Daniele Avenoso, Amal
Alabdulwahab, Michelle Kenyon, Varun Mehra, Pramila Krishnamurthy,
Francesco Dazzi, Ye Ting Leung, Sandra Anteh, Mili Naresh Shah, Andrea
Kuhnl, Robin Sanderson, Piers Patten, Deborah Yallop, Antonio Pagliuca
and Victoria Potter.
King's College Hospital NHS Foundation Trust, Department of Haematological medicine, Denmark Hill, London.
Correspondence to: Dr
Daniele Avenoso, King's College Hospital NHS Foundation Trust,
Department of Haematological medicine, Denmark Hill, London. E-mail:
d.avenoso@nhs.net
Published: July 1, 2023
Received: March 19, 2023
Accepted: June 18, 2023
Mediterr J Hematol Infect Dis 2023, 15(1): e2023041 DOI
10.4084/MJHID.2023.041
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background: The
second decade of this millennium was characterized by a widespread
availability of chimeric antigen receptor T-cell (CAR-T) therapies to
treat relapsed and refractory lymphomas. As expected, the role and
indication of allogeneic haematopoietic stem cell transplant
(allo-HSCT) in the management of lymphoma changed. Currently, a
non-neglectable proportion of patients will be considered candidate for
an allo-HSCT, and the debate of which transplant platform should be
offered is still active.
Objectives: To
report the outcome of patients affected with relapsed/refractory
lymphoma and transplanted following reduced intensity conditioning at
King's College Hospital, London, between January 2009 and April 2021.
Methods: Conditioning was with 150mg/m2 of fludarabine and melphalan of 140mg/m2.
The graft was unmanipulated G-CSF mobilized peripheral blood
haematopoietic stem cells (PBSC). Graft-versus-host disease (GVHD)
prophylaxis consisted of pre-transplant Campath at the total dose of 60
mg in unrelated donors and 30 mg in fully matched sibling donors and
ciclosporin.
Results: One-year
and five years OS were 87% and 79.9%, respectively, and median OS was
not reached. The cumulative incidence of relapse was 16%. The incidence
of acute GVHD was 48% (only grade I/II); no cases of grade III/IV were
diagnosed. Chronic GVHD occurred in 39% of patients. TRM was 12%, with
no cases developed within day 100 and 18 months after the procedure.
Conclusions: The
outcomes of heavily pretreated lymphoma patients are favorable, with
median OS and survival not reached after a median of 49 months. In
conclusion, even if some lymphoma subgroups cannot be treated (yet)
with advanced cellular therapies, this study confirms the role of
allo-HSCT as a safe and curative strategy.
|
Introduction
The
second decade of this millennium was characterized by the widespread
availability of chimeric antigen receptor T-cell (CAR-T) therapies to
treat relapsed and refractory lymphomas, changing these entities'
prognosis and treatment landscape.[1-5]
Also, the
development of antibody-conjugated therapies or bi-specific antibodies
further enriched the treatment algorithm creating more therapeutic
dilemmas in selecting the most effective and safe therapy after the
second relapse and beyond.[6,7]
As expected, the
role and indication of allogeneic haematopoietic stem cell transplant
(allo-HSCT) in the management of lymphoma changed, and it is currently
redefining its role in the treatment pathway of our patients.
Interestingly, the first decade of the millennium can be remembered with a global effort in proving a graft-versus-lymphoma effect and in identifying the safest conditioning regimens before the infusion of the graft.[8]
Different groups previously showed that reduced intensity conditioning
(RIC) regimens could offer the engraftment of the donor immune system
without exposing the patients to an unacceptable risk of
transplant-related mortality (TRM).[9,10,11]
Despite
the new treatment options mentioned before, a not neglectable
proportion of patients, due to the lack of CAR-T products or any other
modern therapy, will be considered a candidate for an allo-HSCT.[12] Therefore, the debate of which transplant platform/conditioning regimen should be offered is still active.
Herein
we report the outcome of patients affected with relapsed/refractory
lymphoma and transplanted following RIC administration.
Material and Methods
Conditioning was administered with 150 mg/m2 of fludarabine and melphalan of 140 mg/m2.
Graft-versus-host
disease (GVHD) prophylaxis consisted of pre-transplant Campath at the
total dose of 60 mg in unrelated donors and 30 mg in fully matched
sibling donors, and ciclosporin 3 mg/Kg from day -1 (therapeutic level
of 150-200) until d+56 and then tapered in the absence of GVHD aiming
to stop it on day +90.
