Cih-En Huang1,6,7, Yi-Yang Chen1, Jung-Jung Chang2, Yu-Ying Wu1, Wei-Ming Chen3, Ying-Hsuan Wang1, Min-Chi Chen4,8, Chang-Hsien Lu1, Chung-Sheng Shi5,6 and Chih-Cheng Chen1,7.
1 Division of Hematology and Oncology, Department of Medicine, Chang Gung Memorial Hospital, Chiayi, Taiwan.
2 Division of Cardiology, Department of Medicine, Chang Gung Memorial Hospital, Chiayi, Taiwan.
3 Division of Gastroenterology and Hepatology, Department of Medicine, Chang Gung Memorial Hospital, Chiayi, Taiwan.
4 Department of Obstetrics and Gynecology, Chang Gung Memorial Hospital, Chiayi, Taiwan.
5 Division of Urology, Department of Surgery, Chang Gung Memorial Hospital, Chiayi, Taiwan.
6 Graduate Institute of Clinical Medical Sciences, College of Medicine, Chang Gung University, Taoyuan, Taiwan.
7 College of Medicine, Chang Gung University, Taoyuan, Taiwan.
8 Department of Public Health and Biostatistics Consulting Center, College of Medicine, Chang Gung University, Taoyuan, Taiwan.
Correspondence to:
Prof. Chih-Cheng Chen. Address: 8, Sec. West, Chia-Pu Road, Pu-Tz City,
Chiayi, Taiwan. Tel: 886-5-3621000, Ext. 2852; Fax: 886-5-3623005.
E-mail:
ccchen1968@gmail.com
Published: May 1, 2023
Received: February 9, 2023
Accepted: April 20, 2023
Mediterr J Hematol Infect Dis 2023, 15(1): e2023030 DOI
10.4084/MJHID.2023.030
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background.
Human platelet antigens (HPAs) are alloantigens associated with
antiplatelet alloantibodies and the risk of immune thrombocytopenia
(ITP). However, few studies have investigated associations among HPAs,
antiplatelet autoantibodies, and cryoglobulins.
Methods.
We enrolled 43 patients with primary ITP, 47 with hepatitis C
virus-associated ITP (HCV-ITP), 21 with hepatitis B virus-associated
ITP (HBV-ITP), 25 controls with HCV, and 1013 normal controls. We
analyzed HPA allele frequencies, including HPA1-6 and 15, antiplatelet
antibodies binding to platelet glycoprotein (GP) IIb/IIIa, Ia/IIa,
Ib/IX, IV, human leukocyte antigen class I, cryoglobulin IgG/A/M, and
their associations with thrombocytopenia.
Results.
In the ITP cohort, HPA2ab, rather than HPA2aa, predicted a low platelet
count. HPA2b was associated with the risk of developing ITP. HPA15b was
correlated with multiple antiplatelet antibodies. In HCV-ITP patients,
HPA3b was correlated with anti-GPIIb/IIIa antibodies. HCV-ITP patients
with anti-GPIIb/IIIa antibodies had a higher positive rate of
cryoglobulin IgG and IgA compared with those without anti-GPIIb/IIIa
antibodies. Overlapping detection was also found among other
antiplatelet antibodies and cryoglobulins. Like the antiplatelet
antibodies, cryoglobulins were associated with clinical
thrombocytopenia, implying their close relationship. Finally, we
extracted cryoglobulins to confirm the exhibition of cryoglobulin-like
antiplatelet antibodies. In contrast, in primary ITP patients, HPA3b
was correlated with cryoglobulin IgG/A/M rather than anti-GPIIb/IIIa
antibodies.
Conclusion.
HPA alleles were associated with antiplatelet autoantibodies and had
different impacts in primary ITP and HCV-ITP patients. HCV-ITP was
considered to be a symptom of mixed cryoglobulinemia in HCV patients.
The pathophysiology may differ between these two groups.
|
Introduction
Human
platelet antigens (HPAs) are alloantigens on platelet surface membrane
glycoproteins (GPs). They are differentiated by single nucleotide
polymorphisms in the genes encoding GPs.[1] To date, 35 antigens
categorized into 29 groups have been identified,[2] among which HPA-1,
HPA-2, HPA-3, HPA-4, HPA-5, and HPA-15 are biallelic, and expressed on
GPIIIa, GPIba, GPIIb, GPIIIa, GPIa, and CD109, respectively. However,
the frequencies of HPA alleles differ between ethnic groups and
geographic areas.[3,4] These antigens are important and characterized
by their immunogenicity of antiplatelet alloantibodies. HPA-associated
alloantibodies have been associated with fetal and neonatal alloimmune
thrombocytopenia, posttransfusion purpura, and platelet transfusion
refractoriness.[1,3]
Immune thrombocytopenia (ITP) can be
categorized as primary and secondary. Secondary ITP is caused by specific
etiologies, like infection and autoimmune disorders. With regards to
infection, hepatitis C virus (HCV) and Helicobacter pylori (H.p.) are well-documented causes of secondary ITP, namely hepatitis C virus-associated immune thrombocytopenia (HCV-ITP), and Helicobacter pylori-associated
immune thrombocytopenia (H.p.-ITP). The Hepatitis B virus (HBV) has
also been associated with thrombocytopenia. However, there is currently
no consensus on the immune modulatory effect of HBV. Primary ITP is
diagnosed by the exclusion of known etiologies.[5] The mechanism of ITP
is complex and may involve the production of antiplatelet
antibodies.[6] HPAs have also been associated with antiplatelet
alloantibodies, and several studies have reported that certain HPAs
could predict the development of ITP.[7,8] However, no reports exist on
the association between HPAs and antiplatelet autoantibodies.
Cryoglobulins
are serum immunoglobulins that precipitate when the temperature is
cooled below 37°C, then redissolve when rewarmed. Cryoglobulinemia is
classified into three types. Type I is associated with monoclonal
immunoglobulin (Ig), and type II and III are mixed cryoglobulinemias
associated with polyclonal IgG and monoclonal IgM or polyclonal IgM,
respectively.[9] HCV is the most common etiology of mixed
cryoglobulinemia, and cryoglobulinemia is a well-documented
extrahepatic manifestation in chronic HCV-infected patients.[10] The
clonal expansion of B cells has been observed in HCV-associated mixed
cryoglobulinemia, which may be driven by antigen selection.[11]
However, whether cryoglobulins bind to a specific antigen or multiple
antigens is unclear. Patients with HCV-ITP have been reported to have
higher rates of cryoglobulinemia and antiplatelet antibodies compared
to those with primary ITP, who have higher rates than the general
population.[12,13] No previous study has investigated the association
between cryoglobulins and antiplatelet antibodies in ITP patients.
Therefore, we conducted this study to explore the clinical associations
between cryoglobulins and thrombocytopenia and evaluate the possible
relationships among HPA alleles, antiplatelet antibodies, and
cryoglobulins.
Patients and Methods
Patients.
We enrolled 111 ITP patients, 25 controls with HCV infection, and 1013
normal controls. The diagnosis of ITP was according to the American
Society of Hematology guidelines when a peripheral platelet count of
< 100 x 109/L was detected.[5,14]
Patients with correctable iron-deficiency anemia due to bleeding were
included. A bone marrow study was performed in patients with
abnormalities in peripheral blood other than thrombocytopenia and
iron-deficiency anemia. Patients with thrombotic events, uncontrolled
active bleeding, acute infection in the past 3 months, active cancer,
and taking medications that could cause thrombocytopenia were excluded.
