Stefanie von Matt1, Ulrike Bacher2, Yara Banz3, Behrouz Mansouri Taleghani2, Urban Novak1 and Thomas Pabst1
1
Department of Medical Oncology; Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland.
2
Department of Hematology and Central Hematology Laboratory;
Inselspital, Bern University Hospital, University of Bern, Bern,
Switzerland.
3 Institute of Pathology, University of Bern, Bern, Switzerland.
Correspondence to:
Prof. Thomas Pabst, MD; Department of Medical Oncology; University
Hospital; 3010 Bern; Switzerland. Tel.: +41 31 632 8430; Fax: +41 31
632 3410; E-mail:
thomas.pabst@insel.ch.
Published: May 1, 2023
Received: December 30, 2022
Accepted: April 16, 2023
Mediterr J Hematol Infect Dis 2023, 15(1): e2023025 DOI
10.4084/MJHID.2023.025
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Introduction:
Autologous stem cell transplantation (ASCT) following high-dose
chemotherapy is applied as salvage therapy in patients with relapsed
disease or as first-line consolidation in high-risk DLBCL with
chemo-sensitive disease. However, the prognosis of relapsing DLBCL
post-ASCT remained poor until the availability of CAR-T cell treatment.
To appreciate this development, understanding the outcome of these
patients in the pre-CAR-T era is essential.
Methods: We retrospectively analyzed 125 consecutive DLBCL patients who underwent HDCT/ASCT.
Results:
After a median follow-up of 26 months, OS and PFS were 65% and 55%.
Fifty-three patients (42%) had a relapse (32 patients, 60%) or
refractory disease (21 patients, 40%) after a median of 3 months
post-ASCT. 81% of relapses occurred within the first year post-ASCT
with an OS of 19% versus 40% at the last follow-up in patients with
later relapses (p=0.0022). Patients with r/r disease after ASCT had
inferior OS compared to patients in ongoing remission (23% versus 96%;
p<0.0001). Patients relapsing post-ASCT without salvage therapy
(n=22) had worse OS than patients with 1-4 subsequent treatment lines
(n=31) (OS 0% versus 39%; median OS 3 versus 25 months; p<0.0001).
Forty-one (77%) of patients relapsing after ASCT died, 35 of which due
to progression.
Conclusions:
Additional therapies can extend OS but mostly cannot prevent death in
DLBCL relapsing/refractory post-ASCT. This study may serve as a
reference to emerging results after CAR-T treatment in this population.
|
Introduction
Diffuse
large B-cell lymphoma, not otherwise specified (DLBCL, NOS) responds
effectively to immunochemotherapy, with R-CHOP (rituximab,
cyclophosphamide, doxorubicin, vincristine, prednisone) being the
first-line standard.[1-6] In up to 60% of patients, this treatment provides definite complete remission.[7,8]
Nevertheless, 30-50% of patients will suffer from relapsed or
progressive disease, mostly within the first two years. The current
treatment of choice for this patient population is salvage chemotherapy
followed by high-dose chemotherapy (HDCT) with peripheral autologous
hematopoietic stem cell transplantation (ASCT).[9-12] The most widely used HDCT regimens are BEAM (carmustine, etoposide, cytarabine, melphalan),[11,13] or BeEAM with bendamustine replacing BCNU.[14,15]
Although
HDCT followed by ASCT is a successful treatment option for many
patients with relapsed DLBCL or high-risk presentation, this treatment
is associated with relevant toxicity; importantly, up to 50% of these
patients will still relapse or are refractory to this treatment.[16-19]
Prognosis of these patients is dismal, and treatment options have been
limited so far. Some r/r patients may not receive further interventions
after HDCT/ASCT due to lack of response to salvage chemotherapy, poor
general condition, or the patient’s request, and they undergo
palliative treatment. For patients eligible for further therapies,
options comprise chemotherapy, immunotherapy, radiotherapy, or
combinations of these, in selected cases, allogeneic hematopoietic stem
cell transplantation, a second ASCT, or, more recently, CAR (chimeric
antigen receptor) T-cell therapy.[11,20]
CAR T-cell therapy is a promising new option for patients with DLBCL
after two or more therapy lines fail. Recent studies have shown
remarkable CR rates of between 40% to 58%.[21-26] On
the other hand, CAR T-cell therapy can be associated with relevant
specific complications, including cytokine release syndrome (CRS) or
CAR T-cell-related encephalopathy syndrome (CRES/ICANS).[21-25,27-29]
We performed a retrospective study to describe the outcome of patients
with DLBCL after HDCT/ASCT and to determine how high-risk or relapsed
DLBCL was managed in clinical practice before the availability of CAR-T
cell treatment.
Patients and Methods
Patients. This
single-center, non-interventional, retrospective study analyzed the
outcome of all consecutive patients with relapsed DLBCL or patients
with high-risk presentation who underwent HDCT/ASCT between May 2005
and February 2019 at the University Hospital of Bern, Switzerland.
Treatment for DLBCL prior to HDCT/ASCT was applied in various referring
centers in Switzerland. Inclusion criteria were the diagnosis of either
high-risk DLBCL or relapsed DLBCL (with the subtypes shown in Table 1),
age of at least 18 years at first diagnosis, and sufficient information
on remission status after HDCT with ASCT. Patients with high-risk
presentation who were consolidated with ACST after first-line therapy
had to have a chemo-sensitive disease and had to achieve partial or
complete remission before consolidation.
 |
- Table
1. Patient characteristics, lymphoma subtypes, stage and international
prognostic index, B-symptoms, CNS infiltration and infiltration of bone
marrow at first diagnosis in patients with or without relapsed or
refractory disease after ASCT.
|
Patients
were divided into two groups depending on their response to HDCT/ASCT.
The first group included patients with r/r disease after ASCT. The
second group comprised patients in ongoing remission without relapse of
DLBCL. All patients gave written informed consent, and this analysis
was approved by the local ethics committee of Bern, Switzerland.
Data source.
Clinical data for this study were collected from the local electronic
patient information system at the University Hospital Bern.
Furthermore, information was obtained from the local Management and
Resource System for Stem Cell Transplantation (MARCELL), providing
specific information on the stem cell transplantation procedures at the
University Hospital Bern.
Methods and Definitions. At first diagnosis, patients were staged according to the Ann Arbor classification,[30] and the international prognostic index (IPI) was used for risk stratification.[31]
Remission status was determined according to the revised response
criteria of the international working group for malignant lymphoma
before ASCT, 100 days after ASCT, and at annual follow-up.[32]
Response was classified as complete remission (CR), partial remission
(PR), stable disease (SD), and progressive disease (PD). CR was defined
as complete disappearance of clinical lymphoma evidence and
disease-related symptoms. PR was defined as a measurable disease
reduction of at least 50% and no occurrence of new lesions. Patients
with SD did not fulfill CR/PR or PD criteria. The occurrence of new
lesions or the increase of previously reported tumor masses by more
than 50% were defined as PD.[32,33]
The primary
endpoints were overall survival and progression-free survival. PFS was
defined as the time from ASCT until the first evidence of
relapse/progression or death from any cause. OS was defined as the time
from ASCT until death from any cause.
Statistical analysis.PFS
and OS were calculated using the Kaplan-Meier method. Survival
differences between subgroups were identified by the log-rank test.
