Chiara Papalini1, Lucia Brescini2, Laura Curci1, Sabrina Bastianelli1, Francesco Barchiesi2, Andrea Giacometti2 and Daniela Francisci1.
1 Infectious Diseases Clinic, Santa Maria della Misericordia Hospital, Università degli Studi di Perugia, Perugia, Italy.
2 Biomedical Sciences and Public Health Department, Università Politecnica delle Marche, Ancona, Italy.
Published: May 1, 2023
Received: January 29, 2023
Accepted: April 17, 2023
Mediterr J Hematol Infect Dis 2023, 15(1): e2023027 DOI
10.4084/MJHID.2023.027
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
To the editor
Kaposi
Sarcoma (KS) in people living with HIV (PLWH) is an AIDS-defining
malignancy implying endothelial cell proliferation. It can involve all
the organs but most frequently appears as purplish or brownish
mucocutaneous lesions. Herpesvirus-8 (HHV-8) is recognized as the
primum movens of the oncogenic pathway. Men who have Sex with Men (MSM)
and African inhabitants of the "KS belt" have the main risk factors for
HHV-8 infection and KS.[1-5] Antiretroviral therapy
(ART) has sensibly modified KS incidence. However, it is still one of
the most frequent neoplasms in PLWH.[1,3,6,7]
The
aim of this study is to report epidemiological and clinical features of
PLWH affected by KS and to analyze which variables, if any, influence
the mortality rate.
This retrospective observational study
included PLWH affected by KS attending the Infectious Diseases Clinics
of S. Maria della Misericordia Hospital of Perugia, Italy, or Torrette
Hospital of Ancona, Italy, from Jan 1, 2002, to Dec 31, 2022. The two
hospitals are the main health centers of two regions of Central Italy,
Umbria, and Marche, and about 800 and 500 PLWH, respectively, visited
their Infectious Diseases Clinics.
KS was diagnosed clinically and
histologically, while data about HHV-8 viremia were unavailable for all
the patients due to the study's retrospective nature.
Every
patient provided consent for using his/her data at the hospital
admission. For each one, we collected information about gender, age at
HIV infection diagnosis, nationality, a risk factor for HIV
acquisition, smoking attitude, HHV-8 positivity, HPV positivity, HCV
antibodies positivity, HBV surface antigen (HBsAg) positivity, date of
KS diagnosis and ART starting, CD4 cell count at the moment of HIV
infection and KS diagnosis, viremia at the moment of HIV infection and
KS diagnosis, CD4/CD8 ratio at KS diagnosis, ART regimen, chemotherapy
administered, 5-year survival and eventually the date of death.
CD4
cell count and CD4/CD8 ratio were measured by cytofluorometry, while
HIV RNA was tested by Real-time quantitative PCR, whose detectability
threshold was 20 copies/ml. When HIV RNA was undetectable or under this
threshold, to calculate the median value, we considered HIV viremia 0
or 19, respectively.
HBsAg and HCV antibodies were detected with
chemiluminescent immunoassay, while HPV DNA on anal or cervical swabs
and plasmatic HHV-8 DNA with semi-quantitative PCR.
Due to their
asymmetrical distribution, continuous variables were presented as the
median with IQR, while the categorical ones were expressed by their
relative frequencies. A comparison was performed between patients who
died and those who survived after 5 years from KS diagnosis. The
Mann-Whitney U-test and the Chi-square test were used to compare
differences between groups, as appropriate. A p-value <0.05 was
considered relevant.
Results
In
the considered period, the number of KS cases remained quite stable,
and there was no increase in the post-SARS-CoV2 pandemic time lapse.