In addition, patients with H.p.-ITP, defined as a positive urea breath
test, positive endoscopic campylobacter-like organism test, or positive
Giemsa staining of a stomach biopsy, were excluded. Patients positive
for both serum hepatitis B surface antigen (HBsAg) and anti-hepatitis C
virus antibody (anti-HCV Ab) and those with advanced cirrhosis
(Child-Pugh Classification B and C) were also excluded. The enrolled
patients were then classified into three groups: primary ITP (which was
diagnosed by excluding known etiologies), HCV-ITP (those positive for
anti-HCV Ab), and HBV-ITP (those positive for HBsAg). We also enrolled
HCV control patients, who were seropositive for anti-HCV Ab and had a
normal platelet count of ≥ 150 x 109/L, and a healthy volunteer group.
After
enrollment, complete blood cell counts and general biochemical data,
including aspartate aminotransferase, alanine aminotransferase,
alkaline phosphatase, prothrombin time, bilirubin, and albumin, were
collected. The platelet count was categorized into five levels: level
1, ≥ 150 x 109/L; level 2, 100 - 149 x 109/L; level 3, 50 - 99 x 109/L; level 4, 30 - 49 x 109/L; and level 5, < 30 x 109/L.
Abdominal sonography was performed to determine cirrhosis status and
spleen size. Spleen size was presented as an index by multiplying the
length of long and short axes over the spleen hilum at a right angle.
In addition, serum and peripheral blood mononuclear cells were
collected and analyzed. This study was performed in accordance with the
Helsinki Declaration and approved by the Review Board at Chang Gung
Memorial Hospital.
HPA allele detection. Peripheral
blood samples from the enrolled subjects were collected in
EDTA-anticoagulant tubes. Buffy coats were isolated by 700g
centrifugation immediately. Peripheral blood mononuclear cells were
extracted from the buffy coats by Ficoll-Hypaque gradient
centrifugation (Thermo Fisher Scientific Inc., MA., USA). DNA
extraction was performed by Trizol-Alcohol precipitation, and the
quantity and quality of the DNA were confirmed according to a nanodrop
concentration ranging from 5 to 40 ng/μl and an A260/A280 ratio between
1.65 and 2.0, respectively.
HPA typing was performed using an
ExProbe SE HPA 1-6, 15, 21 Typing Kit (TBG Diagnostics Limited.,
Melbourne, VIC, Australia), which used sequence-specific primers with
real-time polymerase chain reaction (PCR). The primer panel was mixed
with pre-coated fluorescent dye on plates on an HPA typing tray
according to the manufacturer's instructions. The HPA typing primers
for the allele-specific sequences were amplified and detected by
fluorescence activation with a specific Tm range. Simultaneously, an
internal control primer was amplified with a specific Tm value. The
distinct Tm values of the HPA sequence and internal control determined
the HPA type and integrity of the PCR. PCR was performed using an
Applied Biosystems® 7500 Real-Time PCR System according to the
manufacturer's protocol. The analysis was processed using 7500 Software
v2.3 with the Melting Curve function. In addition, we cooperated with
the Taiwan Blood Services Foundation to include anonymous information
on HPA allele frequencies in 998 Taiwanese blood donors as published by
Pai et al in our healthy controls.[3]
Cryoglobulin examination.
We used the double immunodiffusion method to identify cryoglobulin IgG,
IgA, and IgM. Briefly, fresh blood samples were centrifuged at 3000 rpm
for 10 min at 37°C. The collected plasma was cooled to 4°C for 3 days
and then centrifuged at 3000 rpm for 10 min at 4°C. The deposited
cryocrit at 4ºC was washed and then warmed to 37°C for 2 hours. The
dissolved cryocrit indicated the presence of cryoglobulins. Finally,
the dissolved sample was mixed with anti-human IgG, IgA, and IgM and
then run in agarose gel to identify the cryoglobulins. The data were
presented semi-qualitatively as 1+, 2+, and 3+ by comparing with
controls at a fixed concentration.
Antiplatelet antibody detection.
We used a commercial qualitative enzyme-linked immunosorbent assay
(ELISA) kit (PakPlus assay, Immucor Inc., Norcross, GA, USA) to detect
antiplatelet antibodies in the collected serum. The assay detected
antibodies bound to platelet surface antigens, including GPIIb/IIIa,
Ia/IIa, Ib/IX, IV, and human leukocyte antigen (HLA) class I. Briefly,
we added patients' serum to the wells of a 96-microwell plate coated
with the platelet surface antigens aforementioned so that the present
autoantibodies bound to the specific antigens. Alkaline
phosphatase-labeled anti-human Ig G/A/M were then used to activate
substrate p-nitrophenyl phosphate for detection. ELISA was performed
according to the manufacturer's instructions.
Examination of correlations between cryoglobulin and antiplatelet antibodies.
We selected 5 ITP patients with cryoglobulin (2+)/anti-GPIIb/IIIa
antibody (+), 5 with cryoglobulin (1+)/anti-GPIIb/IIIa antibody (+). We
also selected 13 controls, including 4 with cryoglobulin
(2+)/anti-GPIIb/IIIa antibody (-), 5 with cryoglobulin
(-)/anti-GPIIb/IIIa antibody (+), and 4 who were negative for both
antibodies. To investigate whether the cryoglobulins exhibited the
characteristics of antiplatelet antibodies, we extracted them using a
modified version of the cryoglobulin examination method. The main
difference was that we extracted the cryocrit from the frozen plasma
collected for antiplatelet antibody detection instead of fresh plasm in
cryoglobulin examination. The cryocrit was then manipulated as with the
cryoglobulin examination method. Finally, antiplatelet antibody
detection was performed for the dissolved cryocrit.
Statistical analysis.
Differences in clinical characteristics and study variables between the
ITP and control groups were evaluated using the two-sample t-test,
Wilcoxon rank-sum test, or chi-square test, as appropriate.
Correlations between HPA alleles and antibodies, platelet levels and
antibodies, and antiplatelet antibodies and cryoglobulins were analyzed
using the chi-square test and odds ratios. HPA1-5 and 15 are
bi-allelic, comprised of homozygous aa/bb and heterozygous ab.
Therefore, the impact of a single HPA a or b allele on antibody
production was compared with a non-HPA a or b allele by weighting, with
aa/bb as 2 and ab as 1. Statistical significance was defined as a
two-sided p-value of less than 0.05. All data were analyzed using
Statistical Package for the Social Sciences version 26.0 (SPSS,
Chicago, IL, USA).
Results
Baseline characteristics.