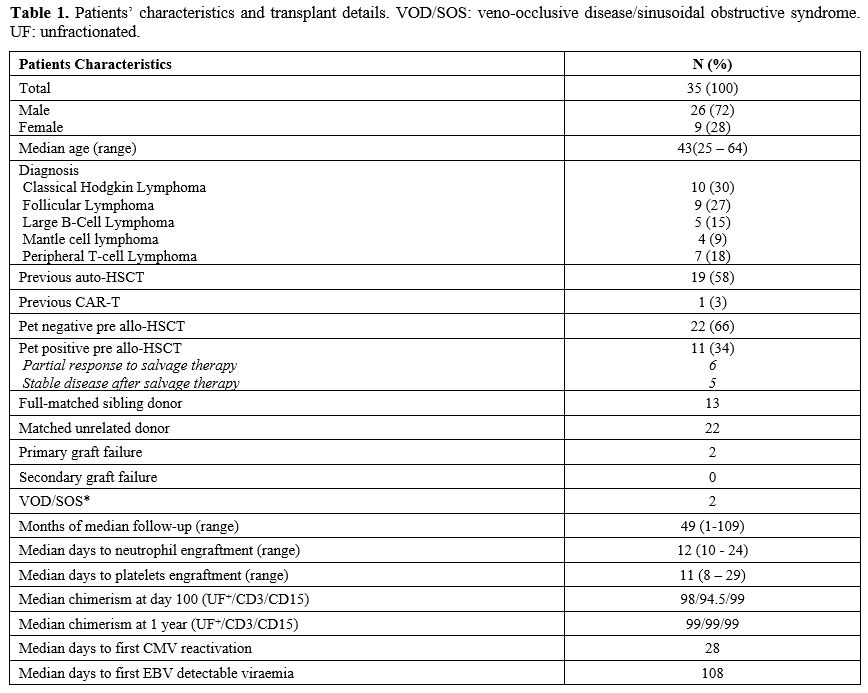 |
- Table
1. Patients' characteristics and transplant details. VOD/SOS:
veno-occlusive disease/sinusoidal obstructive syndrome. UF:
unfractionated.
|
Between
January 2012 and April 2022, 35 patients (9 females and 26 males)
affected with different lymphoma subtypes underwent allo-HSCT at King's
College Hospital in London, United Kingdom. Table 1 summarizes the patients' characteristics and shows the transplant details. Median follow-up was 49 months (range 1-109).
Donors
and recipients were typed using Third Generation Sequencing (TGS) and
Next Generation Sequencing (NGS) techniques for HLA-A, -B, -C, -DRB1,
-DQ at a high-resolution level. Donors were considered mismatched if
<10/10 match was present.
Probabilities of overall survival
(OS) and GVHD-relapse-free survival (GRFS) were calculated using the
Kaplan-Meier method. Relapse incidence (RI) and transplant-related
mortality (TRM) rates were estimated using cumulative incidence (CI)
functions and considered as competing risks. For GvHD, death and
relapse were considered competing events. Univariate analyses were
performed using the log-rank test for OS, GRFS, and Gray's test for RI
and TRM. Statistical analyses were performed with GraphPad Prism
Version 9.4.1.
Results
The graft was unmanipulated G-CSF mobilized peripheral blood haematopoietic stem cells PBSC). A median of 7x106 CD34+/Kg was infused (range 1.8 – 11.2). Median time to neutrophils ≥ 1000/μL was 12 days (10-24), and 11 days (8-29) to platelets ≥ 20.000/μL;
no deaths before engraftment were recorded. Two cases of primary graft
failure occurred; despite autologous reconstitution, these patients
achieved complete remission (CR).
Median unfractionated, CD3+, and CD15 chimerism at 365 days after transplant were 99% and 99%, respectively.
One-year and five years OS were 87% and 79.9%, respectively, and median OS was not reached.
One-year and five years GFRS were 69% and 61%, respectively, with median GRFS not reached (Figure 1A).
The global CI of relapse was 16%, with no late relapses seen beyond 24 months after transplant (Figure 1B);
it is worth highlighting that one of the relapsed patients achieved
durable complete remission following the withdrawal of immune
suppressive therapy, and two patients following the infusion of donor
lymphocyte infusions (DLI).
The overall incidence of acute GVHD
was 48% (15 patients were affected with grade I, and only one patient
had grade II); no grade III/IV cases were diagnosed. The median time to
acute GVHD was 57 days (range 23-112). Chronic GVHD occurred in 39% of
patients; within this group, moderate and severe cases were noted in 4
and 3 patients, respectively. The median time to chronic GVHD was 171
days (range 107-511).
Overall TRM was 12%, with no cases developed within day 100 and 18 months after the procedure (Figure 1C). The leading causes of death were infections (3 cases) and disease progression (1).
CMV
reactivation occurred in 41% with a median time to first CMV
reactivation of 28 days (range -6 – 251). No CMV disease occurred, and
all the patients received pre-emptive therapy per local policy.
