a poorer COVID-19 outcome.[11,12] Indeed, in our previous experience, vaccination improved COVID-19 outcome, but not SARS-CoV-2 incidence.[13]
Second, to date it is the only study to report a long persistence
(until 180 days) of anti-spike IgG antibodies after “booster” dose in
MGUS patients, although a clear-cut relationship between these
antibodies and protection against the virus have not been unequivocally
established. Third, this is the first study including a case-control
analysis of serological response after “booster” dose of
anti-SARS-CoV-2 vaccine in MGUS patients. However, further studies on a
larger number of patients are needed to achieve greater
generalizability of our findings.
Authorship contributions
PM
and NS conceived and led the project. NS conducted database building,
extraction and coding. PM and NS queried and analyzed the data. PM and
NS wrote the main manuscript text and created all tables. All authors
made a substantial intellectual contribution to the study, interpreted
the data, discussed the results and reviewed, edited and approved the
final version of the manuscript.
References
- Kristinsson SY, Björkholm M, Andersson TM, Eloranta S,
Dickman PW, Goldin LR, Blimark C, Mellqvist UH, Wahlin A, Turesson I,
Landgren O. Patterns of survival and causes of death following a
diagnosis of monoclonal gammopathy of undetermined significance: a
population-based study. Haematologica. 2009 Dec;94(12):1714-20.
https://doi.org/10.3324/haematol.2009.010066 PMid:19608666
PMCid:PMC2791946
- Kristinsson SY, Tang M, Pfeiffer RM,
Björkholm M, Goldin LR, Blimark C, Mellqvist UH, Wahlin A, Turesson I,
Landgren O. Monoclonal gammopathy of undetermined significance and risk
of infections: a population-based study. Haematologica. 2012
Jun;97(6):854-8. https://doi.org/10.3324/haematol.2011.054015
PMid:22180421 PMCid:PMC3366650
- Zavidij O, Haradhvala NJ,
Mouhieddine TH, Sklavenitis-Pistofidis R, Cai S, Reidy M, Rahmat M,
Flaifel A, Ferland B, Su NK, Agius MP, Park J, Manier S, Bustoros M,
Huynh D, Capelletti M, Berrios B, Liu CJ, He MX, Braggio E, Fonseca R,
Maruvka YE, Guerriero JL, Goldman M, Van Allen EM, McCarroll SA, Azzi
J, Getz G, Ghobrial IM. Single-cell RNA sequencing reveals compromised
immune microenvironment in precursor stages of multiple myeloma. Nat
Cancer. 2020 May;1(5):493-506.
https://doi.org/10.1038/s43018-020-0053-3 PMid:33409501
PMCid:PMC7785110
- Terpos E, Gavriatopoulou M,
Ntanasis-Stathopoulos I, Briasoulis A, Gumeni S, Malandrakis P, Fotiou
D, Papanagnou ED, Migkou M, Theodorakakou F, Roussou M,
Eleutherakis-Papaiakovou E, Kanellias N, Trougakos IP, Kastritis E,
Dimopoulos MA. The neutralizing antibody response post COVID-19
vaccination in patients with myeloma is highly dependent on the type of
anti-myeloma treatment. Blood Cancer J. 2021 Aug 2;11(8):138.
https://doi.org/10.1038/s41408-021-00530-3 PMid:34341335
PMCid:PMC8327056
- Abella E, Trigueros M, Pradenas E,
Muñoz-Lopez F, Garcia-Pallarols F, Ben Azaiz Ben Lahsen R, Trinité B,
Urrea V, Marfil S, Rovirosa C, Puig T, Grau E, Chamorro A, Toledo R,
Font M, Palacín D, Lopez-Segui F, Carrillo J, Prat N, Mateu L, Clotet
B, Blanco J, Massanella M; VAC-COV-GM-HMAR, KING Cohort Extension and
CoronAVI@S studies. Efficacy of SARS-CoV-2 vaccination in patients with
monoclonal gammopathies: A cross sectional study. Life Sci Alliance.