We enrolled 43 patients with primary ITP, 47 with HCV-ITP, 21 with
HBV-ITP, and 25 controls with HCV infection. Their baseline
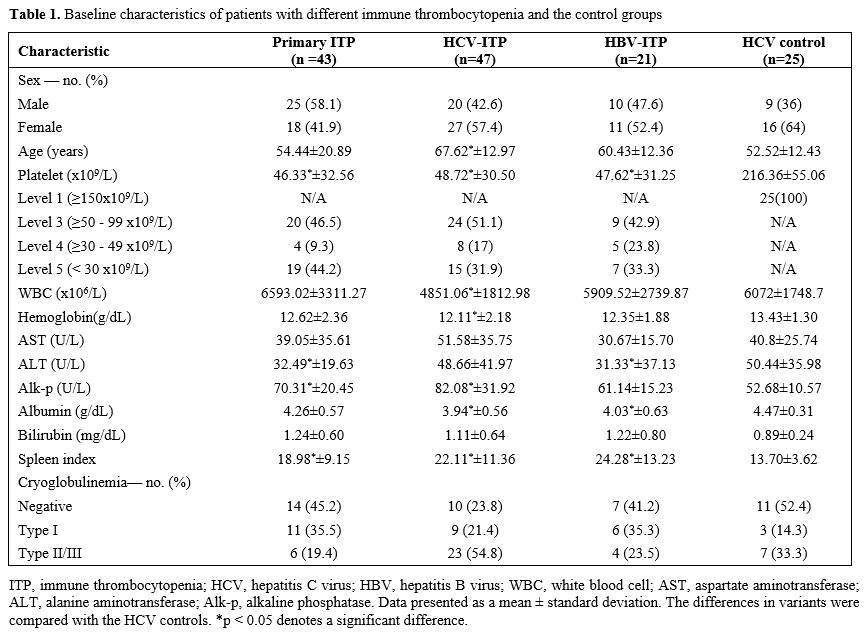
characteristics are presented in Table 1.
Compared with the HCV controls, the whole ITP cohort (primary ITP,
HCV-ITP, and HBV-ITP groups combined) had a significantly larger spleen
index, with the largest value (24.28) in the HBV-ITP group. The HCV-ITP
patients had a higher proportion (54.8%) of type II/III mixed
cryoglobulinemia, while the primary ITP patients had a lower proportion
(19.4%) compared to the HCV controls (33.3%).
 |
- Table
1. Baseline characteristics of patients with different immune thrombocytopenia and the control groups
|
The results of antiplatelet antibody profiles, cryoglobulin profiles, and associated complexity in each group are presented in Supplementary Figure 1.
Some of the antiplatelet antibody results in some patients have been
reported in previous studies.[12,15] In this study, we extended the
analysis to include HPA allele polymorphisms and the presence of
cryoglobulins. The detection rates of total antiplatelet antibodies and
cryoglobulins were higher in the HCV-ITP patients than in other groups (Supplementary Figure 1A and 1C).
Anti-GPIIb/IIIa antibodies and cryoglobulin IgM were these patients'
most commonly detected immunoglobulins. In the complexity analysis of
the three ITP groups, the HBV-ITP group had the lowest rates of the
presence of three or more types of antiplatelet antibodies and
cryoglobulins (Supplementary Figure 1B and 1D).
HPA2ab was associated with lower platelet count, and HPA2b was associated with the risk of developing ITP.
After including the anonymous HPA polymorphism data of 998 Taiwanese
blood donors in addition to our 15 normal controls, there were a total
of 1013 normal controls. Regarding the heterogenicity of HPA
polymorphisms, HPA3 and HPA15 were the most heterogenous in our
population (Table 2). The HBV-ITP patients had a significantly lower proportion of HPA15ab and a higher proportion of HPA15aa (p=0.047, Table 2).
In the analysis of specific allele frequencies, the whole ITP cohort
had a higher HPA2b allele frequency than the normal controls (6.3%
versus 3.5%, p=0.038, Table 3),
and this trend was observed in all three ITP groups. Therefore, we
further explored the HPA alleles and clinical presentations. The
results showed that HPA2ab was associated with a higher rate of severe
thrombocytopenia (level 5 platelet count of <30 x109/L, p=0.023, Supplementary Figure 2A). In addition, the whole ITP cohort with HPA2ab had a significantly lower mean platelet count than those with HPA2aa (31.36 x109/L versus 49.93 x109/L, p=0.037, Supplementary Figure 2B).
These results showed that HPA2b was associated with the risk of ITP and
clinical thrombocytopenia. Because HPA2 determines the alloantigen on
GPIb, we further explored the association between HPA2a/2b and
anti-GPIb/IX antibodies. The results showed no significant difference
in the HPA2b incidence between the patients with or without
anti-GPIb/IX antibodies.
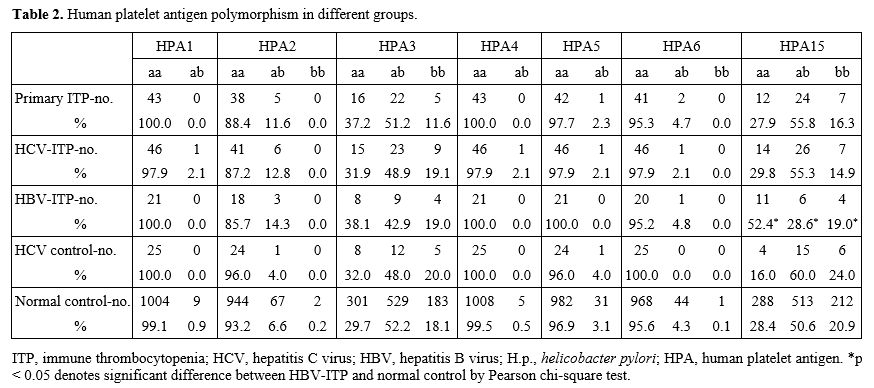 |
Table 2. Human platelet antigen polymorphism in different groups. |
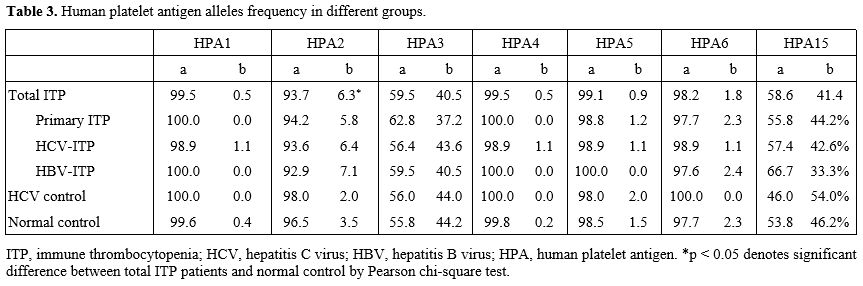 |
Table 3. Human platelet antigen alleles frequency in different groups.
|
HPA15 was associated with the production of many types of antiplatelet antibodies. We
also explored the possible relationships of the most heterogeneous HPA
alleles, HPA3 and HPA15, with antiplatelet antibodies and cryoglobulins
in the complete ITP cohort. The results showed that HPA15b was
associated with higher positive rates of anti-GPIa/IIa, Ib/IX, IV, and
HLA Class 1 antibodies compared with non-HPA15b, whereas HPA15a was
inversely associated with anti-GPIb/IX, IV, and HLA Class 1 antibodies
compared with non-HPA15a (Figure 1A).
It suggested that ITP patients with HPA15b would have more complex
antiplatelet antibody profiles than those without HPA15b, while ITP
patients with HPA15a would have more simple profiles than those without
HPA15a. There was no obvious association between HPA15 and
cryoglobulins. Because HPA alleles consist of aa, ab, and bb, the
comparisons between a/b versus non-a/b and between a versus b make
different results. In the direct comparison between HPA15b versus
HPA15a on antiplatelet antibody production, the results still showed a
significantly higher risk with HPA15b than HPA15a for the production of
anti-GPIb/IX, GPIV, and HLA Class I antibodies at odds ratios of 2.452,
3.841, and 1.939, respectively (Supplementary Table 1). These results reflected the detected number of antiplatelet antibody types in HPA15 alleles. As shown in Figure 1B,
the detection rates of 2 and ≥ 3 types of antiplatelet antibodies were
higher in HPA15b than in HPA15a. In short, HPA15b was associated with
risk for multiple antiplatelet antibodies compared with HPA15a.
