The disease has a complex pathogenesis, with well-recognized mechanisms of increased peripheral autoantibody-mediates destruction of the platelets but also a reduction of appropriate megakaryocyte functionality related to low level of endogenous thrombopoietin and both humoral and cytotoxic immune response targeting the megakaryopoiesis.[1] Moreover, in the last years, the role of Fc gamma receptors (FcγRs), neonatal Fc receptor (FcRn), the spleen tyrosine kinase (Syk), the complement factors, and the desialylation of the platelets have been elucidated, resulting in the development of new drugs and enhanced knowledge of potential new therapeutic targets.[2]
Patients who develop a chronic ITP are defined as “refractory” if they do not respond to two or more treatments, there is no single medication to which they respond, and their platelet count is very low and associated with bleeding.[3] Besides, in these patients, poor quality of life (QoL) related to fatigue, need for multiple hospital visits and blood tests, side effects of chronic treatment, and anxiety of disease worsening have been reported.[4] Therefore, an important goal in refractory ITP is to restore a durable, safe platelet count to avoid possible bleeding, reduce the number of medical checks and ameliorate their QoL.
For refractory patients, the role of combination treatment has been recently described[2-3] using drugs targeting different pathogenic mechanisms at the same time or with sequential use of multiple treatments (i.e., switch of thrombopoietin receptor agonists, or consecutive use of steroid, splenectomy, rituximab), suggesting a synergistic effect of different mechanisms of action in a well-recognized multifaceted immunological disease such as ITP.
We report for the first time the efficacy of sequential use of efgartigimod and romiplostim after the previous failure of two thrombopoietin receptor agonists (TPO-RA) and different immunosuppressive regimens in two multi-refractory patients. We also propose hypothesis about the synergistic effect of the two drugs.
Both patients stated written informed consent to use their anonymized data.
Efgartigimod is a novel human IgG1 Ab fragment that binds to neonatal Fc receptor (FcRn), thus preventing IgG recycling with clinical benefit for patients with autoantibody-mediated diseases such as ITP.[5] Efgartigimod has been recently approved by FDA for myasthenia, and phase II/III studies in ITP are ongoing. Recently, data about the ADVANCE Study (Efficacy and Safety of Intravenous Efgartigimod in Adults with Primary ITP, Phase 3 Double-blind, Placebo-controlled RCT) has been released during the 64th ASH Annual Meeting, demonstrating clinical and statistical improvement of platelet count in refractory ITP patients treated with efgartigimod versus placebo.[6]