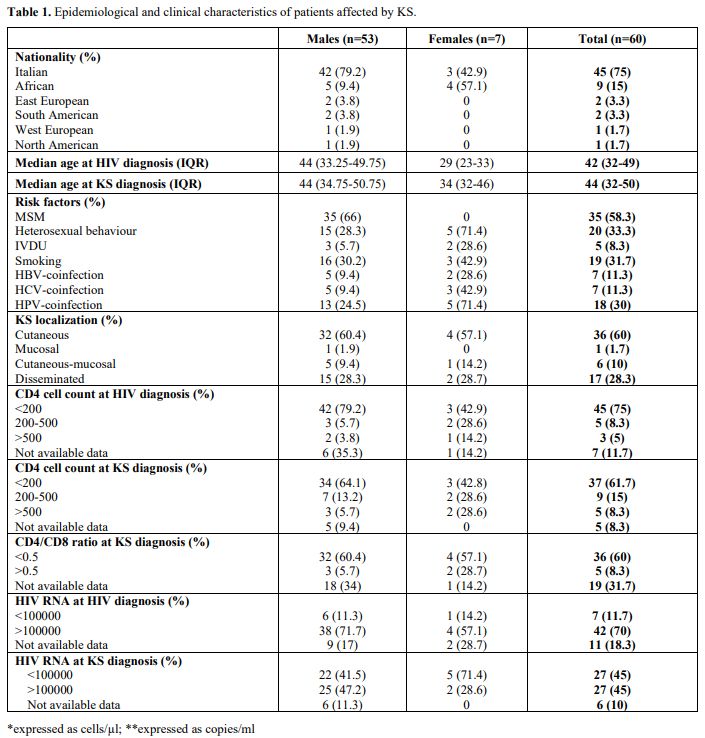
Overall, 60 patients were affected by KS: 53 (88.3%) males and 7
(11.7%) females whose characteristics were summarised in Table 1.
Fourty-5 (75%) were Italians, 9 (15%) were Africans, and 2 (3.3%)
persons were from Eastern Europe, with a median age of 42 and 44 years
at HIV and KS diagnosis, respectively. Some individuals also had other
risk factors for malignancies: 19 (31.7%) smoked, 7 (11.7%), 7 (11.7%),
and 18 (30%) were co-infected with HBV, HCV, and HPV, respectively.
Regarding HIV transmission, 35 (58.3%) were MSM, 20 (33.3%) were
heterosexual people, and 5 (8.3%) were intravenous drug users (IVDU) (Table 1).
 |
- Table
1. Epidemiological and clinical characteristics of patients affected by KS.
|
At
the moment of HIV infection diagnosis, 42 (70%) persons had HIV RNA
over 100,000 copies/ml (median value: 224,000 copies/ml), 45 (75%), and
47 (78.3%) had CD4+ cell count below 200/µl (median value: 63/µl; IQR
19-135) and 350/µl, respectively. In 38 (63.3%) cases, Kaposi Sarcoma
was the first sign of HIV infection, while in 21 (35%), it manifested
later, and the diagnosis date of a patient was unknown (Table 1).
HHV-8
viremia was available for 20 individuals, and 15 resulted positive,
while histology revealed KS in 6 patients. In 34 cases, both data were
lacking.
KS localized on the skin in 36 (60%) patients, while 1
(1.7%), 6 (10%), and 17 (28.3%) had mucosal, cutaneous-mucosal, and
disseminated forms, respectively. Furthermore, 12 patients were
affected by additional cancerous or precancerous lesions: Castleman
disease (2; 3.3%), primitive effusive Lymphoma (1; 1.7%), cervical (2;
3.3%), and anal intraepithelial neoplasms (1; 1.7%), non-Hodgkin
Lymphoma (1; 1.7%), liver (1; 1.7%), prostate (1; 1.7%) and anal cancer
(3; 5%).
At the moment of KS diagnosis, 37 (61.7%) patients had
CD4 cell count <200/µl (median value: 96 cells/µl), 27 (45%) HIV
viremia >100000 copies/ml (median value: 100000 copies/ml), 36 (60%)
CD4/CD8 <0.5 while 17 (28.3%) were already on ART since a median of
212 days (Table 1).