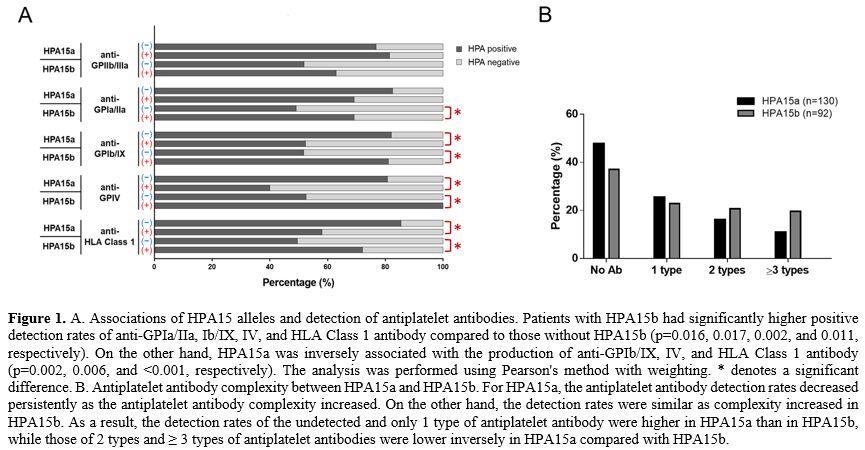 |
- Figure 1. A.
Associations of HPA15 alleles and detection of antiplatelet antibodies.
Patients with HPA15b had significantly higher positive detection rates
of anti-GPIa/IIa, Ib/IX, IV, and HLA Class 1 antibody compared to those
without HPA15b (p=0.016, 0.017, 0.002, and 0.011, respectively). On the
other hand, HPA15a was inversely associated with the production of
anti-GPIb/IX, IV, and HLA Class 1 antibody (p=0.002, 0.006, and
<0.001, respectively). The analysis was performed using Pearson's
method with weighting. * denotes a significant difference. B.
Antiplatelet antibody complexity between HPA15a and HPA15b. For HPA15a,
the antiplatelet antibody detection rates decreased persistently as the
antiplatelet antibody complexity increased. On the other hand, the
detection rates were similar as complexity increased in HPA15b. As a
result, the detection rates of the undetected and only 1 type of
antiplatelet antibody were higher in HPA15a than in HPA15b, while those
of 2 types and ≥ 3 types of antiplatelet antibodies were lower
inversely in HPA15a compared with HPA15b.
|
HPA3 was associated with the production of anti-GPIIb/IIIa antibodies and cryoglobulins.
In the complete ITP cohort, HPA3b was positively associated with
cryoglobulin IgG/IgM and total cryoglobulins production compared with
non-HPA3b, whereas HPA3a was inversely associated with cryoglobulin
IgA/IgM and total cryoglobulins production compared with non-HPA3a (Figure 2A).
Similarly, HPA3b had a higher risk for cryoglobulin IgA and IgM
compared to HPA3a at odds ratios of 1.966 and 1.905, respectively
(Supplementary Table 2). In contrast, there was no significant
association between HPA3a/3b and anti-GPIIb/IIIa antibodies. Because
HPA3 is an important alloantigen on GPIIb, the results could have been
clearer. To exclude the possible different pathophysiology of each ITP
group, we further analyzed the effects of HPA3a/3b on anti-GPIIb/IIIa
antibodies and cryoglobulins in the primary ITP and HCV-ITP patients,
respectively. In the primary ITP patients, HPA3b was associated with
cryoglobulin IgG/A/M production, but HPA3a was inversely
correlated (Figure 2B). On
the other hand, in the HCV-ITP patients, HPA3b was associated with
anti-GPIIb/IIIa antibodies detection, whereas HPA3a was inversely
associated with anti-GPIIb/IIIa antibodies (Figure 2C).
Although the associations of HPA3b on anti-IIb/IIIa antibody in primary
ITP and HPA3b on cryoglobulin IgG/A/M in HCV-ITP did not reach
statistical significance, a trend of the associations still exists.
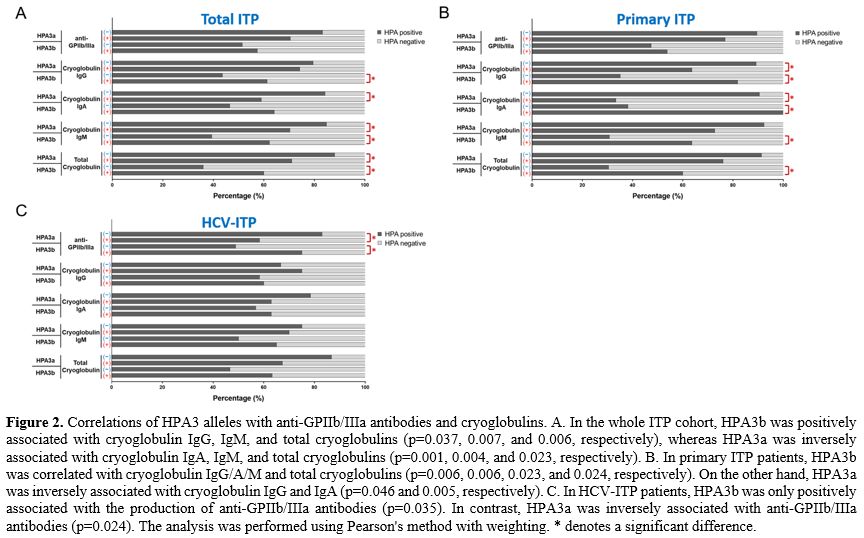 |
- Figure 2. Correlations
of HPA3 alleles with anti-GPIIb/IIIa antibodies and cryoglobulins. A.
In the whole ITP cohort, HPA3b was positively associated with
cryoglobulin IgG, IgM, and total cryoglobulins (p=0.037, 0.007, and
0.006, respectively), whereas HPA3a was inversely associated with
cryoglobulin IgA, IgM, and total cryoglobulins (p=0.001, 0.004, and
0.023, respectively). B. In primary ITP patients, HPA3b was correlated
with cryoglobulin IgG/A/M and total cryoglobulins (p=0.006, 0.006,
0.023, and 0.024, respectively). On the other hand, HPA3a was inversely
associated with cryoglobulin IgG and IgA (p=0.046 and 0.005,
respectively). C. In HCV-ITP patients, HPA3b was only positively
associated with the production of anti-GPIIb/IIIa antibodies (p=0.035).
In contrast, HPA3a was inversely associated with anti-GPIIb/IIIa
antibodies (p=0.024). The analysis was performed using Pearson's method
with weighting. * denotes a significant difference.
|
Positive correlations between anti-GPIIb/IIIa antibodies and cryoglobulin detection.
Because HPA3 alleles were associated with anti-GPIIb/IIIa antibodies
and cryoglobulins production, we hypothesized that there might be
associations between these immunoglobulins. We first explored the
overlapping incidence between these two categories of immunoglobulins.
