Angela Quirino1, Claudia Cicino1, Vincenzo Scaglione2, Nadia Marascio1, Francesca Serapide2, Giuseppe Guido Maria Scarlata1, Rosaria Lionello2, Francesca Divenuto1, Valentina La Gamba2, Grazia Pavia1, Alessandro Russo2, Carlo Torti2, Giovanni Matera1* and Enrico Maria Trecarichi2.
1 Unit of
Clinical Microbiology, Department of Health Sciences, “Magna Graecia”
University of Catanzaro– “Mater Domini” teaching hospital; Catanzaro,
Italy.
2 Unit of Infectious and Tropical Diseases, Department of
Medical and Surgical Sciences, “Magna Graecia” University of Catanzaro
– “Mater Domini” teaching hospital; Catanzaro, Italy.
Published: July 1, 2023
Received: April 17, 2022
Accepted: June 16, 2023
Mediterr J Hematol Infect Dis 2023, 15(1): e2023043 DOI
10.4084/MJHID.2023.043
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
To the editor
The
introduction should briefly place the study in a broad context and
highlight why it is Infections caused by multi-drug resistant (MDR)
Gram-negative bacteria represent one of the main threats to human
health worldwide.[1] Since the beginning of the
SARS-CoV-2 pandemic, a significant increase of severe infections due to
MDR ESKAPE bacteria was observed in our Institution, in particular, due
to carbapenem-resistant Acinetobacter baumannii (CRAB).[2-4]
Cefiderocol (CFDC), approved in 2019 to treat infections sustained by
aerobic Gram-negative bacteria, is a novel siderophore cephalosporin
with broad-spectrum activity and clinical efficacy against CRAB, Pseudomonas aeruginosa and Stenotrophomonas maltophilia.[5,6] However, since its introduction in clinical practice, CFDC-resistant Gram-negative bacterial isolates have been reported.[7]
Herein, we evaluated the in vitro activity of CFDC against CRAB bloodstream strains isolated in our Teaching Hospital during the last three years.
Materials and Methods
This study was conducted in the Microbiology Laboratory “Mater Domini”
Teaching Hospital, Catanzaro, Italy. CRAB isolates were recovered from
blood samples of patients hospitalized between 2020 and 2022 and
diagnosed with CRAB bloodstream infections. Only the first CRAB strain
isolated from each patient was included.
Bacterial isolation and identification and Antimicrobial Susceptibility Testing.
CRAB isolates were identified using matrix-assisted laser-desorption
ionization time-of-flight mass spectrometry (MALDI-TOF) and Vitek®2
System (bioMérieux, Italy). Antibiotic susceptibility tests for
meropenem, amikacin, and trimethoprim/sulfamethoxazole were performed
with the Vitek®2 System (whereas the determination of colistin
resistance was obtained by broth microdilution according to EUCAST
guidelines.[8]
The Kirby-Bauer disc diffusion test on regular un-supplemented
Mueller-Hinton agar (Liofilchem S.R.L., Roseto degli Abruzzi, Italy)
was used to assess sensitivity to CFDC, using discs impregnated with 30
micrograms of drug supplied by Liofilchem® (Liofilchem S.R.L., Roseto
degli Abruzzi, Italy). Of note, EUCAST evaluated (August 2022)
cefiderocol 30 µg disks and regular unsupplemented MH agars from
Liofilchem, and no warning concerning their use was reported (unlike
other products marketed by other manufacturers).[9]
Well-isolated colonies were suspended in saline from an overnight agar
plate to achieve a 0.5 McFarland standard turbidity. The inoculum was
streaked with a sterile cotton swab over the entire area of the
Mueller–Hinton (MH) agar plate. After that, the disc was firmly applied
to the surface of the inoculated agar plate and incubated at 35±1 °C
for 16–20 h. The diameters of the disk areas were read using the
innermost colony-free area. The results were interpreted using the
European Committee on Antimicrobial Susceptibility Testing (EUCAST) and
Clinical and Laboratory Standards Institute (CLSI) experimental
breakpoints.[9,10] The EUCAST defined the clinical breakpoints for CFDC against A. baumannii complex
as susceptible in the range of ≥17mm and resistant of <17 mm, while
the CLSI ranges for the determination of susceptibility and resistance
are ≥15mm and ≤15 mm, respectively.
Ethical Statement.
The present retrospective study is based on clinical isolates stored in
an anonymous archive without association with clinical data. For this
reason, ethics and consent to participate are not applicable. The study
was conducted using retrospectively collected and anonymized data
according to the Declaration of Helsinki and principles of good
clinical practice.
Results
In the last three years, 70 Acinetobacter baumannii were
isolated from blood samples, and 62 were carbapenem-resistant. 62 CRAB
strains were isolated from 62 patients using conventional culture media
and tested for susceptibility to CFDC.
