Ivan Gur*1,Roei Tounek*2,Yaniv Dotan1,2, Elite Vainer Evgrafov1,2, Stav Rakedzon1 and Eyal Fuchs1,2.
1 Rambam Medical Center, Haifa, Israel.
2 The Ruth and Bruce Rappaport Faculty of Medicine, Technion Israel Institute of Technology, Haifa, Israel.
* The authors equally contributed to the work.
Correspondence to: :
Ivan GUR, MD, MPH, MHA. Department of Internal Medicine C, Rambam
Medical Center. 4 HaAlia Street, Haifa 3109601, Israel. Tel:
(+972)-4-777-2661. (+972)-542-555-655. E-mail:
I_GUR@rambam.health.gov.il
Published: January 01, 2024
Received: September 11, 2023
Accepted: December 12, 2023
Mediterr J Hematol Infect Dis 2024, 16(1): e2024006 DOI
10.4084/MJHID.2024.006
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background:
Hospitalized hematological patients often require bronchoalveolar
lavage (BAL). Scarce evidence exists regarding the potential risks in
patients with very severe thrombocytopenia (VST).
Methods: This
retrospective-cohort study included adult hematological in-patients
with VST, defined as platelets<20x103/μL, undergoing BAL during
2012-2021. Mechanically ventilated patients or those with known active
bleeding were excluded. Primary outcomes included major bleeding
halting the BAL or deemed significant by the treating physician, need
for any respiratory support other than low flow O2, or death within 24
hours. Any other bleedings were recorded as secondary
outcomes.
Results: Of the 507 patients included in the final analysis, the 281 patients with VST had lower hemoglobin (Md=0.3, p=0.003), longer prothrombin-time (Md=0.7s, p=0.025),
higher chances of preprocedural platelet transfusion (RR 3.68,
95%CI[2.86,4.73]), and only one primary-outcome event (death of septic
shock 21h postprocedurally) - compared with 3 (1.3%) events (two
bleedings halting procedure and one need for non-invasive-ventilation)
in patients with platelets ≥20x103/μL (p=0.219).
The risk of minor, spontaneously resolved bleeding was higher
(RR=3.217, 95%CI[0.919,11.262]) in patients with VST (4.3% vs 1.3%, p=0.051).
No association was found between the complications recorded and
preprocedural platelets, age, aPTT, P.T., hematological status, or
platelet transfusion.
Conclusions: This data suggests BAL to be safe even when platelet counts are <20x103/μL.
|
Introduction
Patients with hematological malignancies are at increased risk of opportunistic pulmonary infections.[1]
Flexible bronchoscopy-facilitated bronchoalveolar lavage (BAL) is
important in this population's diagnosis and therapy guidance.[2]
Coincidentally, thrombocytopenia is a common occurrence in this
population. Thrombocytopenia, combined with other comorbidities and
hemostatic dysfunction, is a common concern when considering
BAL's safety and cost-benefit ratios in hematological patients.[3] This is particularly true in patients with platelet counts below 50x103/μL,
prompting most providers to consider prophylactic platelet transfusions
(particularly in very low platelet counts below 10x103/μL) in an attempt to mitigate the risk of periprocedural complications.[4]
Nonetheless,
there are no universally accepted platelet count thresholds for BAL or
the decision to transfuse periprocedurally. Previous observational
studies have described vanishingly low complication rates above 20x103/μL[5] or even 10x103/μL.[6,7]
Current accepted guidelines, based on low-level observational data,
agree on the general safety of BAL when the platelet count is above
20x103/μL.[8,9].
In
this study, we aimed to describe the incidence of various
periprocedural complications of BAL in hospitalized hematological
patients with significant thrombocytopenia, assessing potential
predictors of increased risk, in an attempt to assess the safety of
this invasive and yet essential procedure in such a frail population.
Methods
This
retrospective cohort study was conducted in Rambam Health Care Campus
(RMC), a tertiary 1000-bed medical center, the largest medical center
in northern Israel. The Electronic Health Registry (EHR) files of all
patients undergoing BAL between January 1, 2012, and December 31, 2021,
were reviewed.
The study included all adult patients (18 years or
older) undergoing BAL while hospitalized in our hematology ward with a
platelet count below 50x103/μL 24
hours before the procedure. Exclusion criteria were: 1) Active
hemoptysis, epistaxis, or known upper gastrointestinal bleeding in the
24 hours prior to the bronchoalveolar lavage and 2) BAL performed while
the patient is mechanically ventilated.