The primary ITP patients with anti-GPIIb/IIIa antibodies had a higher
positive rate of cryoglobulin IgA than those without anti-GPIIb/IIIa
antibodies (p=0.023, Figure 3A).
On the other hand, the HCV-ITP patients with anti-GPIIb/IIIa antibodies
had significantly higher rates of positive cryoglobulin IgG and IgA
than those without anti-GPIIb/IIIa antibodies (p=0.006 and p=0.026,
respectively, Figure 3B).
Although anti-GPIIb/IIIa antibodies were not statistically associated
with cryoglobulin IgM, most HCV-ITP patients with anti-GPIIb/IIIa
antibodies similarly had a high presentation of cryoglobulin IgM (Figure 3B).
In addition, most HCV-ITP patients with any one positive antiplatelet
antibody had a positive detection for cryoglobulins. Conversely, the
HCV-ITP patients without cryoglobulin nearly had no detectable
antiplatelet antibody (Supplementary Table 3).
Accordingly, the detection of both types of immunoglobulins overlapped
in the HCV-ITP patients but not in the primary ITP patients. It
suggested that cryoglobulins may comprise multiple antiplatelet
antibodies in HCV-ITP patients. These findings also reflected that HPA3
was specifically associated with anti-GPIIb/IIIa antibodies rather than
cryoglobulins in the HCV-ITP patients.
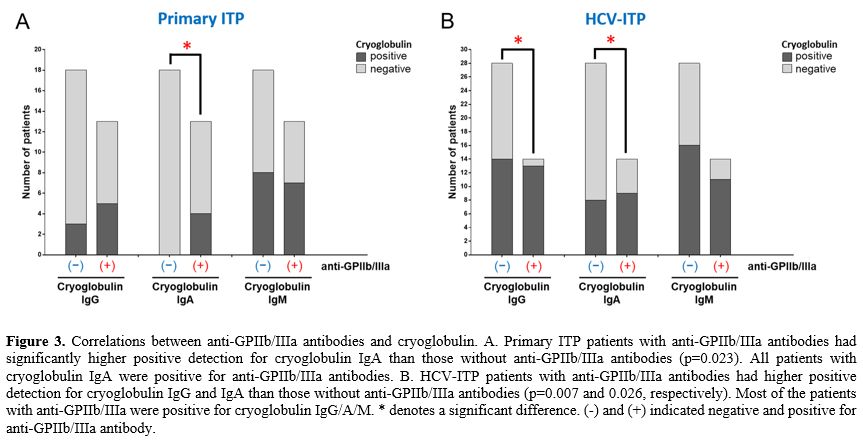 |
- Figure 3. Correlations
between anti-GPIIb/IIIa antibodies and cryoglobulin. A. Primary ITP
patients with anti-GPIIb/IIIa antibodies had significantly higher
positive detection for cryoglobulin IgA than those without
anti-GPIIb/IIIa antibodies (p=0.023). All patients with cryoglobulin
IgA were positive for anti-GPIIb/IIIa antibodies. B. HCV-ITP patients
with anti-GPIIb/IIIa antibodies had higher positive detection for
cryoglobulin IgG and IgA than those without anti-GPIIb/IIIa antibodies
(p=0.007 and 0.026, respectively). Most of the patients with
anti-GPIIb/IIIa were positive for cryoglobulin IgG/A/M. * denotes a
significant difference. (-) and (+) indicated negative and positive for
anti-GPIIb/IIIa antibody.
|
Antiplatelet antibodies and cryoglobulins were significantly associated with platelet levels in HCV-ITP patients.
Because of the high degree of overlap between antiplatelet antibodies
and cryoglobulins, we further explored the clinical impact of these two
categories of antibodies. The antiplatelet antibody profiles and
complexity in the HCV-ITP patients and HCV controls according to
platelet level are shown in Figures 4A and 4B. Antiplatelet antibodies' rates and complexity increased as the platelet
count decreased. Among them, the increases in the rates of anti-GPIb/IX
and total antiplatelet antibodies reached significance (p=0.009 and
0.005, respectively). Similarly, the cryoglobulin IgG, IgA, and IgM
detection rates increased as the platelet count decreased, of which cryoglobulin
IgA reached statistical significance (p=0.018, Figure 4C).
For cryoglobulin complexity, the positive rates of the three types of
cryoglobulins increased as the platelet count decreased, whereas the
rate of undetectable cryoglobulin decreased as the platelet count
decreased (Figure 4D). However,
in the primary ITP patients, correlations between the detection rates
of antiplatelet antibodies and cryoglobulins with platelet levels were
not found (data not shown). Although the primary ITP patients with
cryoglobulin IgA had a lower mean platelet count (33 x109/L) than those without IgA (50.11 x109/L),
the number of cases was too small to make definitive conclusions. These
findings demonstrated that both antiplatelet antibodies and
cryoglobulins were significantly correlated with platelet count in HCV
patients.
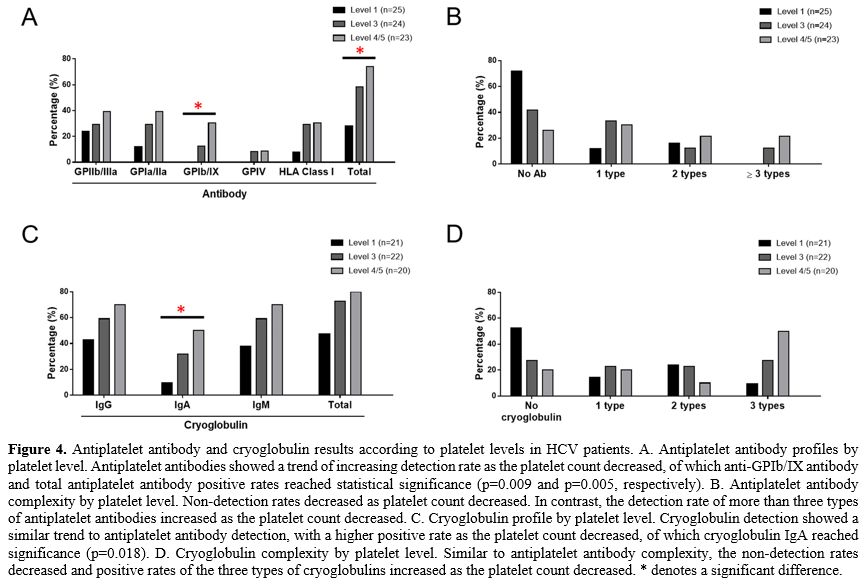 |
- Figure 4. Antiplatelet
antibody and cryoglobulin results according to platelet levels in HCV
patients. A. Antiplatelet antibody profiles by platelet level.
Antiplatelet antibodies showed a trend of increasing detection rate as
the platelet count decreased, of which anti-GPIb/IX antibody and total
antiplatelet antibody positive rates reached statistical significance
(p=0.009 and p=0.005, respectively). B. Antiplatelet antibody
complexity by platelet level. Non-detection rates decreased as platelet
count decreased. In contrast, the detection rate of more than three
types of antiplatelet antibodies increased as the platelet count
decreased. C. Cryoglobulin profile by platelet level. Cryoglobulin
detection showed a similar trend to antiplatelet antibody detection,
with a higher positive rate as the platelet count decreased, of which
cryoglobulin IgA reached significance (p=0.018). D. Cryoglobulin
complexity by platelet level. Similar to antiplatelet antibody
complexity, the non-detection rates decreased and positive rates of the
three types of cryoglobulins increased as the platelet count decreased.
