Howard S. Oster1,2, Ekaterina Sklyar1, Noa Golsdshmidt3 and Moshe Mittelman2,3.
1 Department of Internal Medicine, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel
2 Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel
3 Department of Hematology, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel.
Correspondence to:
Moshe Mittelman MD. Department of Hematology, Tel Aviv Sourasky
Medical Center, Weizmann 6, Tel Aviv 6423906, Israel. E-mail:
moshemt@gmail.com
Published: July 1, 2023
Received: May 10, 2023
Accepted: June 16, 2023
Mediterr J Hematol Infect Dis 2023, 15(1): e2023044 DOI
10.4084/MJHID.2023.044
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
To the editor
The
pathogenesis of myelodysplastic syndromes (MDS)[1] is complex, and
major players include the immune system and inflammatory processes.[2,3]
Despite
the recognition of the involvement of inflammatory processes in MDS
pathogenesis, using inflammatory markers in daily practice as a part of
the diagnostic, prognostic or therapeutic course, is limited. We report
here our observation of C-reactive-protein (CRP) and/or erythrocyte
sedimentation rate (ESR) in patients with MDS at presentation.
Patients and Methods
Records of MDS patients from our center who had BM examination (BME) and
either CRP or ESR labs at presentation, were reviewed. Patients were
excluded if the MDS diagnosis was questionable, or if they had
comorbidities known to be associated with high inflammatory markers.
Normal
level of CRP in our lab is 0-5 mg/L. Thus, values >5 mg/L were
considered high (abnormal). Abnormally high ESR was defined as >25
mm in the first hour.[4]
As controls we used the data of 100
consecutive outpatient subjects (non MDS), undergoing workup including
BME, who were at least 50 yr old and had either CRP or ESR tested at
the time of workup.
The study was approved by the local IRB Helsinki committee.
Results
The
mean age was 74.6 ± 11.2 and 70.5 ± 9.6 yr for the MDS and control
groups, respectively (p=0.001); 43% and 37% of them, respectively, were
females (p=0.47). As expected, the mean hemoglobin (Hb; 10.5 and 11.6
g/dL, respectively, p=0.001), white blood cell count (WBC; 5.1 and 7.5
x109/L, respectively, p<0.001), absolute neutrophil count (ANC; 2.9
and 7.5 x109/L, respectively,
p<0.001) were significantly lower in the MDS patients compared with
controls, while the mean corpuscular volume (MCV) was significantly
higher (96 and 92 Fl, respectively, p=0.005). Platelets were only
slightly and non-significantly lower in the MDS group (164 and 179 x109/L, respectively).
The
mean CRP level was 11.9 [95% CI: 7.1, 16.7] mg/L in the MDS group
compared with 4.1 mg/L [95% CI: 3.1, 5.1] in the controls (Table 1,
p=0.028). The mean ESR was 33.2 [95% CI: 23.1, 43.4] mm and 27.8 [95%
CI: 19.0, 36.6] mm in the MDS and controls, respectively (p=0.277).
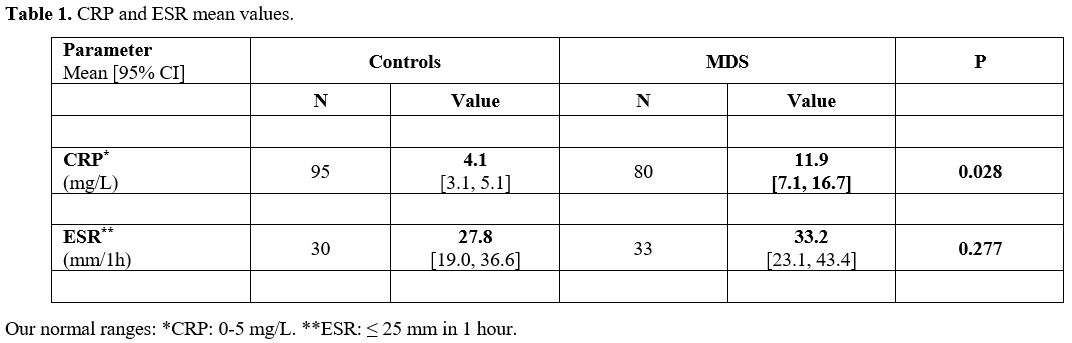 |
- Table
1. CRP and ESR mean values.
|
In the MDS group 31 of 80 patients (38.8%) demonstrated high CRP, compared with only 23/95 (24.2%) controls (Table 2, p=0.049). Elevated ESR was observed in 17/33 (51.5%) in the MDS group and in 13/30 (43.3%) in the control group (Table 2, p=0.616).