Univariate analysis was calculated for the factors: age at first
diagnosis, transformed lymphoma vs. de novo origin, presence of
B-symptoms at first diagnosis, bone marrow infiltration at first
diagnosis, radiotherapy administered during first or second-line
therapy, the interval between first-line therapy until
relapse/progression, the performance of CD34+ cell positive selection,
remission status at ASCT, the interval from ASCT to
relapse/progression, number of therapies prior to HDCT/ASCT, and number
of further therapies after post-ASCT relapse. P values of <0.05 were
assumed to be statistically significant. All data were conducted with
GraphPad Prism, and calculations were done by Excel.
Results
Patient characteristics.
This study included 125 consecutive patients with DLBCL who received
HDCT/ASCT either as first-line consolidation due to high-risk
presentation or as salvage therapy for relapsed DLBCL. Clinical
characteristics at first diagnosis are summarized in Table 1.
63% of the patients were male. The median age at first diagnosis was 58
years (range, 23-76 years). DLBCL NOS (not otherwise specified) was the
most common lymphoma subtype (48%). B-symptoms at first diagnosis were
present in 44 patients (35%), bone marrow infiltration and central
nervous system infiltration were observed in 27 (22%) and 13 patients
(10%), respectively.
Transformed lymphoma was present in 23
patients, with 16 (70%) being initially diagnosed with follicular
lymphoma, five (22%) with CLL, and one patient (4%) each with marginal
zone lymphoma or nodular lymphocyte-predominant Hodgkin lymphoma. 73%
of the patients had advanced-stage disease with Ann Arbor stages III
(29 pts; 24%) or IV (59 pts; 49%). IPI for risk stratification at first
diagnosis was a high-intermediate risk in 35 patients (38%) and high
risk in 27 patients (29%).
As described above, patients were
distributed into two groups depending on the disease control (relapse
or progression) after HDCT/ASCT. Both cohorts were comparable regarding
age, gender, lymphoma subtypes, stages, and IPI at first diagnosis (Table 1).
Previous therapies before ASCT. Details on the treatment given before HDCT/ASCT are presented in Table 2.
Patients had a median of two treatment lines before ASCT (range 1-3).
Fifty-one patients (41%) received HDCT/ASCT after only one line of
therapy due to high-risk presentation, 68 patients (55%) after two, and
6 patients (5%) after three lines of treatment. For first-line
treatment, 93% of patients received the CHOP regimen, and 94% of CHOP
chemotherapies were combined with rituximab.
 |
- Table 2. Overview on therapies used in one to three previous lines before ASCT.
|
High-dose chemotherapy and autologous stem cell transplantation.
HDCT/ASCT was performed after a median interval of 8 months from the
initial diagnosis. Conditioning regimens in 93% were either the BeEAM
(59%) or BEAM (34%). 7% of patients received either melphalan alone or
the combination of carmustine and thiotepa as conditioning treatment.
Detailed information on HDCT and ASCT is presented in Table 3.
 |
- Table 3. Characteristics
of autologous stem cell transplantation (ASCT) in patients with or
without relapsed/ refractory disease after ASCT.
|
Salvage therapy at relapse/progression after HDCT/ASCT.
Fifty-three patients (42%) developed relapse or progression after a
median interval of 3 months from ASCT (range 1 to 145 months).
Thirty-one patients - 58% of all patients with relapse/progression,
respectively - received further therapies. Twenty-one patients were
treated with one therapy line, and ten patients had two to four
treatment lines for relapsing disease after ASCT, with a median of one
therapy line (range 0 to 4 lines). 22 patients had no further therapy
due to poor general condition or by the patient’s wish.
The
following further therapies were administered: cytotoxic chemotherapy
(25 patients), radiotherapy (five patients), second HDCT/ASCT (three
patients), and allogeneic hematopoietic stem cell transplantation
(three patients). Three patients received immunotherapy targeting PD-1
(nivolumab: two patients; pidilizumab: one patient), one patient had
blinatumomab, five patients were given ibrutinib, and one patient
received the antibody-drug conjugate brentuximab vedotin, as listed in Table 4.
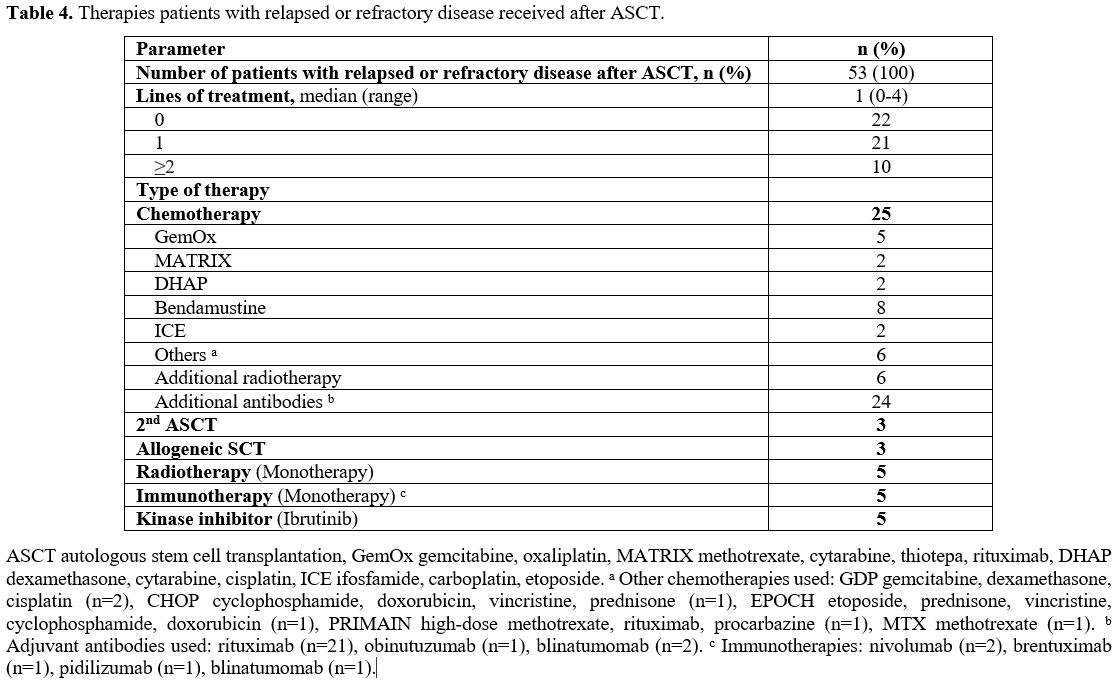 |
- Table 4. Therapies patients with relapsed or refractory disease received after ASCT.
|
Outcome. Details on the outcome of the patients after HDCT/ASCT are depicted in Table 5.
The median follow-up of the entire patient cohort was 26 months.
Forty-four patients (35%) died after a median of six months (range
1-64), 35 (80% of the patients) due to disease progression, six due to
therapy-related reasons (in five cases due to HDCT associated
toxicities, and in one case related to subsequent allogeneic
transplantation) and six from other causes.
