Cosimo Colangelo1, Giorgio Tiecco1, Marco Di Gregorio1, Susanna Capone2, Roberto Luigi Allegri2, Maria De Francesco3, Francesca Caccuri3, Arnaldo Caruso3, Francesco Castelli1 and Emanuele Focà1.
1 Department
of Clinical and Experimental Sciences, Unit of Infectious and Tropical
Diseases, University of Brescia-ASST Spedali Civili, Brescia, Italy
2 Unit of Infectious and Tropical Diseases, ASST Spedali Civili, Brescia, Italy
3
Section of Microbiology, Department of Molecular and Translational
Medicine, University of Brescia-ASST Spedali Civili, P. Le Spedali
Civili, 1, 25123, Brescia, Italy.
Correspondence to:
Emanuele Focà, Department Clinical and Experimental Sciences, Unit of
Infectious and Tropical Diseases, University of Brescia and ASST
Spedali Civili di Brescia, 25123 Brescia, Italy.; Tel.:
+39-0303995677.Email:
emanuele.foca@unibs.it
Published: September 1, 2023
Received: July 18, 2023
Accepted: August 14, 2023
Mediterr J Hematol Infect Dis 2023, 15(1): e2023052 DOI
10.4084/MJHID.2023.052
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
To the editor
Antibiotic
resistance is one of the most relevant problems in hospitals: the
growth of resistant microorganisms in healthcare settings is a
worrisome threat, raising the length of stay, morbidity, and
mortality in patients infected with multidrug resistant bacteria.[1] Moreover, the steady progress in
diagnostic techniques is rising concern about the emergence of new
pathogens which were hardly known in the past years.
Leclercia adecarboxylata is a gram-negative, motile, facultative-anaerobic, oxidase-negative, mesophilic bacillus belonging to the Enterobacteriaceae family.[2] L. adecarboxylata was first described by H. Leclerc in 1962 and was previously known as Enteric group 410 or Escherichia adecarboxylata[3] since Leclercia spp. shares several structural and microbiological properties with the genus Escherichia. Due to those similarities, L. adecarboxylata infections might be more common than what is believed so far since past clinical cases might have been erroneously defined as Escherichia spp.
infections. Moreover, most bacterial assays often could not distinguish
these morphologically and metabolically similar bacteria.[4]
Nevertheless, the availability of more sensitive testing methods (e.g.,
DNA hybridization, computer identification studies) like Matrix
Assisted Laser Desorption/Ionization Time of Flight ("MALDI-TOF") mass
spectrometry allowed a more precise species identification, eventually
leading to the present categorization.[3] L. adecarboxylata
can be found in various specimens and is involved in a wide range of
clinical syndromes commonly related to immunocompromised hosts.
Although most Leclercia spp
isolates show high susceptibility to antibiotics, some multi-resistant
strains have been reported in the literature. Here, we present a
catheter related bloodstream infection caused by a multidrug resistant L. adecarboxylata.
Cae Report
A
38-year-old transgender woman affected by gastric and duodenal diffuse
large B-cell lymphoma in remission was admitted to our Infectious
Diseases Department due to persistent and intense asthenia, weight
loss, and recurring fever episodes. The last rituximab administration
was performed 4 months before, and the antimicrobic prophylaxis was
recently discontinued following bone marrow recovery. The patient
assumed total parenteral nutrition through a tunneled central venous
catheter (CVC) placed 5 months prior to the admission because of
duodenal sub-stenosis subsequent to her hematologic condition.
Moreover, she was affected by chronic hepatitis HBV-correlated, treated
with tenofovir disoproxil fumarate, and several episodes of syphilis
reinfection were recorded following her former sex worker activity. No
HIV or HCV co-infections were detected. The chest CT scan performed in
the Emergency Department showed a parenchymal and nodular thickening.
Considering her risk factors for a healthcare-associated infection,
piperacillin/tazobactam (4.5 g every 6 hours/day) was empirically
started. At the admission, no catheter dysfunction and no signs of
catheter-related infection were recorded, and neither an
anti-methicillin-resistant Staphylococcus aureus (MRSA) nor an antimycotic agent was introduced.