* denotes a significant difference.
|
Cryoglobulins showed characteristics of antiplatelet antibodies.
We extracted the cryocrit from blood samples to detect the presence of
antiplatelet antibodies. The ITP patients with positive anti-GPIIb/IIIa
antibodies always had other antiplatelet antibodies. The data showed
that the patients with cryoglobulin (2+)/anti-GPIIb/IIIa antibody (+)
had significantly higher detection ratios of not only anti-GPIIb/IIIa
antibodies but also anti-GPIa/IIa, Ib/IX, and IV antibodies than
controls (Supplementary Figure 3).
These results confirmed that the cryoglobulins showed characteristics
of antiplatelet antibodies in the primary ITP and HCV-ITP patients who
were strongly positive for cryoglobulins and antiplatelet antibodies.
However, these patients were much fewer in primary ITP than HCV-ITP
patients. These findings are summarized and illustrated in Supplementary Figure 4.
Discussion
This
study demonstrated correlations among HPA alleles, antiplatelet antibodies,
and cryoglobulins in primary and secondary ITP patients. HPA alleles
have been reported to be a risk factor for the development of ITP. Of
HPA1-5 and 15 alleles, HPA2 has been reported to be most strongly
associated with the development of ITP.[7,8,16-18] Consistently with
studies conducted in Egypt and Macedonia,[7,18] we echoed that HPA2b
was a significant risk for ITP development. We further found that the
ITP patients with HPA2b had lower platelet counts than those without
HPA2b. The presence of anti-GPIb/IX antibodies was also significantly
correlated with the level of thrombocytopenia. A few alloantigens are
located on GPIb, of which HPA2 is the most important.[1] Therefore,
HPA2b-associated anti-GPIb/IX antibodies might contribute to the risk
for ITP. However, we found no correlation between HPA2b and
anti-GPIb/IX antibody production, but the limited number of cases
limits the ability to make definite conclusions. There is another
possibility that HPA2b may affect the epitope specificity and the
production of anti-GPIb/IX antibodies.
HPA15 alloimmunization
has been documented in fetal/neonatal alloimmune thrombocytopenia and
platelet transfusion refractoriness but was rarely detected.[19-21]
However, this is the first study to show that HPA15b was significantly
associated with multiple non-epitope antiplatelet antibody production
compared to HPA15a. There were three main findings. First, HPA15 was
strongly associated with anti-HLA class I and pan-antiplatelet
antibodies. Second, cryoglobulins were not associated with HPA15,
becoming a negative control. Third, HPA15 is located on CD109, the only
platelet membrane GP mainly expressed on the surface of platelets and
either white blood cells, especially T lymphocytes.[1,22] CD109 has
been reported to be a coreceptor of transforming growth factor-beta,
which inhibits signal pathways and affects the immune response.
Therefore, we hypothesize that HPA15b may promote the alloimmunization
of multiple GP alloantigens, enhancing the complexity of antiplatelet
antibody production. Significantly the HBV-ITP patients had lower
HPA15b and lower heterogenicity of antiplatelet antibodies.
Anti-GPIIb/IIIa
antibodies were this study's most frequently detectable antiplatelet
antibodies, consistent with a previous study.[23] HPA3 was the most
heterogenous HPA polymorphism, as reported in many other
ethnicities.[4] HPA3 is present on the most prevalent GPIIb on the
platelet cell membrane.[1]
Therefore, the anti-GPIIb/IIIa antibodies
may be related to the high prevalence of GPIIb/IIIa and heterogeneity
of the located alloantigens. Furthermore, we found that HPA3b was
positively associated with anti-GPIIb/IIIa antibodies and cryoglobulin
production but had different impacts in the patients with primary ITP
and
HCV-ITP. Most of the HCV-ITP patients with antiplatelet antibodies were
positive for cryoglobulins. Clinically, cryoglobulins, especially IgA,
similar to antiplatelet antibodies, had an impact on thrombocytopenia.
These results imply that a proportion of cryoglobulins may share the
function of antiplatelet antibodies in HCV-ITP patients. Then we
confirmed this hypothesis by directly extracting cryoglobulins from
patient samples to detect the existence of antiplatelet antibodies.
Accordingly, a large proportion of HPA3b-associated anti-GPIIb/IIIa
antibodies are a part of total cryoglobulins. That explains why HPA3b
is specifically correlated with anti-GPIIb/IIIa antibodies rather than
cryoglobulins in HCV-ITP patients. On the other hand, HPA3b was
specifically associated with cryoglobulins IgG/A/M in the primary ITP
patients. In the primary ITP patients, only some anti-GPIIb/IIIa
antibodies are HPA3-associated cryoglobulin-like antibodies, leading to
the correlation between HPA3 and cryoglobulins rather than
anti-GPIIb/IIIa antibodies. These results are summarized and
illustrated
in Supplementary Figure 4.
Mixed
cryoglobulinemia has been reported to be an extrahepatic manifestation
in HCV patients. This study presented a high detection rate of
cryoglobulinemia in HCV-ITP patients, as reported in a previous
landmark study.[13] In addition, a good correlation between antiplatelet antibodies and
cryoglobulinemia was reported in this study. Regarding the
pathophysiology, mixed cryoglobulinemia has been reported to be an
antigen-derived immune modulator.[11,24] HCV may induce
autoimmunization to many alloantigens and the production of
autoantibodies, leading to mixed cryoglobulinemia. Taken together, we
hypothesize that HPA is one of the complex alloantigens which induce
cryoglobulin-like antiplatelet antibodies. These immunoglobulins are
associated with clinical thrombocytopenia, causing a certain proportion
of HCV-ITP to be a symptom of mixed cryoglobulinemia in HCV patients,
as seen in this study. Cryoglobulin-like antiplatelet antibodies'
detection rate and complexity were closely associated with platelet
count. On the other hand, the pathophysiology of antiplatelet antibody
production has been well-documented in primary ITP patients.[25]
Primary ITP patients mainly have T-cell immune modulation and specific
antiplatelet antibody formation. Although HPA-associated cryoglobulins may
share the characteristics of antiplatelet antibodies to some degree,
the impact of these HPA allele-derived cryoglobulin-like antiplatelet
antibodies was much less prominent and important in primary ITP than in
the HCV-ITP patients, as shown in this study. Actually, it also
reflected the lower positive rates of antiplatelet antibodies and
cryoglobulins in primary ITP patients compared with HCV-ITP patients,
suggesting that different treatment strategies may be required for
patients with these two kinds of ITPs. However, there may also be
similarities between these two ITPs. The roles of HPA2b, cryoglobulin
IgA, and HPA15b seem to be important for thrombocytopenia and
antiplatelet antibody formation.
There are some limitations to
this study. Although we comprehensively demonstrated strong
correlations among HPA, antiplatelet autoantibodies, cryoglobulins, and
associated clinical impact on platelet count, the definite
pathophysiology of how HPA alleles affect antiplatelet antibodies and
cryoglobulin production needed to be clarified. Second, the case number
in each study group was relatively small. However, most of the results
were demonstrated in the complete ITP cohort, including the effect of
the HPA alleles on the risk of ITP or antiplatelet
antibody/cryoglobulin production. The impact of the b allele of HPA2,
HPA3, and HPA15 on antiplatelet antibodies and cryoglobulins was
consistent in the whole ITP cohort and subgroups.