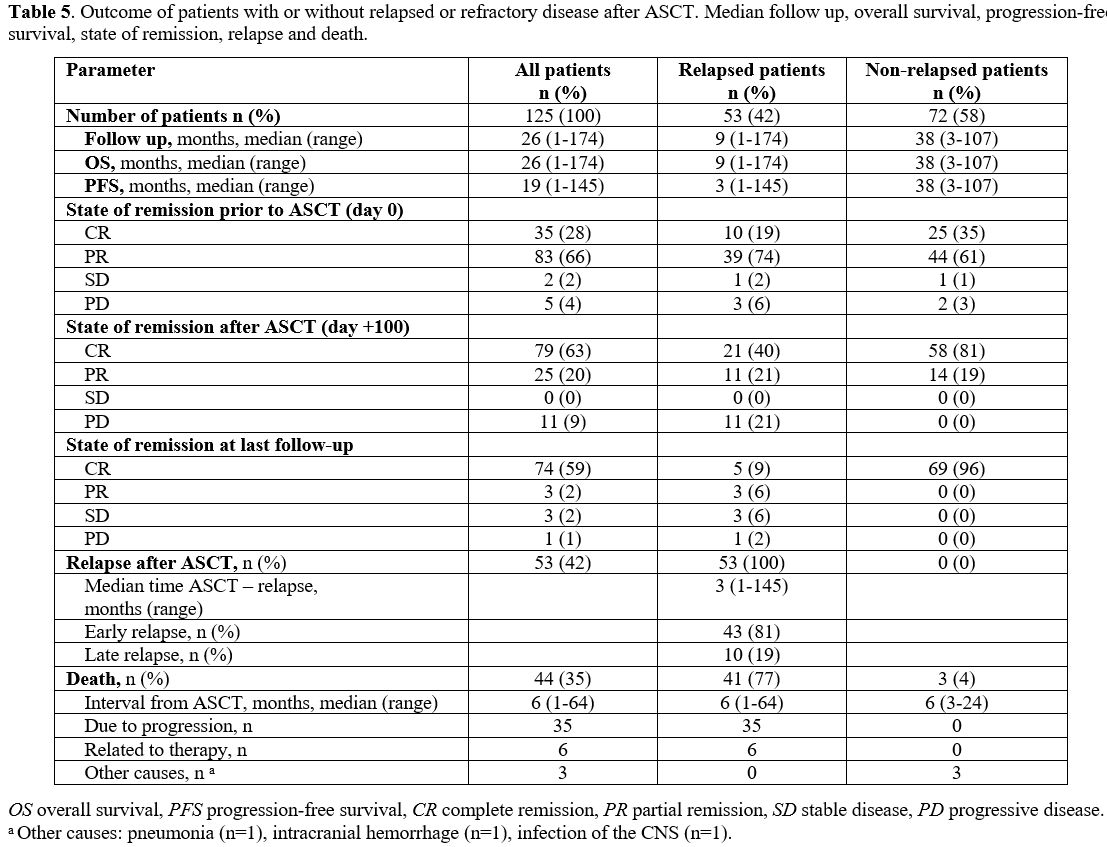 |
- Table 5. Outcome of
patients with or without relapsed or refractory disease after ASCT.
Median follow up, overall survival, progression-free survival, state of
remission, relapse and death.
|
The median OS of the entire population was 26 months, and the OS rate at the last follow-up was 65% (Figure 1C).
As expected, patients with r/r disease after ASCT had worse overall
survival compared to patients in ongoing remission (OS at last
follow-up: 23% vs. 96%; median OS: 9 vs. 38 months; p <0.0001; Figure 1D).
Progression-free survival (PFS) of the entire cohort at the last
follow-up was 55%, with a median duration of response of 19 months (Figure 1A). Relapsed or refractory disease occurred in 42% of patients after a median interval of 3 months.
Four
factors were identified to be associated with survival rates: interval
of relapse from first-line therapy and interval from HDCT/ASCT, number
of therapies prior to HDCT/ASCT, and the number of treatment lines
after post-ASCT relapse. 38% of patients showed an early relapse after
first-line therapy, defined as relapsed disease or progression within
the first year. These patients had inferior OS and PFS compared to
patients who relapsed after 12 months or later (OS rate at 26 months:
48% vs. 75%, p = 0.0008; PFS rate: 33% vs. 69%, p < 0.0001; Figure 3A/B).
When
only patients with r/r disease (n=53) following HDCT/ASCT were
considered, early relapse or progression within the first 12 months
after ASCT occurred in 81% of these patients. 19% of the patients had a
late-onset relapse (an occurrence of relapse ≥ 12 months after ASCT).
Patients with early relapse or progression had lower OS than patients
with late onset of relapse post-ASCT (OS rate at 26 months: 19% vs.
40%, p= 0.0009, Figure 3D).
Comparing
the patients’ outcome regarding the number of therapies prior to HDCT/
ASCT, a significant benefit was observed in those patients who received
HDCT/ASCT after the first-line therapy because of high-risk
presentation (n= 51), compared to those patients who had two to three
lines of treatment prior to HDCT/ASCT (OS rate at 26 months: 78% vs.
55%, p= 0.0161; PFS rate: 75% vs. 42%, p= 0.0054; Figure 4 A/B).
Considering
all patients with r/r disease following HDCT/ASCT, the OS rate was only
23% after a median follow-up of 26 months after ASCT. Patients who
received no other therapy despite relapse/progression after ASCT due to
poor general condition or according to the patient’s wish had an even
worse outcome compared to patients with additional treatment line(s) in
this situation (OS rate at 26 months 0% vs. 39%, p >0.0001, Figure 2A).
42% of patients received no further therapy, 40% were treated with one,
and 19% with two or more therapy lines, corresponding to OS rates of 0%
vs. 24% vs. 70% (p> 0.0001; Figure 2B). Thus, in relapsed patients, the OS was longer with an increasing number of therapy lines.
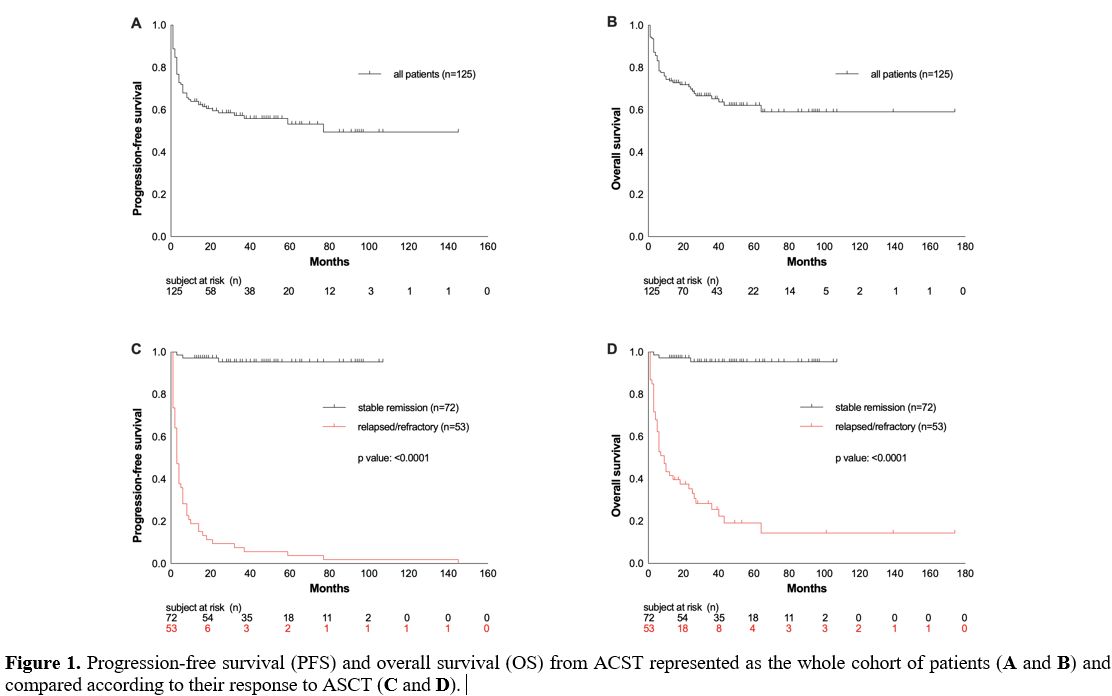 |
Figure 1. Progression-free survival (PFS) and overall survival (OS) from ACST represented as the whole cohort of patients (A and B) and compared according to their response to ASCT (C and D).