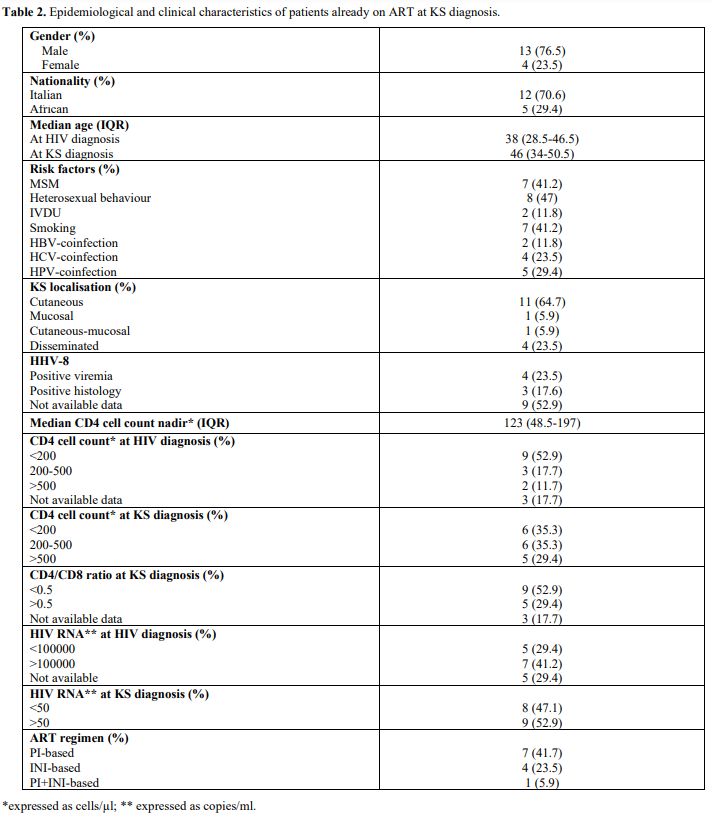
This
special group of people already on treatment included 13 (76.5%) males
with a median CD4 nadir of 123 cells/µl (IQR 48.5-197). Their
epidemiological and clinical data are shown in Table 2.
The median interval from HIV diagnosis to ART starting was 1500 days
(IQR 242.5-4306.5): 7 of them (41.7%) assumed PI-based ART; on the
contrary, 4 (23.5%) the INI-based one.
 |
- Table
2. Epidemiological and clinical characteristics of patients already on ART at KS diagnosis.
|
Thirty-4
patients (56.7%) were treated only with ART; on the other hand, 18
(30%) needed chemotherapy, 4 (6.6%) radiotherapy, and 3 (5%) surgery.
The prevalent ART regimen was nucleoside reverse transcriptase (NRTI)
and protease (PI) inhibitors (31 cases; 51.7%), followed by NRTI plus
integrase inhibitors (INI) (9 cases; 15%), while 2 (3.3%) patients
received NRTI with both PI and INI (Table 1).
Overall, the median interval between HIV diagnosis and ART start was 17
days (IQR 0.25-57.5), and regimens based on NRTI and INI progressively
increased during the considered period.
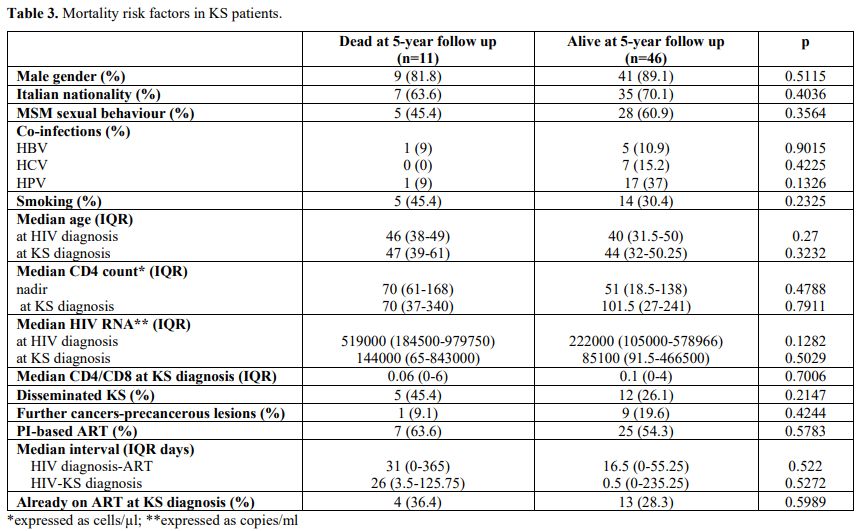
Five years after KS
diagnosis, 46 (76.7%) individuals were still alive: 41 (89.1%) were
male, 28 (60.9%) MSM; 5 (10.9%), 7 (15.2%) and 17 (37%) co-infected by
HBV, HCV, and HPV, respectively; 14 (30.4%) smoked. Their median age
was 40 at HIV diagnosis and 44 at KS diagnosis; 29 (63%) had cutaneous
localization, and 26 (56.5%) were treated only with ART (Table 3).