Romiplostim is a well-known TPO-RA, with many safety and efficacy data collected in ITP and published since its development more than ten years ago.[7]
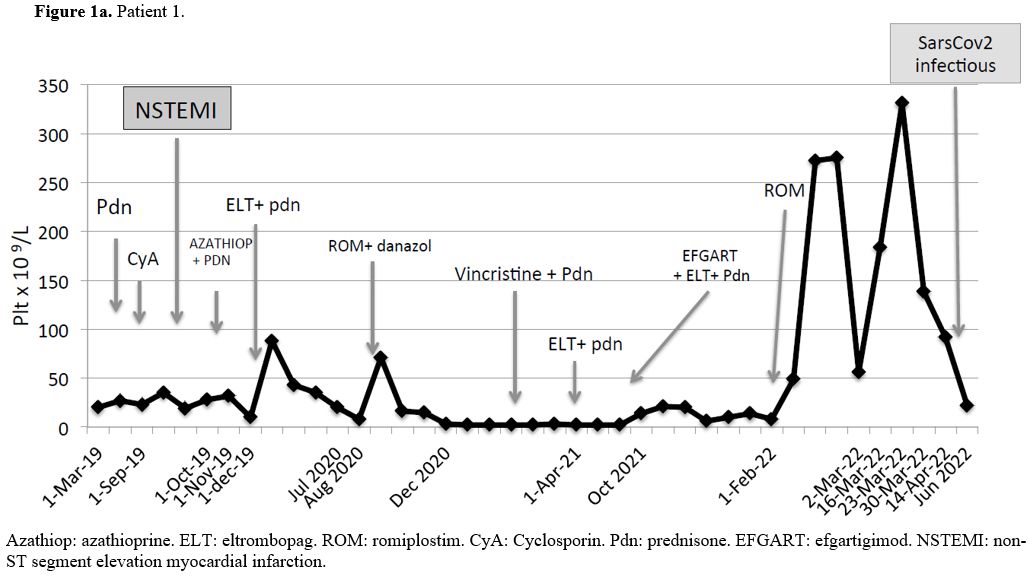
Patient#1 (Figure 1a): male with a history of a previous CABG and PCI at 68 years of age. ITP was diagnosed at 80 years of age. Bone marrow examination and extensive workup ruled out other causes of isolated thrombocytopenia. He was unresponsive to prednisone plus IVIG as the first line, and second-line treatment with cyclosporine was complicated with NSTEMI. The third-line treatment with azathioprine failed. Eltrombopag as single agent was ineffective and we observed only a brief, mild response when eltrombopag was associated with prednisone. The next lines of therapy were romiplostim alone and subsequently associated with danazol, vincristine plus prednisone, and eltrombopag plus prednisone, all without response. Finally, we started efgartigimod associated with eltrombopag and low-dose prednisone without results. Romiplostim was introduced immediately after suspension of efgartigimod and we observed a prompt and complete response, lasting 15 weeks without any adverse event, followed by a relapse triggered by a mild symptomatic SarsCov2 infectious, despite a full course of vaccination (two doses plus one boost of Pfizer-BioNTech vaccine more than three months before). Hence, (not shown in Figure 1a), the patient received fostamatinib without response and now he is treated with chronic administration of prednisone and IgIV on demand if bleeding occurs, with many admissions into the Emergency Rooms.
 |
|
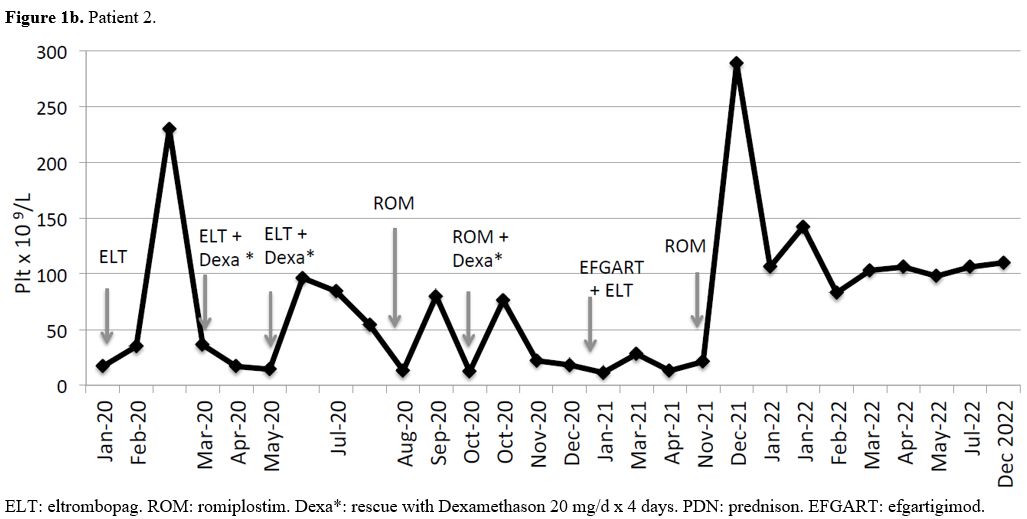
Patient #2 (Figure 1b): female, without comorbidities. ITP was diagnosed at 16 years of age. After the first line with steroids, she had a brief response and remained with a low but safe platelet count for a couple of years (30-40 x 109/L). She received eltrombopag as second line of treatment at 19 years of age, after a bone marrow examination and after having ruled out other causes of isolated thrombocytopenia. She had only a mild and brief response to eltrombopag, with heavy recurrent menstrual bleeding requiring rescue treatment with dexamethason. We switched to romiplostim with a limited and poor response and she needed repeated courses of steroids due to heavy menses leading to anemia.
 |
|
Thereafter she was given efgartigimod associated with eltrombopag without response and she received dexamethason again as rescue therapy.