EBV-detectable
viraemia occurred in 51% of the patients at a median time of 108 days
(range 22 – 713); two monomorphic PTLD cases required treatment with
rituximab achieving CR.
Disease status at the time of transplant
has an impact on OS: patients in complete remission at the time of
transplant had a five years OS of 80% compared to 65% in those with a
partial response at the time of transplant (Figure 1D);
an analogous situation for those who underwent up-front allo-HSCT.
These results confirm that chemo-sensitive disease can benefit from
allo-HSCT even in partial response.
From a histological point of
view, non-large B-cell lymphoma (LBCL) had a better outcome, with
median OS never reached compared to LBCL (Figure 1E).
It is worth highlighting that the two LBCL patients that failed HSCT
underwent the procedure with detectable disease at the PET scan before
transplant, suggesting that high proliferative tumour burden cannot be
controlled with the GVL effect.[13]
 |
- Figure 1. (A) Overall survival (OS) and GVHD-Relapse free survival (GRFS) of the whole cohort. (B) Cumulative incidence of relapse; Figure (C) Cumulative incidence of transplant related mortality (TRM); (D) OS according to disease status at time of transplant; (E)
OS according to histology subtypes. FL= follicular lymphoma, HL=
Hodgkin Lymphoma, LBCL= large B cell lymphoma, MCL= mantle cell
lymphoma, TCL= T-cell lymphoma.
|
Discussion
The
RIC conditioning and the infusion of unmanipulated PBSC are both
effective and safe: the TRM of 12% is dramatically low compared to
early experiences of allo-HSCT in lymphoma.[14]
The
low incidence of early relapse and the absence of late relapse confirms
a durable GVL effect that cures patients heavily pretreated.
The
GVHD prophylaxis with the administration of Campath before the infusion
of the graft and the single agent ciclosporin did not expose the
patient to the risk of severe forms of acute GVHD. Interestingly, only
seven patients affected with chronic GVHD required systemic treatment,
but none developed recurrent infective complications, and none reported
severe impairment of quality of life from that complication. Also, the
in-vivo T-cell depletion did not endanger the GVL effect nor trigger
lethal viral infections.
These encouraging results can offer a
reflection regarding the costs of transplant compared to CAR-T
therapies, which are the current competitors of allograft in the
third-line setting for B-cell CD19 positive NHL.
The
dissimilarities in prices between cellular therapy products and
allo-HSCT should not drive the clinician's choice of the best treatment
to offer to patients; with this assumption, it is important to remember
that none CAR-T product has the same curative trait of allo-HSCT, as
still 60% of B-cell lymphoma patients are not cured with CAR-T therapy.
Despite
that, it is important to highlight that allo-HSCT will not replace
CAR-T as the standard of care in a third-line setting for CD19-positive
lymphoma due to favourable toxicity profile and its efficacy in
progression-free survival and OS.
Lymphoma subtypes still
lacking an available CAR-T product can be cured with allo-HSCT. Our
study confirms the long-term survival of this strategy and, most
importantly, the safety of our RIC transplant platform. Also, the low
TRM should finally eliminate any doubt about the safety of in-vivo T-cell depletion with Campath.
Our
experience showed that MCL and LBCL can also be cured with allo-HSCT;
this manuscript will feed the new debate on when to offer an allo-HSCT
for these entities.
Considering the availability of highly
effective bridging new therapies to CAR-T infusion, it is not uncommon
to meet patients in complete metabolic remission (CMR) in third- or
fourth-line settings.[6,7] Should CD19-positive
lymphoma be in CMR after the third line of therapy consolidated with
CAR-T infusion or with allo-HSCT? Only a randomized prospective
clinical trial will provide the answer to this dilemma.[15]
Also,
there is evidence of the safety and efficacy of CAR-T infusion after
allo-HSCT; therefore, his strategy should not be considered the
condition sine qua non to preclude CAR-T therapies.[16]
Our
experience shows that the FMC allo-HSCT platform is highly effective
and safe, with low TRM and GVHD rates, and cheaper than CAR-T therapy.
In
conclusion, RIC allo-HSCT can still cure lymphomas, and prospective
clinical trials are needed to further define its role, particularly in
sequencing new strategies for managing CD-19-positive lymphomas.
Acknowledgments
The
authors want to thank all the staff of the haematology department at
King's College Hospital for the care provided to all patients.
References
- Schuster, S. J. et al. Tisagenlecleucel in Adult
Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med.