A
diagnostic bronchoscopy was also performed, but both microbiological
tests (serology and cultures) and molecular biology assays performed on
the bronchoalveolar lavage gave negative results. However, L. adecarboxylata
was isolated from either peripheral and CVC blood culture performed at
the hospital admission. Catheter-related bloodstream infection (CRBSI)
was then diagnosed since a blood culture drawn from the line was
positive 4 hours earlier than the peripheral vein. This result was also
consistent with the anamnestic data concerning suboptimal domiciliary
management of the CVC, as she referred a sporadic nonsterile handling
of the catheter entry site (for instance, contact with tap water). The
antibiogram showed resistance to amoxicillin/clavulanate, fosfomycin,
and trimethoprim-sulfamethoxazole (Table 1),
so piperacillin/tazobactam (MIC ≤1) was maintained, and the catheter
was promptly replaced with a peripherally inserted central catheter
(PICC). A progressive clinical improvement was observed with a
significant reduction in inflammatory markers. On day 14, the targeted
systemic antibiotic therapy was discontinued. An
esophagogastroduodenoscopy was later performed to assess the severity
of the duodenal stenosis. A mass-forming inflammatory non-lymphomatous
tissue was observed, and on day 21, a duodenal prosthesis was placed.
In the following days, the patient was discharged with a semi-liquid
diet and parenteral nutrition to recover a complete oral feeding.
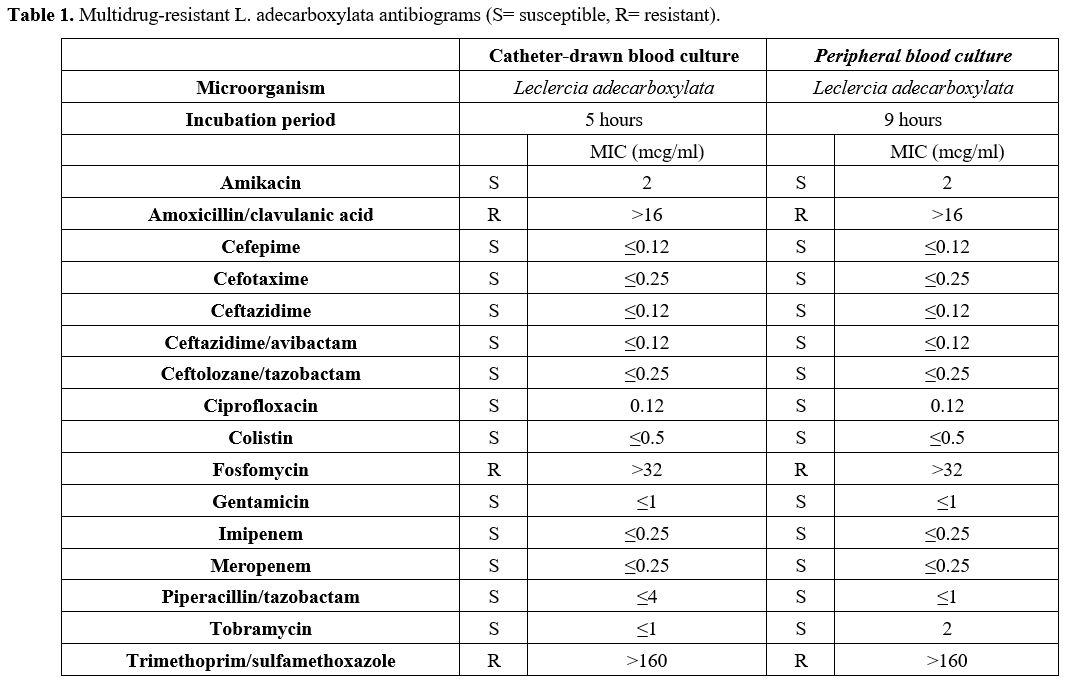 |
- Table 1. Multidrug-resistant L. adecarboxylata antibiograms (S= susceptible, R= resistant).