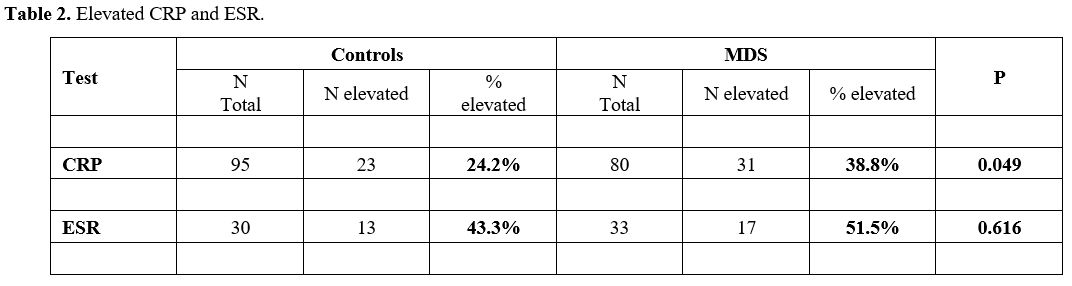 |
- Table
2. Elevated CRP and ESR.
|
The 31 MDS patients and 23 controls with elevated CRP were stratified according to the severity of the abnormal CRP (Figure 1).
1) Mildest CRP elevation (5<CRP≤15 mg/L, blue): 48.4% (MDS) vs 87.0%
(controls); 2) Moderate elevation (15<CRP≤25 mg/L, yellow): 22.6% vs
8.7%, respectively; 3) Highest elevation (CRP>25 mg/L, red): 29.0%
vs 4.3%, respectively. P=0.0038 for the trend. This demonstrates that
in the control group the vast majority have milder elevation (left bar,
blue), while in the MDS group, most are either in the moderate or
highest elevation group (right bar, yellow and red).
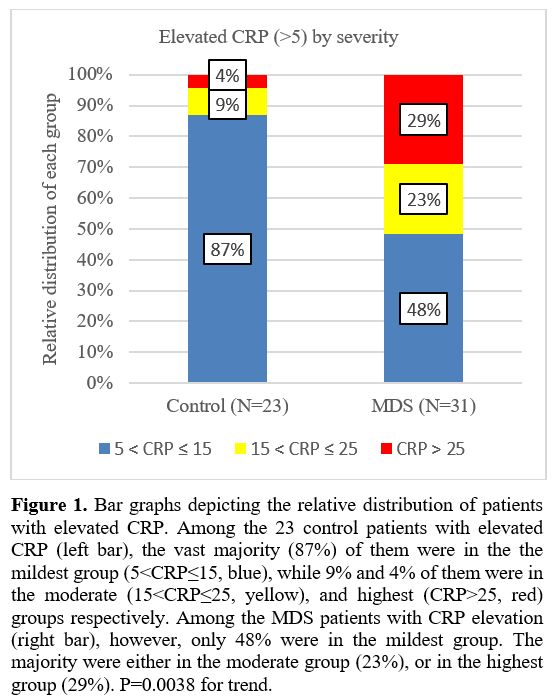 |
- Figure
1. Bar graphs depicting the relative distribution of patients with
elevated CRP. Among the 23 control patients with elevated CRP (left
bar), the vast majority (87%) of them were in the the mildest group
(5<CRP≤15, blue), while 9% and 4% of them were in the moderate
(15<CRP≤25, yellow), and highest (CRP>25, red) groups
respectively. Among the MDS patients with CRP elevation (right bar),
however, only 48% were in the mildest group. The majority were either
in the moderate group (23%), or in the highest group (29%). P=0.0038
for trend.
|
Discussion
The
involvement of the immune system and inflammatory processes in MDS
pathogenesis has gained attention over the last decades. Cytokine
abnormalities have been reported,[2] including high
levels of interleukin (IL)-6, tumor necrosis factor (NTF)-α, and IL-10,
as well as low levels of transforming growth factor (TGF)-β1, and
S100A4. Sallman and List focused on the role of inflammation in the
pathogenesis of MDS.[3] Aberrant innate immune
activation and pro-inflammatory signaling within the malignant clone
and the bone marrow (BM) microenvironment were identified as key
pathogenic drivers of MDS. In particular, S100A9-mediated NOD-like
receptor protein 3 (NLRP3) inflammasome activation directs an
inflammatory, lytic form of cell death termed pyroptosis that underlies
many of the hallmark features of the disease.
Aging has been
found to be a major player. Over the last decade the biological
phenomenon age-related clonal hematopoiesis (ARCH) has been
well-described.[5,6] Emerging data suggest that the
association between clonal hematopoiesis and nonmalignant comorbidity
may be bidirectional. Macrophage and inflammasome activation in clonal
hematopoiesis contribute to the etiology of inflammaging conditions,
such as atherosclerosis, and the systemic inflammation caused by
age-related inflammatory comorbidity, may also drive clonal expansion
and selection in the pathogenesis of myeloid neoplasia.[7] Thus, "inflammaging", the inflammatory process facilitated by aging, becomes important in MDS pathogenesis.[7-9]
Despite
the recognition that inflammatory processes and markers play a role in
the pathogenesis of MDS, little has been done so far to use it as an
assisting tool in daily practice. Inflammatory markers can help in
establishing diagnosis, staging and prognostication, and might also
serve as potential therapeutic targets.
In our study, we
provide another piece of evidence for the involvement of inflammation
in MDS at presentation. The work suggests the importance of using these
simple, readily available markers in clinical practice. The mean CRP
level was higher in MDS patients at presentation compared with controls
(Table 1). Moreover, a higher
percentage of MDS patients than controls had abnormal CRP and ESR.