|
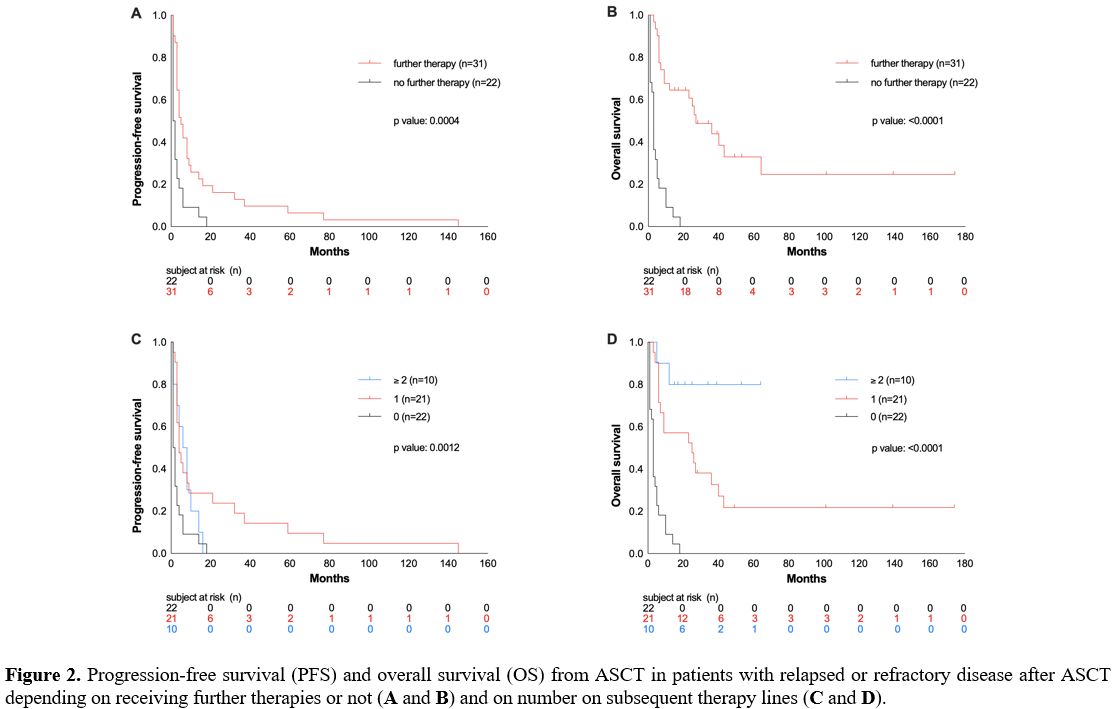 |
Figure
2. Progression-free survival (PFS) and overall survival (OS) from ASCT
in patients with relapsed or refractory disease after ASCT depending on
receiving further therapies or not (A and B) and on number on subsequent therapy lines (C and D).
|
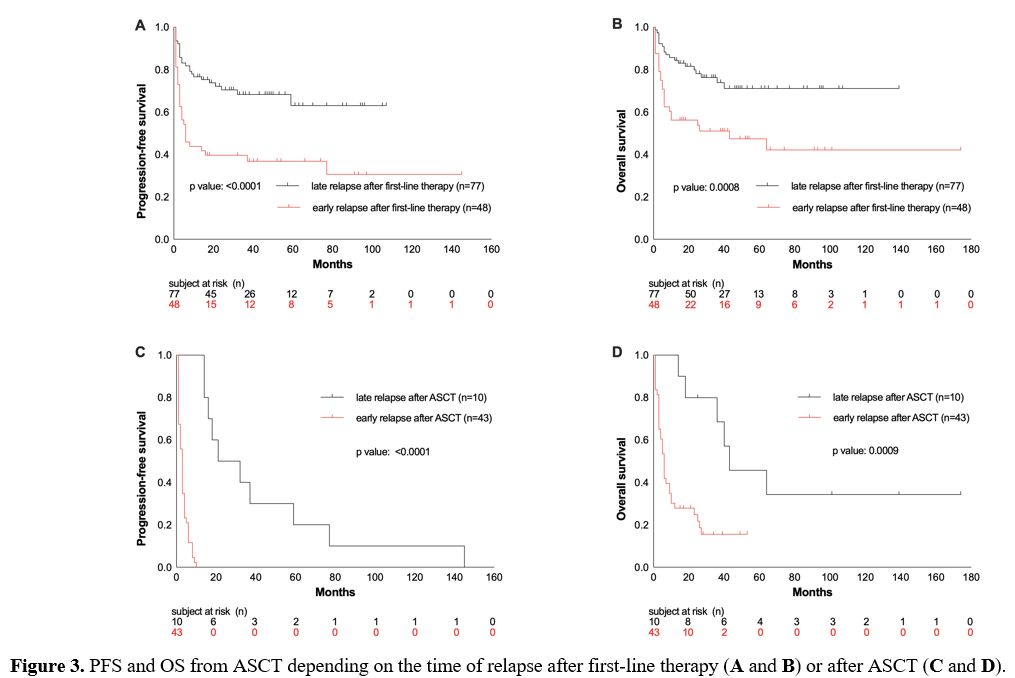 |
Figure 3. PFS and OS from ASCT depending on the time of relapse after first-line therapy (A and B) or after ASCT (C and D).