In conclusion,
platelet antibodies' pathophysiology and associated characteristics
differed in the primary ITP and HCV-ITP patients. This may lead to
different management strategies and responses to treatment. ITP may be
considered a complication of cryoglobulinemia in a certain proportion
of HCV patients.
Conclusions
The
antiplatelet antibodies pathophysiology and associated characteristics
differed in the primary ITP and HCV-ITP patients. This may lead to
different management strategies and responses to treatment. ITP may be
considered a complication of cryoglobulinemia in a certain proportion
of HCV patients.
References
- Curtis BR, McFarland JG. Human platelet antigens - 2013. Vox Sang 2014; 106:93-102. https://doi.org/10.1111/vox.12085 PMid:24102564
- Bertrand
G, Danger Y, Croisille L, Le Toriellec E, Verite F, Renac V, Bierling
P. A new platelet alloantigen (Efs. Transfusion 2019; 59:2463-4. https://doi.org/10.1111/trf.15283 PMid:30942487
- Pai
SC, Burnouf T, Chen JW, Lin LI. Human platelet antigen alleles in 998
Taiwanese blood donors determined by sequence-specific primer
polymerase chain reaction. Biomed Res Int 2013; 2013:973789. https://doi.org/10.1155/2013/973789 PMid:23865077 PMCid:PMC3705808
- Kazemi
MH, Malakootikhah F, Momeni-Varposhti Z, Falak R, Delbandi AA, Tajik N.
Human platelet antigen 1-6, 9 and 15 in the Iranian population: An
anthropological genetic analysis. Sci Rep 2020; 10:7442. https://doi.org/10.1038/s41598-020-64469-4 PMid:32366900 PMCid:PMC7198494
- Neunert
C, Terrell DR, Arnold DM, Buchanan G, Cines DB, Cooper N, Cuker A,
Despotovic JM, George JN, Grace RF, Kühne T, Kuter DJ, Lim W, McCrae
KR, Pruitt B, Shimanek H, Vesely SK. American Society of Hematology
2019 guidelines for immune thrombocytopenia. Blood Adv 2019; 3:3829-66.
https://doi.org/10.1182/bloodadvances.2019000966 PMid:31794604 PMCid:PMC6963252
- Zufferey A, Kapur R, Semple JW. Pathogenesis and Therapeutic Mechanisms in Immune Thrombocytopenia (ITP). J Clin Med 2017; 6. https://doi.org/10.3390/jcm6020016 PMid:28208757 PMCid:PMC5332920
- Eyada
TK, Amin DG, Samih I, Khedr SM. Human platelet antigen 1, 2 and 5 gene
polymorphisms in Egyptians and their potential association with
susceptibility to immune thrombocytopenic purpura in Egyptian patients.
Hematology 2018; 23:111-6. https://doi.org/10.1080/10245332.2017.1365435 PMid:28823219
- Thude
H, Gatzka E, Anders O, Barz D. Allele frequencies of human platelet
antigen 1, 2, 3, and 5 systems in patients with chronic refractory
autoimmune thrombocytopenia and in normal persons. Vox Sang 1999;
77:149-53. https://doi.org/10.1046/j.1423-0410.1999.7730149.x PMid:10545851
- Cacoub P, Comarmond C, Domont F, Savey L, Saadoun D. Cryoglobulinemia Vasculitis. Am J Med 2015; 128:950-5. https://doi.org/10.1016/j.amjmed.2015.02.017 PMid:25837517
- Cacoub P, Saadoun D. Extrahepatic Manifestations of Chronic HCV Infection. N Engl J Med 2021; 384:1038-52. https://doi.org/10.1056/NEJMra2033539 PMid:33730456
- Charles
ED, Green RM, Marukian S, Talal AH, Lake-Bakaar GV, Jacobson IM, Rice
CM, Dustin LB. Clonal expansion of immunoglobulin M+CD27+ B cells in
HCV-associated mixed cryoglobulinemia. Blood 2008; 111:1344-56. https://doi.org/10.1182/blood-2007-07-101717 PMid:17942751 PMCid:PMC2214737
- Huang
CE, Chen WM, Wu YY, Shen CH, Hsu CC, Li CP, Chen MC, Chang JJ, Chen YY,
Lu CH, Shi CS, Chen CC. Comparison of antiplatelet antibody profiles
between hepatitis C virus-associated immune thrombocytopenia and
primary immune thrombocytopenia. Platelets 2020:1-8. https://doi.org/10.1080/09537104.2020.1820975 PMid:32967492
- Rajan
SK, Espina BM, Liebman HA. Hepatitis C virus-related thrombocytopenia:
clinical and laboratory characteristics compared with chronic immune
thrombocytopenic purpura. Br J Haematol 2005; 129:818-24. https://doi.org/10.1111/j.1365-2141.2005.05542.x PMid:15953010
- Neunert
C, Lim W, Crowther M, Cohen A, Solberg L, Jr., Crowther MA, American
Society of H. The American Society of Hematology 2011 evidence-based
practice guideline for immune thrombocytopenia. Blood 2011;
117:4190-207. https://doi.org/10.1182/blood-2010-08-302984 PMid:21325604
- Huang
CE, Chang JJ, Wu YY, Huang SH, Chen WM, Hsu CC, Lu CH, Hung CH, Shi CS,
Lee KD, Chen CC, Chen MC. Different impacts of common risk factors
associated with thrombocytopenia in patients with hepatitis B virus and
hepatitis C virus infection. Biomed J 2022; 45:788-97. https://doi.org/10.1016/j.bj.2021.09.001 PMid:34508913 PMCid:PMC9661505
- Castro
V, Oliveira GB, Origa AF, Annichino-Bizzacchi JM, Arruda VR. The human
platelet alloantigen 5 polymorphism as a risk for the development of
acute idiopathic thrombocytopenia purpura. Thromb Haemost 2000;
84:360-1. https://doi.org/10.1055/s-0037-1614027 PMid:10959720
- Carmo
JCD, Klippel PS, Cordeiro SDC, Fernandes A, Pinto RM, Weber SS, Fantin
C. Molecular typing of human platelet antigens in immune
thrombocytopenia patients in northern Brazil. Rev Bras Hematol Hemoter
2017; 39:122-6. https://doi.org/10.1016/j.bjhh.2017.01.003 PMid:28577648 PMCid:PMC5457461
- Pavkovic
M, Stojanovic A, Karanfilski O, Cevreska L, Spiroski M. Association of
polymorphisms in human platelet antigens with idiopathic
thrombocytopenic purpura in Macedonians. Prilozi 2012; 33:135-46.