2022 Aug 12;5(12):e202201479. https://doi.org/10.26508/lsa.202201479
PMid:35961779 PMCid:PMC9375155
- Storti P, Marchica V,
Vescovini R, Franceschi V, Russo L, Notarfranchi L, Raimondi V, Toscani
D, Burroughs Garcia J, Costa F, Dalla Palma B, Iannozzi NT, Sammarelli
G, Donofrio G, Giuliani N. Immune response to SARS-CoV-2 mRNA
vaccination and booster dose in patients with multiple myeloma and
monoclonal gammopathies: impact of Omicron variant on the humoral
response. Oncoimmunology. 2022 Sep 6;11(1):2120275.
https://doi.org/10.1080/2162402X.2022.2120275 PMid:36105747
PMCid:PMC9467550
- Konishi Y, Sklavenitis-Pistofidis R, Yue H,
Ferrari F, Redd RA, Lightbody ED, Russo M, Perry J, Horowitz E, Justis
AV, Shayegh NA, Savell A, Prescott J, Varmeh S, Nowak RP, Hamilton M,
Auclair D, Marinac CR, Trippa L, Fischer ES, Ghobrial IM. Attenuated
response to SARS-CoV-2 vaccine in patients with asymptomatic precursor
stages of multiple myeloma and Waldenstrom macroglobulinemia. Cancer
Cell. 2022 Jan 10;40(1):6-8.
https://doi.org/10.1016/j.ccell.2021.12.003 PMid:34895486
PMCid:PMC8654583
- Gung C, McGuire R, George M, Abdulkareem A,
Belden KA, Porcu P, Martinez-Outschoorn U, Binder AF, Chervenova I,
Alpdogan O. Antibody Response to SARS-CoV-2 Vaccination in Patients
With Lymphoproliferative Disorders and Plasma Cell Dyscrasias:
Anti-Lymphoma Therapy as a Predictive Biomarker of Response to
Vaccination. Front Oncol. 2022 Jul 7;12:840451.
https://doi.org/10.3389/fonc.2022.840451 PMid:35875166 PMCid:PMC9300919
- Wu AHB, Wang CC, Ong CM, Lynch KL. Adequate Antibody Response to
COVID-19 Vaccine in Patients with Monoclonal Gammopathies and Light
Chain Amyloidosis. Lab Med. 2022 May 5;53(3):314-319.
https://doi.org/10.1093/labmed/lmab113 PMid:35026018 PMCid:PMC8807219
- Jolliffe DA, Faustini SE, Holt H, Perdek N, Maltby S,
Talaei M, Greenig M, Vivaldi G, Tydeman F, Symons J, Davies GA, Lyons
RA, Griffiths CJ, Kee F, Sheikh A, Shaheen SO, Richter AG, Martineau
AR. Determinants of Antibody Responses to SARS-CoV-2 Vaccines:
Population-Based Longitudinal Study (COVIDENCE UK). Vaccines (Basel).
2022 Sep 23;10(10):1601. doi: 10.3390/vaccines10101601. https://doi.org/10.3390/vaccines10101601
PMid:36298466 PMCid:PMC9610049
- Rognvaldsson S, Eythorsson E,
Thorsteinsdottir S, Vidarsson B, Onundarson PT, Agnarsson BA,
Sigurdardottir M, Thorsteinsdóttir I, Olafsson I, Runolfsdottir HL,
Helgason D, Emilsdottir AR, Agustsson AS, Bjornsson AH, Kristjansdottir
G, Thordardottir AR, Indridason OS, Jonsson A, Gislason GK, Olafsson A,
Steingrimsdottir H, Kampanis P, Hultcrantz M, Durie BGM, Harding S,
Landgren O, Palsson R, Love TJ, Kristinsson SY. Monoclonal gammopathy
of undetermined significance and COVID-19: a population-based cohort
study. Blood Cancer J. 2021 Dec 1;11(12):191. Doi:
10.1038/s41408-021-00580-7. https://doi.org/10.1038/s41408-021-00580-7 PMid:34853309
PMCid:PMC8635472
- Sgherza N, Curci P, Rizzi R, Strafella V, Di
Gennaro D, Vitucci A, Palma A, Rossi AVR, Albano F, Stefanizzi P,
Tafuri S, Musto P. Incidence and outcome of SARS-CoV-2 infection in
patients with monoclonal gammopathy of undetermined significance: a
case-control study. Haematologica. 2022 Feb 1;107(2):555-557. doi:
10.3324/haematol.2021.279895. https://doi.org/10.3324/haematol.2021.279895 PMid:34732044
PMCid:PMC8804555
- Sgherza N, Di Gennaro D, Curci P, Rizzi R,
Roccotelli D, Croce M, Avantaggiato M, Ruga L, Strafella V, Vitucci A,
Palma A, Russo Rossi AV, Troiano T, Larocca AMV, Chironna M, Tafuri S,
Albano F, Musto P. SARS-CoV-2 infection incidence and outcome before
and after fully vaccination in patients with monoclonal gammopathy of
undetermined significance. Hemasphere. 2022 Nov 9;6(12):e800. doi:
10.1097/HS9.0000000000000800. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9649266/ PMID: 36382051; PMCID: PMC9649266.