Univariate analysis did not show any statistically relevant risk factor influencing mortality.
 |
- Table 3. Mortality risk factors in KS patients.
|
Discussion
This
study, carried out in 2 Italian hospitals, confirmed that KS is not a
rare disease among PLWH, as shown in the 2020 report, too[1,6,7]
and its frequency has remained constant through the years. Literature
enlightened male gender, homosexual behaviour, and sub-Saharan and
Mediterranean origin being KS risk factors in PLWH [2-5].
Similarly, in our study, males and MSM were the most affected
population groups, while Africans were a minority in our sample. This
distribution reflects the characteristics of patients observed in our
centers, mostly males, Italians, and MSM, as shown in previous
publications.[8-10] The burden of risk factors changes
in different settings; in sub-Saharan Africa, male predominance in KS
is less pronounced, while extremely relevant is a history of sexually
transmitted infections (STIs).[5,11]
Concerning STIs, only 11.7% of our cases were co-infected with HBV and
HCV, and they did not show a higher mortality rate. Also, HPV infection
and smoking regarded just a minority of our population and did not
result to be negative prognostic factors in this analysis.
Unfortunately, too many patients were not tested for HPV and were not
asked about their smoking attitude.
Previously it has been
demonstrated that high viral load, low nadir CD4 count, and CD4/CD8
ratio were associated with KS occurrence.[3,4,12]
More than 70% of our patients had a CD4 count <350 cells/µl either
at HIV or at KS diagnosis, and the median value of CD4 nadir was 63
cells/µl. Similar frequency for viral load and CD4/CD8 ratio. None of
these immunological and viral parameters resulted in enhancing
mortality rate in our sample. Low CD4 cell quantity and high frequency
of concomitant diagnosis of HIV infection and KS confirmed the
increasing number of late HIV diagnoses reported by Istituto Superiore
di Sanità.[13]
Cutaneous and disseminated forms
were the most common in this study, while mucosal ones were localized
in the oral cavity except in 2 cases: 1 in the anus and 1 in the ocular
conjunctive. Rohrmus B et al. reported a higher death rate in oral KS
than in cutaneous KS.[14] Our 5 cases of oral KS are
too few to make the same comparison, and our analysis focused on
disseminated versus not disseminated KS finding no difference in
mortality rate. In addition, 12 KS patients also had other
cancerous or precancerous lesions. In particular, 3 were affected by
other HHV-8-related neoplasms: 2 by Castleman disease and 1 by
primitive effusive Lymphoma. Unfortunately, KS diagnosis was supported
by positive HHV-8 viremia and histology only in 15 and 6 individuals,
respectively. All the 5 persons having negative HHV-8 viremia were
affected by cutaneous KS.
Except for 3 individuals whose data were
unavailable, every patient received ART, and 18, mostly with
disseminated KS, required chemotherapy, too. PI was often included in
the ART regimen according to the evidence of some literature.[15,16] However, there is still debate about this aspect,[17] and also our study failed to prove any benefit of PI-based ART on mortality rate.
Finally,
it is worth noticing that KS diagnosis occurred after HIV diagnosis in
21 patients, and 17 were already on ART when KS was discovered. This
late diagnosis of patients on ART therapy is unsurprising because an
increase of KS incidence in the first 6 months of ART has been
estimated to coincide with the immune reconstitution.[18,19]
In our sample, the 17 patients with a new diagnosis of KS had been on
treatment for about 200 days. This, together with an uncertain
adherence, could explain why, despite therapy, 52.9% of them did not
reach viral suppression at KS diagnosis. Moreover, the main
characteristic of this particular group of PLWH was the longer median
interval between HIV diagnosis and ART starting (1500 versus 17 days)
due to the higher CD4 count (median CD4 nadir 123 versus 63 cells/µl),
and this aspect could represent an additional risk factor for KS.[5]
ART advent and its early starting significantly lowered KS occurrence.[3,4,20] However, our analysis did not show relevant differences in HIV diagnosis-ART starting interval between dead and alive groups.
Limitations
of this study are the small sample size, the number of people lost to
follow-up, and missing data due to the retrospective nature of this
work.
Conclusion
Despite
the ART era, KS is not a rare disease and keeps high lethality.