Separation of the elevated CRP values into 3 levels of severity
demonstrated that despite the small numbers in each subgroup, most of
the MDS patients had a CRP with highest levels of elevation, while the
vast majority of controls had CRP values in the mildest group. Since we
excluded patients with active infections or immune diseases (often with
exceedingly high CRP levels), the results might be more significant.
Our
study suffers from several limitations. The retrospective nature of the
study and the relatively small number of tested individuals are the
most significant. The small numbers do not allow separation of the
patients into risk categories. Also, the possibility that some patients
(and controls) could have had another unrecognized reason for the high
CRP or ESR, could shift the results and the conclusions.
Nevertheless,
this preliminary study points to the involvement of the
inflammatory-immune system in MDS pathogenesis and calls for the use of
these markers in practice. Future studies will examine the
use of these inflammatory markers in patient prognosis and will
hopefully lead to another group of potential therapeutic targets as
well.
Acknowledgements
The
authors wish to acknowledge the assistance of Yochi Akiva for technical
support, and Zmira Silman, MA, for statistical assistance.
References
- Malcovati L, Hellstrom-Lindberg E, Bowen D, Ades L,
Cermak J, Del Canizo C, Della Porta MG, Fenaux P, Gattermann N, Germing
U, Jansen JH, Mittelman M, Mufti G, Platzbecker U, Sanz GF, Selleslag
D, Skov-Holm M, Stauder R, Symeonidis A, van de Loosdrecht AA, de Witte
T, Cazzola M, European Leukemia N: Diagnosis and treatment of primary
myelodysplastic syndromes in adults: recommendations from the European
LeukemiaNet. Blood 2013;122:2943-2964. https://doi.org/10.1182/blood-2013-03-492884 PMid:23980065 PMCid:PMC3811170
- Nielsen
AB, Hansen JW, Orskov AD, Dimopoulos K, Salem M, Grigorian M,
Bruunsgaard H, Gronbaek K: Inflammatory Cytokine Profiles Do Not Differ
Between Patients With Idiopathic Cytopenias of Undetermined
Significance and Myelodysplastic Syndromes. Hemasphere 2022;6:e0713. https://doi.org/10.1097/HS9.0000000000000713 PMid:35495296 PMCid:PMC9038488
- Sallman
DA, List A: The central role of inflammatory signaling in the
pathogenesis of myelodysplastic syndromes. Blood 2019;133:1039-1048. https://doi.org/10.1182/blood-2018-10-844654 PMid:30670444 PMCid:PMC7022316
- Miller
A, Green M, Robinson D: Simple rule for calculating normal erythrocyte
sedimentation rate. Br Med J (Clin Res Ed) 1983;286:266. https://doi.org/10.1136/bmj.286.6361.266 PMid:6402065 PMCid:PMC1546487
- Genovese
G, Kahler AK, Handsaker RE, Lindberg J, Rose SA, Bakhoum SF, Chambert
K, Mick E, Neale BM, Fromer M, Purcell SM, Svantesson O, Landen M,
Hoglund M, Lehmann S, Gabriel SB, Moran JL, Lander ES, Sullivan PF,
Sklar P, Gronberg H, Hultman CM, McCarroll SA: Clonal hematopoiesis and
blood-cancer risk inferred from blood DNA sequence. N Engl J Med
2014;371:2477-2487. https://doi.org/10.1056/NEJMoa1409405 PMid:25426838 PMCid:PMC4290021
- Jaiswal
S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, Lindsley
RC, Mermel CH, Burtt N, Chavez A, Higgins JM, Moltchanov V, Kuo FC,
Kluk MJ, Henderson B, Kinnunen L, Koistinen HA, Ladenvall C, Getz G,
Correa A, Banahan BF, Gabriel S, Kathiresan S, Stringham HM, McCarthy
MI, Boehnke M, Tuomilehto J, Haiman C, Groop L, Atzmon G, Wilson JG,
Neuberg D, Altshuler D, Ebert BL: Age-related clonal hematopoiesis
associated with adverse outcomes. N Engl J Med 2014;371:2488-2498. https://doi.org/10.1056/NEJMoa1408617 PMid:25426837 PMCid:PMC4306669
- Weeks
LD, Marinac CR, Redd R, Abel G, Lin A, Agrawal M, Stone RM, Schrag D,
Ebert BL: Age-related diseases of inflammation in myelodysplastic
syndrome and chronic myelomonocytic leukemia. Blood 2022;139:1246-1250.
https://doi.org/10.1182/blood.2021014418 PMid:34875037 PMCid:PMC8874362
- Franceschi
C, Garagnani P, Parini P, Giuliani C, Santoro A: Inflammaging: a new
immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol
2018;14:576-590. https://doi.org/10.1038/s41574-018-0059-4 PMid:30046148
- Trowbridge
JJ, Starczynowski DT: Innate immune pathways and inflammation in
hematopoietic aging, clonal hematopoiesis, and MDS. The Journal of
experimental medicine 2021;218. https://doi.org/10.1084/jem.20201544 PMid:34129017 PMCid:PMC8210621
[TOP]