Immediately after the suspension of efgartigimod we started romiplostim as single agent and we recorded a prompt and complete response, still ongoing after 33 weeks, without adverse events or bleeding and with a complete recovery from anemia.
We describe two patients of very different ages, sex, and comorbidities, both having a refractory ITP, with previous failure of many different lines of therapy, including single agents (steroid, TPO-RA, immunosuppressant drugs) and combination strategies. In these two patients, efgartigimod was at first ineffective, but the sequential introduction of romiplostim (already used in both patients many months before, without results) increased platelet count.
Efgartigimod selectively acts by targeting IgG recycling mediated by blocking neonatal Fc receptor and accelerates the destruction of IgG, reducing pathogenic IgG and IgG immune complexes.
Romiplostim is an IgG Fc fragment connected with four TPO-R binding domains, thus clinical trials involving efgartigimod in ITP do not allow the concomitant use of this specific TPO-RA. Consequently data about a combination of efgartigimod and romiplostim are lacking.
We expected an enhancement in the clearance of romiplostim when administering the drug immediately after suspension of efgartigimod, as it is well described that efgartigimod ends its effect on IgG recycling after eight weeks of suspension. Nonetheless, we observed a prompt and high response since the first administration of romiplostim. This response appears to be lasting many weeks (15 and 33 weeks, respectively), thus opening a different and unrecognized scenario of immunomodulation as the result of the sequential administration of the two drugs. In one patient, the response ceased after 15 weeks triggered by a viral infection as it frequently happens in multi-refractory ITP patients.
It is worth underlining that in both patients efgartigimod had previously been administered together with eltrombopag, as eltrombopag was ineffective as single treatment. However, this association of drugs failed. Thus, a “class effect” of a synergistic power of TPO-RA and efgartigimod cannot be claimed.
The chemical structure of romiplostim is completely different from that of eltrombopag, and this might be the reason of a different effect of the two drugs combined or sequentially used with efgartigimod. It is otherwise suggested that the Fc fragment of romiplostim may play an important role in immune surveillance and regulation of immune response, acting as “Tregitops” (T regulatory cell epitopes) sequence.[8]
Tregitopes are natural T cell epitopes derived from immunoglobulin G, and this mechanism might explain the regulatory effects of intravenous immunoglobulin therapy in ITP. In addition, antigen-specific adaptive tolerance induction might be boosted when antigens are administered in combination with regulatory T cell epitopes, such as Tregitopes.
Tregitopes have many important properties because they bind to multiple MHC class II molecules, suppressing the effector T cell responses to co-delivered antigen and regulating Treg-associated cytokines and chemokines.
Previous studies in murine models have demonstrated the ability of Tregitopes to induce antigen-specific tolerance leading to a reduction of immune responses to allergens in vitro and in vivo.[9]
A suggestive mice model of type 1 diabetes showed that the co-administration of the target antigen and Tregitope peptides completely suppressed the development of diabetes.[9] Moreover, recently, the Fc domain of recombinant FVIII-Fc fusion protein seems to reduce immunogenicity, confer immunomodulatory and anti-inflammatory properties, and induce tolerance in hemophiliac patients.[10]
Based on the mechanisms mentioned above, it is conceivable that in these two multi-refractory ITP patients, the use of efgartigimod had initially reduced the circulating autoantibody against the surface antigens of the platelets without any clinical effect while enhancing the availability of the target antigens. The immediate sequential use of romiplostim just after the withdrawal of the FcRn inhibitor might have induced the mechanism of antigen-specific adaptive tolerance through the properties of the Fc fragment of romiplostim. On the other hand, this TPO-RA was previously unable to give results when it was used without previous exposure to efgartigimod, suggesting a complementary effect of the two drugs administered in this specific sequence.
Efgartigimod has just begun its journey in ITP, and its potential as immunomodulatory in this disease is far from being completely understood. In addition, data about the sequential use of different target therapies are needed in multi-refractory ITP patients. However, the availability of new drugs with different mechanisms of action will allow clinicians to better manage even the patients with the most challenging disease.