|
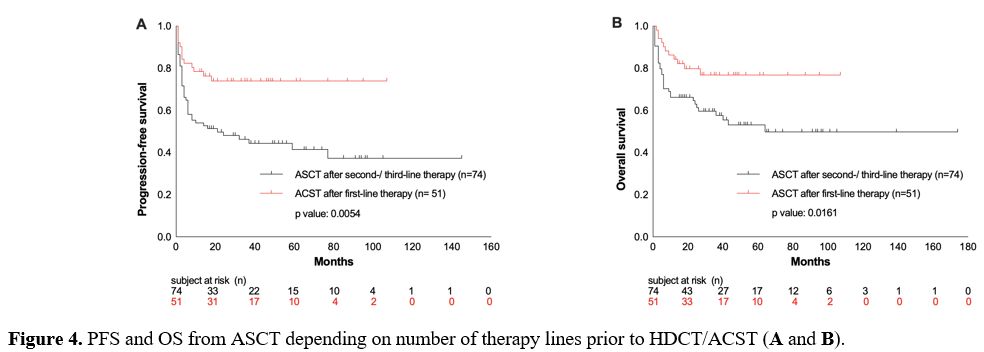 |
Figure 4. PFS and OS from ASCT depending on number of therapy lines prior to HDCT/ACST (A and B). |
No differences were
detected in OS and PFS rates of specific subsets when data were
adjusted for the following variables: de novo versus transformed
lymphoma (OS p=0.7369; PFS p=0.7909), age higher/lower than 60 years
(OS p= 0.3617; PFS p= 0.2655), B-symptoms present at first diagnosis
yes/no (OS p=0.9446; PFS p=0.7619), bone marrow infiltration present at
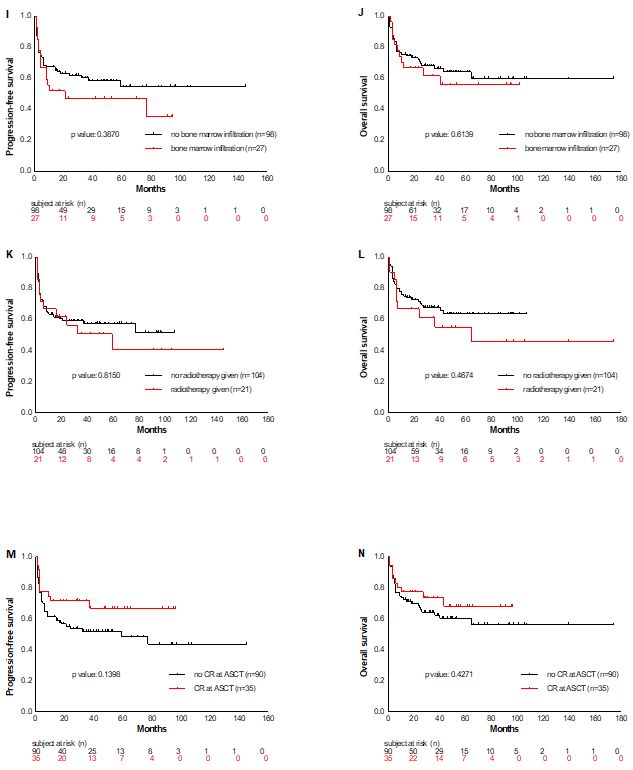
first diagnosis yes/no (OS p=0.6139; PFS p=0.3870), radiotherapy
administered before ASCT yes/no (OS p=0.4674; PFS p=0.8150), and CR at
ASCT yes/no (OS p=0.4271; PFS p=0.1398) (Supplementary material, Figure S1).
Discussion
Considering the introduction of CAR-T cell therapies for aggressive lymphatic malignancies in Europe and elsewhere,[24,25]
we aimed to further characterize the outcomes of patients with DLBCL
with a specific focus on those with failure after HDCT/ASCT. We
retrospectively investigated a cohort of 125 patients with DLBCL
treated with HDCT/ASCT in a single academic/ tertiary center and
studied, in particular, the subset of patients who relapsed or
developed progression following HDCT/ASCT and who could have benefited
from CAR-T therapy, had it then been available.
Confirming reports
by others, the present study demonstrates that HDCT, followed by ASCT,
provides excellent long-term outcomes in patients with relapsed or
refractory DLBCL, achieving stable remission after this salvage therapy
option.[9,34,35] In our analysis
comprising 125 recipients of HDCT/ASCT due to relapsed DLBCL or
high-risk presentation, the ORR was 61% for the total cohort. That 55%
of patients in ongoing remission following HDCT/ASCT showed encouraging
OS and PFS of 96% with a median duration of 38 months.
In
contrast, the prognosis for patients with r/r disease after HDCT/ASCT
is poor, especially in those with characteristics such as high IPI or
early relapse within 12 months following ASCT.[11,34]
In our study, 42% of patients developed relapses or showed progression
after HDCT/ASCT. Although 58% of these patients with failure of
HDCT/ASCT received other therapeutic approaches, 77% rapidly died after
a median interval of 6 months, mostly due to lymphoma progression.
Likewise, in the CORAL study, 29% of patients with r/r DLBCL after ASCT
had poor survival, with a median OS of 10 months and a 1-year OS of
39.1%.[35]
We evaluated the impact of various
parameters on the outcomes of our HDCT/ASCT cohort. Significant impact
on survival could only be verified for the duration of the response to
first-line therapy, the duration of response after HDCT/ASCT, the
number of therapies prior to HDCT/ASCT, and the number of subsequent
therapies after post-ASCT relapse. We documented a median interval to
progression following ASCT of only 3 months, and 81% of relapses
occurred within 12 months after ASCT, demonstrating that DLBCL relapses
are associated with rapid kinetics, early manifesting after HDCT/ASCT.
Furthermore, the OS rate was only 19% in patients with early relapses
(<12 months) post-ASCT, as compared to late relapses (≥12 months
after ASCT) with an OS rate of 40%. These results correspond with
previous studies demonstrating early relapses following ASCT in 65-80%
of patients, associated with a significantly worse OS compared to later
time points of relapse.[12,36] Other parameters such as the histopathological origin (transformed vs. de novo DLBCL) [37-41] and CD34+ selection[42-45] had no significant impact on the prognosis of the recipients of HDCT/ASCT in our cohort.
Treatment
options for patients with r/r DLBCL following ASCT were so far limited.
Allogeneic stem cell transplantation may provide a certain graft versus
lymphoma effect.[46-48] However, only a few DLBCL
patients are, in fact, candidates for this approach due to its high
transplant-related mortality and high relapse rates. Only three
patients in our cohort received an allogeneic SCT, which is
representative of the limited use of this option for r/r DLBCL patients.
CAR-T cell therapies, recently introduced, offer patients with r/r DLBCL a promising new option with CR rates of up to 58%.[24,49,50] In several studies, ORR of 52-85% with 40-58% CR rates were achieved by CAR-T cell therapy in patients with r/r DLBCL.[21,24-26,51,52] However, long-term outcomes will still be awaited in the next decades.
In addition, novel immunotherapies such as polatuzumab vedotin,[53] tafasitamab,[54]
glofitamab, or mosunetuzumab are additional promising new options for
DLBCL patients ineligible for HDCT/ASCT or for those whom CAR-T therapy
is no option due to its toxicity, or due to its logistic or financial
obstacles.
An obvious limitation of our study is its
retrospective single-center design covering a large timespan, including
various DLBCL subtypes, heterogeneity in conditioning regimen, and
inevitable lack of some data in a few patients. Nevertheless, our study
demonstrates the adverse prognosis of DLBCL patients after HDCT/ASCT
failure and the limited efficacy of subsequent therapeutic approaches,
including a second HDCT/ASCT, allogeneic SCT, radiation, cytotoxic
treatment, and traditional monoclonal antibody therapies. Our study
emphasizes the urgent need to make CAR-T cell therapies available to
all patients with r/r DLBCL following HDCT/ASCT failure.[21]
References
- Pfreundschuh M, Schubert J, Ziepert M, et al. Six
versus eight cycles of bi-weekly CHOP-14 with or without rituximab in
elderly patients with aggressive CD20+ B-cell lymphomas: a randomised
controlled trial (RICOVER-60). Lancet Oncol. 2008;9(2):105-116.
doi:10.1016/S1470-2045(08)70002-0 https://doi.org/10.1016/S1470-2045(08)70002-0 PMid:18226581
- Pfreundschuh
M, Trümper L, Österborg A, et al. CHOP-like chemotherapy plus rituximab
versus CHOP-like chemotherapy alone in young patients with
good-prognosis diffuse large-B-cell lymphoma: a randomised controlled
trial by the MabThera International Trial (MInT) Group. Lancet Oncol.