The range of diameters and the percentage of susceptible and resistant isolates are shown in Table 1,
using interpretations of breakpoints recommended by EUCAST and CLSI.
CRAB isolates showed higher susceptibility when CLSI breakpoints were
applied compared to the EUCAST breakpoints. In particular, seven
isolates with a zone diameter ≥15 mm were susceptible according to CLSI
guidelines but resistant according to EUCAST breakpoints. When EUCAST
breakpoints were applied, the overall susceptibility rate to CFDC was
(45/62) 72.5%, whereas it was (52/62) 83.8% using CLSI breakpoints.
Antibiotic susceptibility results for amikacin, colistin, and
trimethoprim/ sulfamethoxazole of CRAB isolates according to
susceptibility pattern to CFDC based on EUCAST breakpoints are shown in
Table 2.
 |
Table 1. Summary of cefiderocol disk diffusion susceptibility among analysed carbapenem-resistant Acinetobacter baumannii bacterial isolates. |
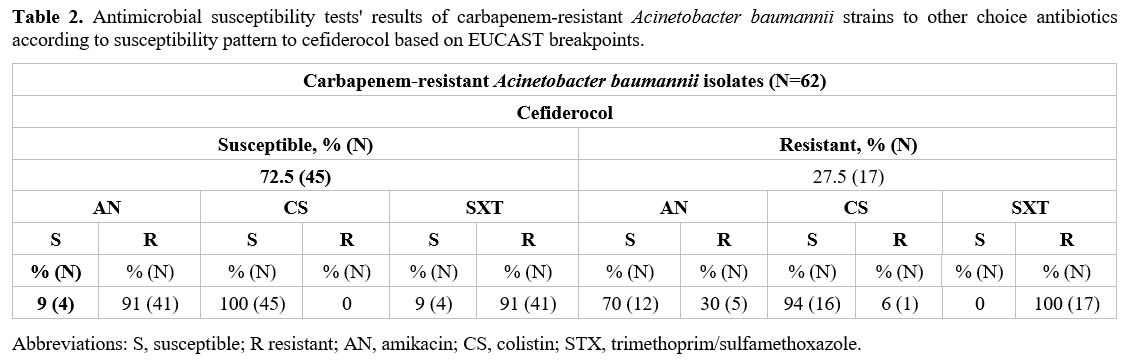 |
Table 2. Antimicrobial susceptibility tests' results of carbapenem-resistant Acinetobacter baumannii strains to other choice antibiotics according to susceptibility pattern to cefiderocol based on EUCAST breakpoints.
|
Discussion
In
the present study, we tested the susceptibility of CRAB isolates to
CFDC by using the Kirby-Bauer disc diffusion test on regular
un-supplemented Mueller-Hinton agar, considered the gold standard
method for this purpose. Then we compared the results to interpretation
breakpoints recommended by EUCAST and CLSI guidelines.[10,11]
According to EUCAST breakpoints, in our study, resistance to CFDC was
observed in 17/62 (27.4%) isolates, whereas by using CLSI guidelines,
10/62 (16.1%) isolates resulted in being resistant to CFDC. In previous
epidemiological studies investigating in vitro efficacy of CFDC against CRAB isolates, the overall rates of resistance ranged from 3.1% to 47.4%.[12-20]
Therefore, the present results were within the range of literature
data, although the range of resistance rates in previous studies was
very wide.[12-20]
Furthermore, susceptibility results were interpreted according to breakpoints recommended by EUCAST guidelines in some studies[13,15,16,19] and by CLSI guidelines in others.[12,17]
In two of these studies, a disk diffusion test according to EUCAST
breakpoints was used to assess the susceptibility of CRAB strains to
CFDC, and the resistance rates to CFDC were 5.3%14 and 22.1%,[16]
respectively. Compared to these studies, we found a higher resistance
rate to CFDC among CRAB isolates. However, the study of Carcione et
al., although conducted in Italy and during a period similar to ours,
was based on a total of 19 isolates, and the small sample analyzed
could at least partially explain the difference in resistance rate
using DD (5%; 1/20) compared to our study.[14]
Conversely, the study of Ghebremedhin et al. was conducted in a
different country (Germany) from 2014 to 2021, so their results could
be difficultly comparable to ours.[16]
Furthermore, Morris et al. performed DD tests for CFDC by using
30-µg discs produced by two different manufacturers (i.e., FDA-cleared
HardyDisks and MASTDISCS [RUO]) on standard MH agar on 14 CRAB strains
and compared results by interpreting them according to both EUCAST and
CLSI breakpoints; they found rates of resistance which were widely
different depending on the type of disc used and interpretation
breakpoints used: 35.7% and 57.1% by using HardyDisks discs and 7.2%
and 64.7% by using MASTDISCS (RUO) discs, according to CLSI and EUCAST
breakpoints, respectively; therefore, authors concluded that DD methods
(at least with the methodology used in their study) performed poorly
for CRAB.[17] Based on these findings, it is possible
to speculate that it is difficult to compare our results to those
reported in the literature due to the heterogeneity of techniques used
to assess the resistance of CRAB to CFDC and different local
epidemiology or study periods. Because high rates of clinical failure
were reported in patients affected by CFDC-resistant Gram-negative
bacteria,[20] optimizing microbiological procedures
to assess resistance as part of routine clinical practice is a
mandatory task that should be the object of further investigations.