|
Discussion and Literature Review
Leclercia adecarboxylata is a gram-negative bacillus member of the Enterobacteriaceae family with many structural and microbiological properties in common with the genus Escherichia.[2,3]
The reclassification of this bacteria was achieved thanks to more
sensitive testing methods such as DNA hybridization and computer
identification studies.[2]
L. adecarboxylata has been recently recognized as an emerging pathogen[3,5] for which, thanks to the currently available diagnostic methods, it is possible to obtain an accurate identification.[3] Moreover, several analyses enlighten an ever-increasing number of multidrug-resistant strains[4,5,10] that should highlight the implications of this bacterial infection. L. adecarboxylata
is a ubiquitous microorganism that may be found in aquatic environments
and soil, as well as in the commensal gut flora of certain animals.[2]
In our case, an exposition to an aquatic environment was identified
(use of water to rinse the CVC), similar to a few cases reported in the
literature.[6] Moreover, L. adecarboxylata
might also be isolated from blood culture, skin wounds, peritoneal
fluid, abscesses (e.g., peritonsillar and periovarian), feces, urine,
and synovial fluid.[8] Several underlying conditions might favor L. adecarboxylata
infections: for instance, wounds may act as a direct entry into the
tissue, thus easing the pathogenicity, as well as catheters may be used
as gateways in catheter-related bacteremia or peritonitis could be
developed in patients undergoing dialysis or chemotherapy.[4]
The isolates more commonly mentioned in the literature show a high susceptibility to antibiotics.[4]
They could be controlled with a variety of antibiotics, such as
B-lactams, without witnessing therapeutic failures or needing
second-line treatments.[10] Considering the EUCAST breakpoint for Enterobacterales and given the contemporary resistance to at least [1] antibiotic of 3 different classes showed in our L. adecarboxylata
antibiogram, we consider peculiar our results since, to our best
knowledge, only a few cases of resistant strains have been reported.[2] A more comprehensive evaluation regarding natural antimicrobial susceptibility patterns was reported by Stock et al. from 94 L. adecarboxylata strains
collected from several human specimens: the bacteria were naturally
resistant to numerous antibiotic molecules, such oxacillin,
clarithromycin, erythromycin, roxithromycin, ketolides, rifampin,
glycopeptides, streptogramins, fusidic acid, lincosamides, penicillin G
and fosfomycin but susceptible to most B-lactams, quinolones,
aminoglycosides, tetracyclines, nitrofurantoine, folate pathway
inhibitors, azithromycin and chloramphenicol. In addition,
Extended-spectrum beta-lactamase (ESBL) and New Delhi
metallo-beta-lactamase 1 (NDM)-producing L. adecarboxylata
are also described. Three cases of ESBL producer isolates were
reported: the first case was described from a patient with acute
myeloid leukemia,[2] the second in a 47-year-old female with breast cancer,[10] and the third one in a 50-year-old female with end-stage renal disease.[5] Regarding NDM-producing L. adecarboxylata,
two cases were reported: the first regarding a patient hospitalized for
a foot trauma-related injury, and the second concerned an outbreak of
25 patients in intravenous total parental nutrition.[2]
L. adecarboxylata
might cause monomicrobial infection in immunocompromised patients,
while it is thought that this pathogen generally requires other
coinfecting bacteria to establish infection in immunocompetent
subjects.[4] However, some cases of monomicrobial
infection were described in immunocompetent patients even without
significant underlying comorbidities: only in one case the patient
report a clinical history of chronic disease,[8] while in the other cases, no history indicative of a clinically compromised state was observed.[9] Prevalently, L. adecarboxylata infections are described in adults, but a wound infection and peritonitis were reported in two immunocompetent children.[2] L. adecarboxylata is implicated in several clinical syndromes, such as endocarditis,[2] bacteremia,[4,8] wound infection and cellulitis,[6] pharyngeal and peritonsillar abscesses,[9] urinary tract infections,[3] pneumonia[3] and peritonitis.[3]
Most of these infections, as reported in our case, have been linked to
immunosuppression and the simultaneous presence of central vascular
catheter.[8] Additionally, as it appears from several reports, catheters could be considered important reservoirs for L. adecarboxylata bloodstream infection.[5,7,10] As a matter of fact, L. adecarboxyalta is not a fastidious pathogen: our strain grows both on blood and MacConkey agar.
Regarding treatment options, there are no shared guidelines or recommendations for L. adecarboxylata infections. Most isolates described are sensitive to tested antibiotics.[4] However, as described by Spiegelhauer et al., several strains of L. adecarboxylata
displayed resistance to ampicillin (9/30 isolates resistant) and
fosfomycin (8/10 isolates resistant), so these antibiotics should not
be used as first-line for treatment. Stock et al. described the natural
susceptibility patterns of L. adecarboxylata, showing that most isolated strains were sensible to B-lactams. Thus, Leclercia could be treated with this antibiotic class.[10]
In our case, considering the multi-resistance pattern, we successfully
treated our patient with the administration of piperacillin/tazobactam.