380, 45-56 (2019). https://doi.org/10.1056/NEJMoa1804980 PMid:30501490
- Neelapu,
S. S. et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory
Large B-Cell Lymphoma. N. Engl. J. Med. 377, 2531-2544 (2017). https://doi.org/10.1056/NEJMoa1707447 PMid:29226797 PMCid:PMC5882485
- Kamdar,
M. et al. Lisocabtagene maraleucel versus standard of care with salvage
chemotherapy followed by autologous stem cell transplantation as
second-line treatment in patients with relapsed or refractory large
B-cell lymphoma (TRANSFORM): results from an interim analysis of an
open-label, randomised, phase 3 trial. Lancet Lond. Engl. 399,
2294-2308 (2022). https://doi.org/10.1016/S0140-6736(22)00662-6 PMid:35717989
- Locke,
F. L. et al. Axicabtagene Ciloleucel as Second-Line Therapy for Large
B-Cell Lymphoma. N. Engl. J. Med. 386, 640-654 (2022). https://doi.org/10.1056/NEJMoa2116133 PMid:34891224
- Wang,
M. et al. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory
Mantle-Cell Lymphoma. N. Engl. J. Med. 382, 1331-1342 (2020). https://doi.org/10.1056/NEJMoa1914347 PMid:32242358 PMCid:PMC7731441
- Castaneda-Puglianini,
O. & Chavez, J. C. Bispecific antibodies for non-Hodgkin's
lymphomas and multiple myeloma. Drugs Context 10, 2021-2-4 (2021). https://doi.org/10.7573/dic.2021-2-4 PMid:34603459 PMCid:PMC8462994
- Sehn,
L. H. et al. Polatuzumab Vedotin in Relapsed or Refractory Diffuse
Large B-Cell Lymphoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol.
38, 155-165 (2020). https://doi.org/10.1200/JCO.19.00172 PMid:31693429 PMCid:PMC7032881
- Peniket,
A. J. et al. An EBMT registry matched study of allogeneic stem cell
transplants for lymphoma: allogeneic transplantation is associated with
a lower relapse rate but a higher procedure-related mortality rate than
autologous transplantation. Bone Marrow Transplant. 31, 667-678 (2003).
https://doi.org/10.1038/sj.bmt.1703891 PMid:12692607
- Hari,
P. et al. Allogeneic transplants in follicular lymphoma: higher risk of
disease progression after reduced-intensity compared to myeloablative
conditioning. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow
Transplant. 14, 236-45 (2008). https://doi.org/10.1016/j.bbmt.2007.11.004 PMid:18215784 PMCid:PMC2531158
- Genadieva-Stavrik,
S. et al. Myeloablative versus reduced intensity allogeneic stem cell
transplantation for relapsed/refractory Hodgkin's lymphoma in recent
years: a retrospective analysis of the Lymphoma Working Party of the
European Group for Blood and Marrow Transplantation. Ann. Oncol. Off.
J. Eur. Soc. Med. Oncol. 27, 2251-2257 (2016). https://doi.org/10.1093/annonc/mdw421 PMid:28007754
- Thomson,
K. J. et al. Favorable long-term survival after reduced-intensity
allogeneic transplantation for multiple-relapse aggressive
non-Hodgkin's lymphoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol.
27, 426-432 (2009). https://doi.org/10.1200/JCO.2008.17.3328 PMid:19064981
- Snowden,
J. A. et al. Indications for haematopoietic cell transplantation for
haematological diseases, solid tumours and immune disorders: current
practice in Europe, 2022. Bone Marrow Transplant. 57, 1217-1239 (2022).
https://doi.org/10.1038/s41409-022-01691-w PMid:35589997 PMCid:PMC9119216
- Sammassimo,
S. et al. A Cellular Therapy with Haploidentical Peripheral
Hematopoietic STEM CELL Transplantation MAY be a Therapeutic Option in
Patients with Relapsed Lymphoma with Chemorefractory Disease. Blood
132, 2189 (2018). https://doi.org/10.1182/blood-2018-99-119752
- Sureda,
A. et al. Reduced-Intensity Conditioning Compared With Conventional
Allogeneic Stem-Cell Transplantation in Relapsed or Refractory
Hodgkin's Lymphoma: An Analysis From the Lymphoma Working Party of the
European Group for Blood and Marrow Transplantation. J. Clin. Oncol.
26, 455-462 (2008). https://doi.org/10.1200/JCO.2007.13.2415 PMid:18086796
- Dreger,
P. et al. CAR T cells or allogeneic transplantation as standard of care
for advanced large B-cell lymphoma: an intent-to-treat comparison.
Blood Adv. 4, 6157-6168 (2020). https://doi.org/10.1182/bloodadvances.2020003036 PMid:33351108 PMCid:PMC7756983
- Zurko,
J. et al. Allogeneic transplant following CAR T-cell therapy for large
B-cell lymphoma. Haematologica 108, 98-109 (2023). https://doi.org/10.3324/haematol.2022.281242 PMid:35833303 PMCid:PMC9827150