This study is affected by several limitations: i) this is a monocentric
study, and the size of samples analysed is relatively small; ii)
previous therapy with CFDC was not evaluated for patients carrying
CFDC-resistant CRAB isolates; iii) analysis of the molecular
characterization of resistance mechanisms was not conducted; iv) clonal
analysis of CRAB isolates to identify possible local outbreak was not
performed; this last limitation may have influenced the resistance rate
reported in our study.
Conclusions
Our
results showed a relatively high resistance rate to CFDC among clinical
CRAB isolates compared to previous reports. However, several
differences in methods, breakpoints interpretation guidelines, and
local epidemiology should be considered.
Author Statement
Conceptualization,
EMT and AQ; methodology, NM and AR; software, CC GGMS; investigation,
VS, FS, RL, and VLB data curation, FD and GP; writing—original draft
preparation, EMT and AQ; writing, EMT and AQ; supervision, CT and GM.
References
- Antimicrobial Resistance Collaborators. Global
burden of bacterial antimicrobial resistance in 2019: a systematic
analysis. Lancet. 2022, 399,629, 655.
- Reale,
M., Strazzulla, A., Quirino, A., Rizzo, C., Marano, V., Postorino, M.
C., Mazzitelli, M., Greco, G., Pisani, V., Costa, C., et al. Patterns
of multi-drug resistant bacteria at first culture from patients
admitted to a third level University hospital in Calabria from 2011 to
2014: implications for empirical therapy and infection control. Infez
Med. 2017, 25, 98,107.
- Scaglione,
V., Reale, M., Davoli, C., Mazzitelli, M., Serapide, F., Lionello, R.,
La Gamba, V., Fusco, P., Bruni, A., Procopio, D., et al. Prevalence of
Antibiotic Resistance Over Time in a Third-Level University Hospital.
Microb Drug Resist. 2022, 28, 425,435. https://doi.org/10.1089/mdr.2021.0109 PMid:34910885 PMCid:PMC9058886
- Serapide,
F., Quirino, A., Scaglione, V., Morrone, H. L., Longhini, F., Bruni,
A., Garofalo, E., Matera, G., Marascio, N., Scarlata, G. G. M. et al.
Is the Pendulum of Antimicrobial Drug Resistance Swinging Back after
COVID-19?. Microorganisms. 2022, 10, 957. https://doi.org/10.3390/microorganisms10050957 PMid:35630400 PMCid:PMC9146770
- Hsueh
SC, Lee YJ, Huang YT, Liao CH, Tsuji M, Hsueh PR. In vitro activities
of cefiderocol, ceftolozane/tazobactam, ceftazidime/avibactam and other
comparative drugs against imipenem-resistant Pseudomonas aeruginosa and
Acinetobacter baumannii, and Stenotrophomonas maltophilia, all
associated with bloodstream infections in Taiwan. J Antimicrob
Chemother. 2019,74,380, 386. https://doi.org/10.1093/jac/dky425 PMid:30357343
- Trecarichi,
E. M., Quirino, A., Scaglione, V., Longhini, F., Garofalo, E., Bruni,
A., Biamonte, E., Lionello, R., Serapide, F., Mazzitelli, M., et al.,
Successful treatment with cefiderocol for compassionate use in a
critically ill patient with XDR Acinetobacter baumannii and
KPC-producing Klebsiella pneumoniae: a case report. The Journal of
antimicrobial chemotherapy, 2019, 74, 3399,3401. https://doi.org/10.1093/jac/dkz318 PMid:31369095
- Wang
C, Yang D, Wang Y, Ni W. Cefiderocol for the Treatment of
Multidrug-Resistant Gram-Negative Bacteria: A Systematic Review of
Currently Available Evidence. Frontiers in pharmacology, 2022, 13,
896971. https://doi.org/10.3389/fphar.2022.896971 PMid:35496290 PMCid:PMC9039133
- The
European Committee on Antimicrobial Susceptibility Testing. Breakpoint
tables for interpretation of MICs and zone diameters. Version 13.0,
2023. http://www.eucast.org
- EUCAST warnings concerning antimicrobial susceptibility testing products or procedures (August 2022) https://www.eucast.org/ast-of-bacteria/warnings
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoints for cefiderocol from EUCAST https://www.eucast.org/eucast_news/news_singleview/?tx_ttnews%5Btt_news%5D=493&cHash=22779384b74c8cf2c55aa3f7fd69d173
- CLSI m100-ed32:2022 performance standards for antimicrobial susceptibility testing, 32nd edition
- Abdul-Mutakabbir,
J. C., Nguyen, L., Maassen, P. T., Stamper, K. C., Kebriaei, R., Kaye,
K. S., Castanheira, M., & Rybak, M. J. In Vitro Antibacterial
Activity of Cefiderocol against Multidrug-Resistant Acinetobacter
baumannii. Antimicrobial agents and chemotherapy, 2021, 65, e0264620 https://doi.org/10.1128/AAC.02646-20 PMid:34125590 PMCid:PMC8370208
- Candel,
F. J., Santerre Henriksen, A., Longshaw, C., Yamano, Y., & Oliver,
A. In vitro activity of the novel siderophore cephalosporin,
cefiderocol, in Gram-negative pathogens in Europe by site of infection.