Individuals examined in our study were mostly Italians, MSM, with high
HIV viral load, low CD4 count and CD4/CD8 ratio. Most of them were
affected by cutaneous KS and treated with PI-based ART. Analyzing
epidemiological and clinical aspects, immune and viral parameters, and
type and timing of ART, we did not find any statistically relevant risk
factor influencing mortality rate. However, the sample size is too
small to generalize these results, and further studies may be needed.
References
- Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020:
GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers
in 185 Countries. CA Cancer J Clin. 2021;71:209-49. https://doi.org/10.3322/caac.21660 PMid:33538338
- Martin
JN, Ganem DE, Osmond DH, Page-Shafer KA, Macrae D, Kedes DH. Sexual
Transmission and the Natural History of Human Herpesvirus 8 Infection.
N Engl J Med. 1998;338:948-54. https://doi.org/10.1056/NEJM199804023381403 PMid:9521982
- Poizot-Martin
I, Obry-Roguet V, Duvivier C, Lions C, Huleux T, Jacomet C, Ferry T,
Cheret A, Allavena C, Bani-Sadr F, Palich R, Cabié A, Fresard A,
Pugliese P, Delobel P, Lamaury I, Hustache-Mathieu L, Brégigeon S,
Makinson A, Rey D; Dat'AIDS study group. Kaposi sarcoma among people
living with HIV in the French DAT'AIDS cohort between 2010 and 2015. J
Eur Acad Dermatol Venereol. 2020;34(5):1065-73. https://doi.org/10.1111/jdv.16204 PMid:31953902 PMCid:PMC7318618
- Liu
Z, Fang Q, Zuo J, Minhas V, Wood C, Zhang T. The world-wide incidence
of Kaposi's sarcoma in the HIV/AIDS era. HIV Med. 2018;19(5):355-364.
Epub 2018 Jan 25 https://doi.org/10.1111/hiv.12584 PMid:29368388
- Grabar S, Costagliola D. Epidemiology of Kaposi's Sarcoma. Cancers (Basel). 2021;13(22):5692 https://doi.org/10.3390/cancers13225692 PMid:34830846 PMCid:PMC8616388
- Ibrahim
Khalil A, Franceschi S, de Martel C, Bray F, Clifford GM. Burden of
Kaposi sarcoma according to HIV status: A systematic review and global
analysis. Int J Cancer. 2022;150(12):1948-57. https://doi.org/10.1002/ijc.33951 PMid:35085400
- AIDS-defining
Cancer Project Working Group for IeDEA and COHERE in EuroCoord.
Comparison of Kaposi sarcoma risk in human immunodeficiency
virus-positive adults across 5 continents: a multiregional multicohort
study. Clin Infect Dis. 2017;65:1316-26.
- Papalini
C, Paciosi F, Schiaroli E, Pierucci S, Busti C, Bozza S, Mencacci A,
Francisci D. Seroprevalence of anti-SARS-CoV2 Antibodies in Umbrian
Persons Living with HIV. Mediterr J Hematol Infect Dis.
2020;12(1):e2020080. https://doi.org/10.4084/mjhid.2020.080 PMid:33194154 PMCid:PMC7643777
- Schiaroli
E, De Socio GV, Gabrielli C, Papalini C, Nofri M, Baldelli F, Francisci
D. Partial Achievement of the 90-90-90 UNAIDS Target in a Cohort of HIV
Infected Patients from Central Italy. Mediterr J Hematol Infect Dis.