- Mandelbaum
M, Koren D, Eichelberger B, Auerbach L, Panzer S. Frequencies of
maternal platelet alloantibodies and autoantibodies in suspected
fetal/neonatal alloimmune thrombocytopenia, with emphasis on human
platelet antigen-15 alloimmunization. Vox Sang 2005; 89:39-43. https://doi.org/10.1111/j.1423-0410.2005.00662.x PMid:15938738
- Inoue
H, Sakamoto R, Nishimiya H, Sakamoto H, Terasu S, Aminaka R, Koh Y,
Takihara Y, Hirayama F, Kuroishi A. Minor impact of patient
alloantibodies against human platelet antigen (HPA)-15 in the
effectiveness of platelet transfusion: A pilot study. Transfusion 2021;
61:738-43. https://doi.org/10.1111/trf.16181 PMid:33166416
- Matsuhashi
M, Tsuno NH, Sone S, Mishima Y, Nagura Y, Watanabe-Okochi N, Ikeda T,
Kashiwase K, Fukuda S, Iriyama T, Hyodo H, Yamashita T, Kamei Y, Arai
S, Minami M, Fujii T, Kurokawa M, Tozuka M, Takahashi K, Santoso S. The
role of alloantibodies against human platelet antigen-15 in multiply
platelet transfused patients. Transfusion 2014; 54:1093-9. https://doi.org/10.1111/trf.12455 PMid:24147542
- Hwang
SM, Kim MJ, Chang HE, Hong YJ, Kim TS, Song EY, Park KU, Song J, Han
KS. Human platelet antigen genotyping and expression of CD109 (human
platelet antigen 15) mRNA in various human cell types. Biomed Res Int
2013; 2013:946403. https://doi.org/10.1155/2013/946403 PMid:23509816 PMCid:PMC3583088
- Brighton
TA, Evans S, Castaldi PA, Chesterman CN, Chong BH. Prospective
evaluation of the clinical usefulness of an antigen-specific assay
(MAIPA) in idiopathic thrombocytopenic purpura and other immune
thrombocytopenias. Blood 1996; 88:194-201. https://doi.org/10.1182/blood.V88.1.194.194 PMid:8704174
- Dustin
LB, Charles ED. Primary, post-primary and non-specific immunoglobulin M
responses in HCV infection. Antivir Ther 2012; 17:1449-52. https://doi.org/10.3851/IMP2222 PMid:23322600 PMCid:PMC3737728
- Audia
S, Mahevas M, Nivet M, Ouandji S, Ciudad M, Bonnotte B. Immune
Thrombocytopenia: Recent Advances in Pathogenesis and Treatments.
Hemasphere 2021; 5:e574. https://doi.org/10.1097/HS9.0000000000000574 PMid:34095758 PMCid:PMC8171374
Supplementary tables
 |
Supplementary Table 1. Odds ratio of HPA15b versus HPA15a for detection of antiplatelet antibodies in the whole ITP patients. |
 |
Supplementary Table 2.
Odds ratio of HPA3b versus HPA3a for detection of anti-GPIIb/IIIa
antibodies and cryoglobulins in the whole ITP patients. |
 |
Supplementary Table 3. The correlation between antiplatelet antibodies and total cryoglobulins in the HCV-ITP patients.
|
Supplementary figures
 |
Supplementary Figure 1. Detection results of antiplatelet antibodies and cryoglobulins.
A. Antiplatelet antibody profiles. Anti-GPIIb/IIIa antibodies were the
most common antiplatelet antibody. Compared with HCV
controls, HCV-ITP patients had higher positive rates of anti-Ib/IX
antibodies, anti-HLA Class I antibodies, and all antiplatelet
antibodies (p = 0.012, 0.040, and 0.002, respectively). B. Antiplatelet
antibody complexity. More than three types of antiplatelet antibodies
were detected in the primary and secondary ITP patients, but they were
not detected in the HCV control. C. Cryoglobulin profile. Cryoglobulin
IgM was the most frequently detected immunoglobin, whereas
cryoglobulin IgA was the least detected immunoglobin in ITP patients.
The positive detection rates of cryoglobulin IgA, IgM and total
cryoglobulin were significantly higher in HCV-ITP patients than in HCV
control (p = 0.018, 0.049, and 0.023, respectively). D. Cryoglobulin
complexity. All three types of cryoglobulins were most frequentlydetected in HCV-ITP patients. * denotes a significant difference.. |
 |
Supplementary Figure 2. Correlations of HPA2 with platelet level.
A. In the whole ITP cohort, patients with HPA2ab had a significantly
higher percentage of severe thrombocytopenia (level 5) than those with
HPA2aa (p=0.023). B. The ITP patients with HPA2ab had a
significantly lower mean platelet level than those with HPA2aa (31.36
x109 /L vs. 49.93 x109 /L, p=0.037). * denotes a significant difference |
 |
Supplementary Figure 3. Detection of antiplatelet antibodies in cryoglobulins.
We selected 5 ITP patients with cryoglobulin (2+: IgG 2+/IgA 1+ or
2+/IgM2+) and positive anti-GPIIb/IIIa antibodies, 5 with cryoglobulin
(1+: IgG 1+/IgA 0 or 1+/IgM 1+) and positive anti-GPIIb/IIIa
antibodies, and 13 controls including 4 with cryoglobulin (2+: IgG
2+/IgM 2+) and negative anti-GPIIb/IIIa antibody, 5 with negative
cryoglobulin and positive anti-GPIIb/IIIa antibodies, and 4 with
both negative cryoglobulin and antiGPIIb/IIIa antibody. The ITP
patients with positive anti-GPIIb/IIIa antibodies always had multiple
antiplatelet antibodies simultaneously. A and B, the patients with
cryoglobulin (2+)/anti-GPIIb/IIIa antibody (+) had significantly higher
detection ratios of anti-GPIIb/IIIa antibodies than the controls and
the ITP patients with cryoglobulin (1+)/anti-GPIIb/IIIa antibody (+).
C, D, E, and F. The anti-GPIa/IIa, GPIb/IX, and IV antibodies were
detected with a higher ratio in the patients with cryoglobulin
(2+)/antiGPIIb/IIIa antibody (+) than the controls. The data suggests
that cryoglobulins shared the characteristics of antiplatelet
antibodies in the ITP patients with strongly positive cryoglobulins and
antiplatelet antibodies.*, **, and *** denote a significant difference of p < 0.05, < 0.01, and < 0.001, respectively. |
 |
Supplementary Figure 4. Summary of the associations among HPA alleles, antiplatelet antibodies, and cryoglobulins in primary ITP and HCV-ITP patients.
According to the results of this study, we found that HPA2b
was associated with thrombocytopenia. However, it was not clear
whether the production of HPA2b-associated anti-GPIb/IX antibodies or
the specificity of these antibodies was responsible for clinical
thrombocytopenia. HPA15b promoted the complexity of antiplatelet
antibody profiles in ITP patients. On the other hand, HPA3b was
associated with anti-GPIIb/IIIa antibody production in the HCVITP
patients, but with cryoglobulin IgG/A/M in the primary ITP
patients. The correlations between antiplatelet antibodies and
cryoglobulins were different in primary ITP and HCV-ITP patients. In
HCVITP patients, the positive rates of antiplatelet antibodies
were lower than cryoglobulins. Most of the HCVITP patients with
positive antiplatelet antibodies had positive cryoglobulins.
Clinically, the cryoglobulins, similar to antiplatelet antibodies, were
correlated with thrombocytopenia. In laboratory, the cryoglobulins
exhibited characteristics of antiplatelet antibodies. HCV-ITP may be
considered as one of cryoglobulinemia-related complications. However,
the associations between antiplatelet antibodies and cryoglobulins
were not as strong in the primary ITP patients as in the HCV-ITP
patients. The correlation between cryoglobulin and clinical
thrombocytopenia was not obvious in the primary ITP patients. This
implies that the pathophysiology of antiplatelet antibody formation is
different between HCV-ITP patientsand primary ITP patients.
|
[TOP]