2006;7(5):379-391. doi:10.1016/S1470-2045(06)70664-7 https://doi.org/10.1016/S1470-2045(06)70664-7 PMid:16648042
- Habermann
TM, Weller EA, Morrison VA, et al. Rituximab-CHOP versus CHOP alone or
with maintenance rituximab in older patients with diffuse large B-cell
lymphoma. J Clin Oncol. 2006;24(19):3121-3127.
doi:10.1200/JCO.2005.05.1003 https://doi.org/10.1200/JCO.2005.05.1003 PMid:16754935
- Sehn
LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHOP
plus rituximab therapy dramatically improved outcome of diffuse large
B-cell lymphoma in British Columbia. J Clin Oncol.
2005;23(22):5027-5033. doi:10.1200/JCO.2005.09.137 https://doi.org/10.1200/JCO.2005.09.137 PMid:15955905
- Tilly
H, Gomes da Silva M, Vitolo U, et al. Diffuse large B-cell lymphoma
(DLBCL): ESMO Clinical Practice Guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 2015;26(Supplement 5):vii78-vii82.
doi:10.1093/annonc/mdv304 https://doi.org/10.1093/annonc/mdv304 PMid:26314773
- Feugier
P, Van Hoof A, Sebban C, et al. Long-term results of the R-CHOP study
in the treatment of elderly patients with diffuse large B-cell
lymphoma: A study by the groupe d'etude des lymphomes de l'adulte. J
Clin Oncol. 2005;23(18):4117-4126. doi:10.1200/JCO.2005.09.131 https://doi.org/10.1200/JCO.2005.09.131 PMid:15867204
- Coiffier
B, Lepage E, Brière J, et al. CHOP Chemotherapy plus Rituximab Compared
with CHOP Alone in Elderly Patients with Diffuse Large-B-Cell Lymphoma.
N Engl J Med. 2002;346(4):235-242. doi:10.1056/NEJMoa011795 https://doi.org/10.1056/NEJMoa011795 PMid:11807147
- Coiffier
B, Sarkozy C. Diffuse large B-cell lymphoma: R-CHOP failure-what to do?
Hematology. 2016;2016(1):366-378. doi:10.1182/asheducation-2016.1.366 https://doi.org/10.1182/asheducation-2016.1.366 PMid:27913503 PMCid:PMC6142522
- Philip
T, Guglielmi C, Hagenbeek A, et al. Autologous Bone Marrow
Transplantation as Compared with Salvage Chemotherapy in Relapses of
Chemotherapy-Sensitive Non-Hodgkin's Lymphoma. N Engl J Med.
2002;333(23):1540-1545. doi:10.1056/nejm199512073332305 https://doi.org/10.1056/NEJM199512073332305 PMid:7477169
- Camus
V, Tilly H. Managing early failures with R-CHOP in patients with
diffuse large B-cell lymphoma. Expert Rev Hematol.
2017;10(12):1047-1055. doi:10.1080/17474086.2016.1254547 https://doi.org/10.1080/17474086.2016.1254547 PMid:27791447
- Gisselbrecht
C, Van Den Neste E. How I manage patients with relapsed/refractory
diffuse large B cell lymphoma. Br J Haematol. 2018;182(5):633-643.
doi:10.1111/bjh.15412 https://doi.org/10.1111/bjh.15412 PMid:29808921 PMCid:PMC6175435
- Nagle
SJ, Woo K, Schuster SJ, et al. Outcomes of patients with
relapsed/refractory diffuse large B-cell lymphoma with progression of
lymphoma after autologous stem cell transplantation in the rituximab
era. Am J Hematol. 2013;88(10):890-894. doi:10.1002/ajh.23524 https://doi.org/10.1002/ajh.23524 PMid:23813874
- Friedberg JW. Relapsed / Refractory Diffuse Large B-Cell Lymphoma. Am Soc Hematol. Published online 2011:498-505. https://doi.org/10.1182/asheducation-2011.1.498 PMid:22160081
- Gilli
S, Novak U, Taleghani BM, et al. BeEAM conditioning with
bendamustine-replacing BCNU before autologous transplantation is safe
and effective in lymphoma patients. Ann Hematol. 2017;96(3):421-429.
doi:10.1007/s00277-016-2900-y https://doi.org/10.1007/s00277-016-2900-y PMid:28011985
- Prediletto
I, Farag SA, Bacher U, et al. High incidence of reversible renal
toxicity of dose-intensified bendamustine-based high-dose chemotherapy
in lymphoma and myeloma patients. Bone Marrow Transplant.
2019;54(12):1923-1925. doi:10.1038/s41409-019-0508-2 https://doi.org/10.1038/s41409-019-0508-2 PMid:30890768
- Betticher
C, Bacher U, Legros M, et al. Prophylactic corticosteroid use prevents
engraftment syndrome in patients after autologous stem cell
transplantation. Hematol Oncol. 2021;39(1):97-104. doi:10.1002/hon.2813
https://doi.org/10.1002/hon.2813 PMid:32979278
- Vose
JM, Bierman PJ, Anderson JR, et al. Progressive disease after high-dose
therapy and autologous transplantation for lymphoid malignancy:
clinical course and patient follow-up. Blood. 1992;80(8):2142-2148. https://doi.org/10.1182/blood.V80.8.2142.2142 PMid:1356515
- Robinson
SP, Boumendil A, Finel H, et al. Autologous stem cell transplantation
for relapsed/refractory diffuse large B-cell lymphoma: Efficacy in the
rituximab era and comparison to first allogeneic transplants. A report
from the EBMT Lymphoma Working Party. Bone Marrow Transplant.
2016;51(3):365-371. doi:10.1038/bmt.2015.286 https://doi.org/10.1038/bmt.2015.286 PMid:26618550
- Eicher
F, Mansouri Taleghani B, Schild C, Bacher U, Pabst T. Reduced survival
after autologous stem cell transplantation in myeloma and lymphoma
patients with low vitamin D serum levels. Hematol Oncol.