2020;12(1):e2020017. https://doi.org/10.4084/mjhid.2020.017 PMid:32180912 PMCid:PMC7059746
- Papalini
C, Lagi F, Schiaroli E, Sterrantino G, Francisci D. Transgender people
living with HIV: characteristics and comparison to homosexual and
heterosexual cisgender patients in two Italian teaching hospitals. Int
J STD AIDS. 2021;32(2):194-198. Epub 2020 Dec 16 https://doi.org/10.1177/0956462420950573 PMid:33327898
- Stolka
K, Ndom P, Hemingway-Foday J, Iriondo-Perez J, Miley W, Labo N, Stella
J, Abassora M, Woelk G, Ryder R, Whitby D, Smith JS. Risk factors for
Kaposi's sarcoma among HIV-positive individuals in a case control study
in Cameroon. Cancer Epidemiol. 2014;38(2):137-43. Epub 2014 Mar 13 https://doi.org/10.1016/j.canep.2014.02.006 PMid:24631417 PMCid:PMC4075442
- Caby,
F.; Guiguet, M.; Weiss, L.; Winston, A.; Miro, J.M.; Konopnicki, D.; Le
Moing, V.; Bonnet, F.; Reiss, P.; Mussini, C, Poizot-Martin I, Taylor
N, Skoutelis A, Meyer L, Goujard C, Bartmeyer B, Boesecke C, Antinori
A, Quiros-Roldan E, Wittkop L, Frederiksen C, Castagna A, Thurnheer MC,
Svedhem V, Jose S, Costagliola D, Mary-Krause M, Grabar S; (CD4/CD8
ratio and cancer risk) project Working Group for the Collaboration of
Observational HIV Epidemiological Research Europe (COHERE) in
EuroCoord. CD4/CD8 Ratio and the Risk of Kaposi Sarcoma or Non-Hodgkin
Lymphoma in the Context of Efficiently Treated Human Immunodeficiency
Virus (HIV) Infection: A Collaborative Analysis of 20 European Cohort
Studies. Clin Infect Dis. 2020;73:50-59. https://doi.org/10.1093/cid/ciaa1137 PMid:34370842
- Regine
V, Pugliese L, Boros S, Santaquilani M, Ferri M, Suligoi B.
Aggiornamento delle nuove diagnosi di infezione da HIV e dei casi di
AIDS in Italia al 31 dicembre 2021. Notiziario. 2022;35(11)
- Rohrmus B, Thoma-Greber EM, Rocken M, Bogner JR. Outlook in oral and cutaneous Kaposi's sarcoma. Lancet. 2000;356:2160. https://doi.org/10.1016/S0140-6736(00)03503-0 PMid:11191549
- Gantt
S, Cattamanchi A, Krantz E, Magaret A, Selke S, Kuntz SR, Huang ML,
Corey L, Wald A, Casper C. Reduced human herpesvirus-8 oropharyngeal
shedding associated with protease inhibitor-based antiretroviral
therapy. J Clin Virol. 2014;60(2):127-32 https://doi.org/10.1016/j.jcv.2014.03.002 PMid:24698158 PMCid:PMC4020979
- Leitch
H, Trudeau M, Routy JP. Effect of protease inhibitor-based highly
active antiretroviral therapy on survival in HIV-associated advanced
Kaposi's sarcoma patients treated with chemotherapy. HIV Clin Trials.
2003;4:107-14 https://doi.org/10.1310/VQXJ-41X6-GJA2-H6AG PMid:12671778
- Bower
M, Fox P, Fife K, Gill J, Nelson M, Gazzard B. Highly active
antiretroviral therapy (HAART) prolongs time to treatment failure in
Kaposi's sarcoma. AIDS. 1999;13:2105-11 https://doi.org/10.1097/00002030-199910220-00014 PMid:10546864
- Mocroft
A, Kirk O, Clumeck, N, Gargalianos-Kakolyris P, Trocha H, Chentsova N,
Antunes F, Phillips AN, Lundgren J. The changing pattern of Kaposi
sarcoma in patients with HIV, 1994-2003. Cancer. 2004;100:2644-54. https://doi.org/10.1002/cncr.20309 PMid:15197808
- Cancer
ProjectWorking Group for the Collaboration of Observational HIV
Epidemiological Research Europe Study in EuroCoord. Changing Incidence
and Risk Factors for Kaposi Sarcoma by Time Since Starting
Antiretroviral Therapy: Collaborative Analysis of 21 European Cohort
Studies. Clin. Infect. Dis. 2016, 63, 1373-79. https://doi.org/10.1093/cid/ciw562 PMid:27535953 PMCid:PMC5091347
- Group
I.S.S. Lundgren, JD, Babiker AG, Gordin, F, Emery S, Grund B, Sharma S,
Avihingsanon A, Cooper DA, Fätkenheuer G, Llibre JM, Molina JM, Munderi
P, Schechter M, Wood R, Klingman KL, Collins S, Lane HC, Phillips AN,
Neaton JD. Initiation of Antiretroviral Therapy in Early Asymptomatic
HIV Infection. N Engl J Med. 2015;373:795-807. https://doi.org/10.1056/NEJMoa1506816 PMid:26192873 PMCid:PMC4569751
[TOP]