Clinical microbiology and infection : the official publication of the
European Society of Clinical Microbiology and Infectious Diseases,
(2022). 28(3), 447,e1, 447,e6. https://doi.org/10.1016/j.cmi.2021.07.018 PMid:34298176
- Carcione,
D., Siracusa, C., Sulejmani, A., Migliavacca, R., Mercato, A., Piazza,
A., Principe, L., Clementi, N., Mancini, N., Leoni, V., et al., In
Vitro Antimicrobial Activity of the Siderophore Cephalosporin
Cefiderocol against Acinetobacter baumannii Strains Recovered from
Clinical Samples. Antibiotics (Basel, Switzerland), 2021 10(11), 1309. https://doi.org/10.3390/antibiotics10111309 PMid:34827247 PMCid:PMC8614976
- Cercenado,
E., Cardenoso, L., Penin, R., Longshaw, C., Henriksen, A. S., &
Pascual, A. In vitro activity of cefiderocol and comparators against
isolates of Gram-negative bacterial pathogens from a range of infection
sources: SIDERO WT 2014-2018 studies in Spain. Journal of global
antimicrobial resistance, 2021 26, 292, 300. https://doi.org/10.1016/j.jgar.2021.06.011 PMid:34274538
- Ghebremedhin,
B., & Ahmad-Nejad, P. In-Vitro Efficacy of Cefiderocol in
Carbapenem-Non-Susceptible Gram-Negative Bacilli of Different Genotypes
in Sub-Region of North Rhine Westphalia, Germany. Pathogens (Basel,
Switzerland), 10 2021 (10), 1258. https://doi.org/10.3390/pathogens10101258 PMid:34684208 PMCid:PMC8540251
- Morris,
C. P., Bergman, Y., Tekle, T., Fissel, J. A., Tamma, P. D., &
Simner, P. J. Cefiderocol Antimicrobial Susceptibility Testing against
Multidrug-Resistant Gram-Negative Bacilli: a Comparison of Disk
Diffusion to Broth Microdilution. Journal of clinical microbiology,
2020, 59(1), e01649,20 https://doi.org/10.1128/JCM.01649-20 PMid:32938734 PMCid:PMC7771458
- Naas
T, Lina G, Santerre Henriksen A, Longshaw C, Jehl F. In vitro activity
of cefiderocol and comparators against isolates of Gram-negative
pathogens from a range of infection sources: SIDERO-WT-2014-2018
studies in France [published correction appears in JAC Antimicrob
Resist. JAC Antimicrob Resist. 2021;3(2),dlab0 81 https://doi.org/10.1093/jacamr/dlab081 PMid:34223140 PMCid:PMC8251251
- Wang,
Y., Li, Y., Zhao, J., Guan, J., Ni, W., & Gao, Z. Susceptibility of
cefiderocol and other antibiotics against carbapenem-resistant,
Gram-negative bacteria. Annals of translational medicine, (2022).
10(5), 261.
https://doi.org/10.21037/atm-22-889 PMid:35402576 PMCid:PMC8987888
- Bleibtreu,
A., Dortet, L., Bonnin, R. A., Wyplosz, B., Sacleux, S. C., Mihaila,
L., Dupont, H., Junot, H., Bunel, V., Grall, N et al., Susceptibility
Testing Is Key for the Success of Cefiderocol Treatment: A
Retrospective Cohort Study. Microorganisms,2021 9(2), 282. https://doi.org/10.3390/microorganisms9020282 PMid:33573148 PMCid:PMC7911443