2020;38(4):523-530. doi:10.1002/hon.2774 https://doi.org/10.1002/hon.2774 PMid:32594534
- Skrabek
P, Assouline S, Christofides A, et al. Emerging therapies for the
treatment of relapsed or refractory diffuse large B cell lymphoma. Curr
Oncol. 2019;26(4):253-265. doi:10.3747/co.26.5421 https://doi.org/10.3747/co.26.5421 PMid:31548805 PMCid:PMC6726277
- Sermer D, Brentjens R. CAR T‐cell therapy: Full speed ahead. Hematol Oncol. 2019;37(S1):95-100. doi:10.1002/hon.2591 https://doi.org/10.1002/hon.2591 PMid:31187533
- Chavez
JC, Locke FL. A Possible Cure for Refractory DLBCL: CARs Are Headed in
the Right Direction. Mol Ther. 2017;25(10):2241-2243.
doi:10.1016/j.ymthe.2017.09.005 https://doi.org/10.1016/j.ymthe.2017.09.005 PMid:28941574 PMCid:PMC5628929
- Lekakis
LJ, Moskowitz CH. The Role of Autologous Stem Cell Transplantation in
the Treatment of Diffuse Large B-cell Lymphoma in the Era of CAR-T Cell
Therapy. HemaSphere. 2019;3(6). doi:10.1097/HS9.0000000000000295 https://doi.org/10.1097/HS9.0000000000000295 PMid:31976472 PMCid:PMC6924546
- Schuster
SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in Adult Relapsed or
Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med.
2018;380(1):45-56. doi:10.1056/NEJMoa1804980 https://doi.org/10.1056/NEJMoa1804980 PMid:30501490
- Neelapu
SS, Locke FL, Bartlett NL, et al. Axicabtagene Ciloleucel CAR T-Cell
Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med.
2017;377(26):2531-2544. doi:10.1056/NEJMoa1707447 https://doi.org/10.1056/NEJMoa1707447 PMid:29226797 PMCid:PMC5882485
- Abramson
JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients
with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001):
a multicentre seamless design study. The Lancet.
2020;396(10254):839-852. doi:10.1016/S0140-6736(20)31366-0 https://doi.org/10.1016/S0140-6736(20)31366-0 PMid:32888407
- Titov
A, Petukhov A, Staliarova A, et al. The biological basis and clinical
symptoms of CAR-T therapy-associated toxicites. Cell Death Dis.
2018;9(9). doi:10.1038/s41419-018-0918-x https://doi.org/10.1038/s41419-018-0918-x PMid:30181581 PMCid:PMC6123453
- Bishop
MR, Maziarz RT, Waller EK, et al. Tisagenlecleucel in
relapsed/refractory diffuse large B-cell lymphoma patients without
measurable disease at infusion. Blood Adv. 2019;3(14):2230-2236.
doi:10.1182/bloodadvances.2019000151 https://doi.org/10.1182/bloodadvances.2019000151 PMid:31332046 PMCid:PMC6650727
- Pabst
T, Joncourt R, Shumilov E, et al. Analysis of IL-6 serum levels and CAR
T cell-specific digital PCR in the context of cytokine release
syndrome. Exp Hematol. 2020;88:7-14.e3.
doi:10.1016/j.exphem.2020.07.003 https://doi.org/10.1016/j.exphem.2020.07.003 PMid:32673688
- Carbone
PP, Kaplan HS, Musshoff K, Smithers DW, Tubiana M. Report of the
Committee on Hodgkin's Disease Staging Classification. Cancer Res.
1971;31(11):1860.
- Ziepert
M, Hasenclever D, Kuhnt E, et al. Standard international prognostic
index remains a valid predictor of outcome for patients with aggressive
CD20 + B-cell lymphoma in the rituximab era. J Clin Oncol.
2010;28(14):2373-2380. doi:10.1200/JCO.2009.26.2493 https://doi.org/10.1200/JCO.2009.26.2493 PMid:20385988
- Cheson
BD, Pfistner B, Juweid ME, et al. Revised response criteria for
malignant lymphoma. J Clin Oncol. 2007;25(5):579-586.
doi:10.1200/JCO.2006.09.2403 https://doi.org/10.1200/JCO.2006.09.2403 PMid:17242396
- Cheson
BD, Horning SJ, Coiffier B, et al. Report of an international workshop
to standardize response criteria for non-Hodgkin's lymphomas. NCI
Sponsored International Working Group. J Clin Oncol Off J Am Soc Clin
Oncol. 1999;17(4):1244. doi:10.1200/JCO.1999.17.4.1244 https://doi.org/10.1200/JCO.1999.17.4.1244 PMid:10561185
- Crump
M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large
B-cell lymphoma: Results from the international SCHOLAR-1 study. Blood.
2017;130(16):1800-1808. doi:10.1182/blood-2017-03-769620 https://doi.org/10.1182/blood-2017-03-769620 PMid:28774879 PMCid:PMC5649550
- Van
Den Neste E, Schmitz N, Mounier N, et al. Outcomes of diffuse large
B-cell lymphoma patients relapsing after autologous stem cell
transplantation: An analysis of patients included in the CORAL study.
Bone Marrow Transplant. 2017;52(2):216-221. doi:10.1038/bmt.2016.213 https://doi.org/10.1038/bmt.2016.213 PMid:27643872
- González-Barca
E, Boumendil A, Blaise D, et al. Outcome in patients with diffuse large
B-cell lymphoma who relapse after autologous stem cell transplantation
and receive active therapy. A retrospective analysis of the Lymphoma
Working Party of the European Society for Blood and Marrow
Transplantation (. Bone Marrow Transplant. 2020;55(2):393-399.
doi:10.1038/s41409-019-0650-x https://doi.org/10.1038/s41409-019-0650-x PMid:31541205
- Wagner-Johnston
ND, Link BK, Byrtek M, et al. Outcomes of transformed follicular
lymphoma in the modern era: a report from the National LymphoCare Study
(NLCS). Blood. 2015;126(7):851-857. doi:10.1182/blood-2015-01-621375 https://doi.org/10.1182/blood-2015-01-621375 PMid:26105149 PMCid:PMC4543911
- Sorigue
M, Garcia O, Baptista MJ, et al. Similar prognosis of transformed and
de novo diffuse large B-cell lymphomas in patients treated with
immunochemotherapy. Med Clínica Engl Ed. 2017;148(6):243-249.
doi:https://doi.org/10.1016/j.medcle.2017.03.004 https://doi.org/10.1016/j.medcle.2017.03.004
- Ghesquières
H, Berger F, Felman P, et al. Clinicopathologic Characteristics and
Outcome of Diffuse Large B-Cell Lymphomas Presenting With an Associated
Low-Grade Component at Diagnosis. J Clin Oncol. 2006;24(33):5234-5241.
doi:10.1200/JCO.2006.07.5671 https://doi.org/10.1200/JCO.2006.07.5671 PMid:17043351
- Guirguis
HR, Cheung MC, Piliotis E, et al. Survival of patients with transformed
lymphoma in the rituximab era. Ann Hematol. 2014;93(6):1007-1014.
doi:10.1007/s00277-013-1991-y https://doi.org/10.1007/s00277-013-1991-y PMid:24414374
- Montoto
S, Fitzgibbon J. Transformation of Indolent B-Cell Lymphomas. J Clin
Oncol. 2011;29(14):1827-1834. doi:10.1200/JCO.2010.32.7577 https://doi.org/10.1200/JCO.2010.32.7577 PMid:21483014
- Berger
MD, Branger G, Leibundgut K, et al. CD34+ selected versus unselected
autologous stem cell transplantation in patients with advanced-stage
mantle cell and diffuse large B-cell lymphoma. Leuk Res.