References
- Ricciardi W., Giubbini G., Laurenti P. Surveillance
and control of antibiotic resistance in the Mediterranean region.
Mediterr J Hematol Infect Dis 2016, 8(1): e2016036 https://doi.org/10.4084/mjhid.2016.036 PMid:27413528 PMCid:PMC4928537
- Malik K, Davie R, Withers A, Faisal M, Lawal F. A
case of Leclercia adecarboxylata endocarditis in a 62-year-old man.
IDCases. 2021 Mar 27;24: e01091. doi: 10.1016/j.idcr. 2021.e01091. https://doi.org/10.1016/j.idcr.2021.e01091 PMid:33889491 PMCid:PMC8047457
- Zayet
S, Lang S, Garnier P, Pierron A, Plantin J, Toko L, Royer PY, Villemain
M, Klopfenstein T, Gendrin V. Leclercia adecarboxylata as Emerging
Pathogen in Human Infections: Clinical Features and Antimicrobial
Susceptibility Testing. Pathogens. 2021 Oct 28;10(11):1399. doi:
10.3390/pathogens10111399. https://doi.org/10.3390/pathogens10111399 PMid:34832555 PMCid:PMC8619052
- Spiegelhauer
MR, Andersen PF, Frandsen TH, Nordestgaard RLM, Andersen LP. Leclercia
adecarboxylata: a case report and literature review of 74 cases
demonstrating its pathogenicity in immunocompromised patients. Infect
Dis (Lond). 2019 Mar;51(3):179-188. doi: 10.1080/23744235.2018.1536830.
Epub 2018 Nov 29. https://doi.org/10.1080/23744235.2018.1536830 PMid:30488747
- Alosaimi
RS, Muhmmed Kaaki M. Catheter-Related ESBL-Producing Leclercia
adecarboxylata Septicemia in Hemodialysis Patient: An Emerging
Pathogen? Case Rep Infect Dis. 2020 Jan 21;2020:7403152. doi:
10.1155/2020/7403152. https://doi.org/10.1155/2020/7403152 PMid:32089912 PMCid:PMC6996699
- Broderick
A, Lowe E, Xiao A, Ross R, Miller R. Leclercia adecarboxylata
folliculitis in a healthy swimmer-An emerging aquatic pathogen? JAAD
Case Rep. 2019 Aug 5;5(8):706-708. doi:
10.1016/j.jdcr.2019.06.007. https://doi.org/10.1016/j.jdcr.2019.06.007 PMid:31440563 PMCid:PMC6698442
- De
Mauri A, Chiarinotti D, Andreoni S, Molinari GL, Conti N, De Leo M.
Leclercia adecarboxylata and catheter-related bacteraemia: review of
the literature and outcome with regard to catheters and patients. J Med
Microbiol. 2013 Oct;62(Pt 10):1620-1623. doi: 10.1099/jmm.0.059535-0.
Epub 2013 Jul 23. https://doi.org/10.1099/jmm.0.059535-0 PMid:23882033
- Shaikhain
T, Al-Husayni F, Al-Fawaz S, Alghamdi EM, Al-Amri A, Alfares M.
Leclercia adecarboxylata Bacteremia without a Focus in a
Non-Immunosuppressed Patient. Am J Case Rep. 2021 Mar 30;22:e929537.
doi: 10.12659/AJCR.929537. https://doi.org/10.12659/AJCR.929537 PMid:33782375 PMCid:PMC8019838
- Bali
R, Sharma P, Gupta K, Nagrath S. Pharyngeal and peritonsillar abscess
due to Leclercia adecarboxylata in an immunocompetant patient. J Infect
Dev Ctries. 2013 Jan 15;7(1):46-50. doi: 10.3855/jidc.2651. https://doi.org/10.3855/jidc.2651 PMid:23324820
- Shin
GW, You MJ, Lee HS, Lee CS. Catheter-related bacteremia caused by
multidrug-resistant Leclercia adecarboxylata in a patient with breast
cancer. J Clin Microbiol. 2012 Sep;50(9):3129-32. doi:
10.1128/JCM.00948-12. Epub 2012 Jul 3. https://doi.org/10.1128/JCM.00948-12 PMid:22760051 PMCid:PMC3421816
[TOP]