2015;39(6):561-567. doi:10.1016/j.leukres.2015.03.004 https://doi.org/10.1016/j.leukres.2015.03.004 PMid:25890431
- Gribben
JG, Freedman AS, Neuberg D, et al. Immunologic Purging of Marrow
Assessed by PCR before Autologous Bone Marrow Transplantation for
B-Cell Lymphoma. N Engl J Med. 1991;325(22):1525-1533.
doi:10.1056/NEJM199111283252201 https://doi.org/10.1056/NEJM199111283252201 PMid:1944436
- Sharp
JG, Kessinger A, Mann S, et al. Outcome of high-dose therapy and
autologous transplantation in non-Hodgkin's lymphoma based on the
presence of tumor in the marrow or infused hematopoietic harvest. J
Clin Oncol. 1996;14(1):214-219. doi:10.1200/JCO.1996.14.1.214 https://doi.org/10.1200/JCO.1996.14.1.214 PMid:8558200
- Williams
C D, Goldstone, A H,R M Pearce R M, Philip T, Hartmann O, Colombat P,
Santini G, Foulard L, C GN. Purging of bone marrow in autologous bone
marrow transplantation for non-Hodkin's lymphoma: a case-matches
comparsion with unpurged cases by the European Blood and Marrow
Transplant Lymphoma Registry. J Clin Oncol. 1996;14(9):2454-2464. https://doi.org/10.1200/JCO.1996.14.9.2454 PMid:8823323
- Klyuchnikov
E, Bacher U, Kroll T, et al. Allogeneic hematopoietic cell
transplantation for diffuse large B cell lymphoma: Who, when and how.
Bone Marrow Transplant. 2014;49(1):1-7. doi:10.1038/bmt.2013.72 https://doi.org/10.1038/bmt.2013.72 PMid:23708703
- Doocey
RT, Toze CL, Connors JM, et al. Allogeneic haematopoietic stem-cell
transplantation for relapsed and refractory aggressive histology
non-Hodgkin lymphoma. Br J Haematol. 2005;131(2):223-230.
doi:10.1111/j.1365-2141.2005.05755.x https://doi.org/10.1111/j.1365-2141.2005.05755.x PMid:16197454
- Van
Kampen RJW, Canals C, Schouten HC, et al. Allogeneic stem-cell
transplantation as salvage therapy for patients with diffuse large
B-cell non-Hodgkin's lymphoma relapsing after an autologous stem-cell
transplantation: An analysis of the European Group for Blood and Marrow
Transplantation registry. J Clin Oncol. 2011;29(10):1342-1348.
doi:10.1200/JCO.2010.30.2596 https://doi.org/10.1200/JCO.2010.30.2596 PMid:21321299
- Locke
FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of
axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a
single-arm, multicentre, phase 1-2 trial. Lancet Oncol.
2019;20(1):31-42. doi:10.1016/S1470-2045(18)30864-7 https://doi.org/10.1016/S1470-2045(18)30864-7 PMid:30518502
- Abramson
JS, Gordon LI, Palomba ML, et al. Updated safety and long term clinical
outcomes in TRANSCEND NHL 001, pivotal trial of lisocabtagene
maraleucel (JCAR017) in R/R aggressive NHL. J Clin Oncol.
2018;36(15_suppl):7505-7505. doi:10.1200/JCO.2018.36.15_suppl.7505 https://doi.org/10.1200/JCO.2018.36.15_suppl.7505
- Kochenderfer
JN, Somerville RPT, Lu T, et al. Lymphoma Remissions Caused by
Anti-CD19 Chimeric Antigen Receptor T Cells Are Associated With High
Serum Interleukin-15 Levels. J Clin Oncol Off J Am Soc Clin Oncol.
2017;35(16):1803-1813. doi:10.1200/JCO.2016.71.3024 https://doi.org/10.1200/JCO.2016.71.3024 PMid:28291388 PMCid:PMC5455597
- Locke
FL, Neelapu SS, Bartlett NL, et al. Phase 1 Results of ZUMA-1: A
Multicenter Study of KTE-C19 Anti-CD19 CAR T Cell Therapy in Refractory
Aggressive Lymphoma. Mol Ther. 2017;25(1):285-295.
doi:10.1016/j.ymthe.2016.10.020 https://doi.org/10.1016/j.ymthe.2016.10.020 PMid:28129122 PMCid:PMC5363293
- Sehn
LH, Herrera AF, Flowers CR, et al. Polatuzumab vedotin in relapsed or
refractory diffuse large B-cell lymphoma. J Clin Oncol.
2020;38(2):155-165. doi:10.1200/JCO.19.00172 https://doi.org/10.1200/JCO.19.00172 PMid:31693429 PMCid:PMC7032881
- Salles
G, Duell J, González Barca E, et al. Tafasitamab plus lenalidomide in
relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a
multicentre, prospective, single-arm, phase 2 study. Lancet Oncol.
2020;21(7):978-988. doi:10.1016/S1470-2045(20)30225-4 https://doi.org/10.1016/S1470-2045(20)30225-4 PMid:32511983
Online Supplement
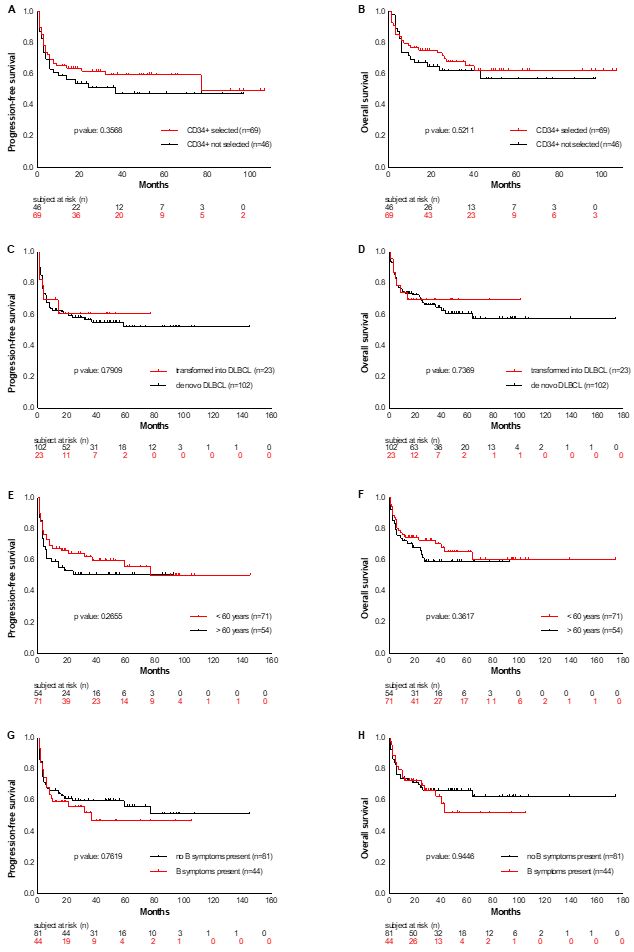 |
Supplementary Figure
S1. PFS and OS analyzed according to various parameters like presence
or absence of CD34+ stem cell selection (Figure A and B), transformed
versus de novo lymphoma (Figure C and D), age at first diagnosis <
versus ≥ 60 years (Figure E and F), presence or absence of b symptoms
(Figure G and H) and bone marrow infiltration (Figure I and J) at first
diagnosis, radiotherapy given during first- or second line therapy
(Figure K and L) and remission state at ASCT (Figure M and N).
|
[TOP]