Physician's notes,
admission and discharge reports, imaging interpretations, and
background diagnoses were manually and individually reviewed for each
patient in this study. Additional demographic, clinical, and laboratory
data, including date of birth, vital signs, and laboratory results upon
presentation, were mined using the MD-Clone® interface (version 4.25 or
older). Machine-mined data was assessed for accuracy and relevance by
the investigator reviewing the EHR.
The primary outcome was any
major complication in the 24 hours after the initiation of BAL,
including any of the following: 1) clinically significant major
bleeding, either resulting in the premature termination of the BAL and
/ or necessitating packed red blood cells transfusion peri procedurally
and / or deemed by any of the treating physicians as potentially
life-threatening; 2) The need for ventilatory support other than low
flow conventional supplementary oxygen therapy (COT), including high
flow (>10 L/min) oxygen therapy or any positive pressure ventilation
(both invasive and noninvasive) or 3) death from any cause. The
secondary outcomes were defined as any non-major bleeding within the
first 24 hours post-procedurally, including self-limiting bleeding
visualized during bronchoscopy and any epistaxis, whether said bleeding
resolved spontaneously, or hemostatic measures (such as nasal packing,
intraluminal epinephrine, tranexamic acid or cold saline injections)
were required. Additional secondary outcomes included a decrease of ≥5
mmHg in mean arterial pressure (MAP) in the lowest measurement 24 hours
post-BAL (compared with MAP measured immediately prior to BAL); an
increase of ≥10 beats per minute (bpm) in heart rate (similarly defined
as the highest resting heart rate documented in the 24 hours after
bronchoscopy minus the heart rate immediately before bronchoscopy), a
decrease of ≥1 mg/dL in hemoglobin (similarly defined) and a decrease
of ≥5% capillary hemoglobin saturation (SpO2) as measured by pulse
oximetry within 24 hours (similarly defined). In addition, we recorded
the diagnostic yield of the BAL, i.e., whether any pathogens were
recovered and the type of infectious syndrome (e.g., invasive pulmonary
aspergillosis, bacterial pneumonia, or atypical infection such as
legionellosis) supported by the BAL results.
Per institutional
protocols, BAL was performed transnasally unless technically
infeasible, in which case the transoral approach was implemented. This
study was reviewed and approved by the RMC institutional ethics
committee (RMB-22-0017).
Statistical Analysis. Standard
descriptive statistics were used to summarize population
characteristics. We used a chi-square test for categorical variables, a
Mann-Witney U test for nonparametric variables, and a student's
unpaired t-test for normally distributed continuous variables. Tukey's
correction was applied when applicable to adjust for multiple
comparisons. Categorical variables were described using proportions and
percentages, nonparametric variables with median and interquartile
range (IQR), and normally distributed continuous variables as mean with
standard deviation (S.D.).
Multivariate logistic regression
modeling was performed using Pearl and Reed's method. We used the
Pearson correlation coefficient to determine possible correlations
between independent variables; only variables that were not co-related
(Pv>0.1 on univariate analysis) were included in the model. A
2-sided Pv<0.05 was considered statistically significant for all
tests. All calculations were performed using PASW software version 29.0
(IBM, Chicago, IL).
Results
Of
720 adult (age 18 or older) hematological patients undergoing BAL while
hospitalized during the study period, only 553 (76.8) had a platelet
count below 50x103/μL.
Nine (1.6%) patients were excluded due to active hemoptysis, epistaxis,
or upper gastrointestinal bleeding in the 24 hours prior to
bronchoscopy, and 37 (6.7%) patients were mechanically ventilated while
undergoing BAL. A Consolidated Standards of Reporting Trials (CONSORT)
diagram summarizing the data mining and filtering process is presented
in Figure 1.
 |
- Figure 1. Study Design. The study phases are presented in accordance with the CONSORT guidelines. Abbreviations: EHR - electronic healthcare registry, BAL - Bronchoalveolar Lavage.
|
Of the 507 patients included in the final analysis, 281 (55.4%) had a platelet count below 20x103/μL, and 210 (41.4%) were females. The mean age was 55.3 years (median 59, Sd 15.1), and the mean platelet count was 20.4x103/μL
(median 18, Sd 12). Regarding the background hematological diagnosis,
the most common diagnosis was acute myeloid leukemia, affecting 264
(52.1%) of patients. Overall 324 (63.9%) had acute leukemia, 6 (1.2%)
chronic leukemia, 11 (2.1%) indolent lymphomas, 66 (13%) other
lymphomas, 46 (9.1%) multiple myeloma, 40 (7.9%) myelodysplastic
syndrome, 10 (1.9%) myeloproliferative disorders, and 7 (1.4%) aplastic
anemia. Only the rates of myelodysplastic syndrome were significantly
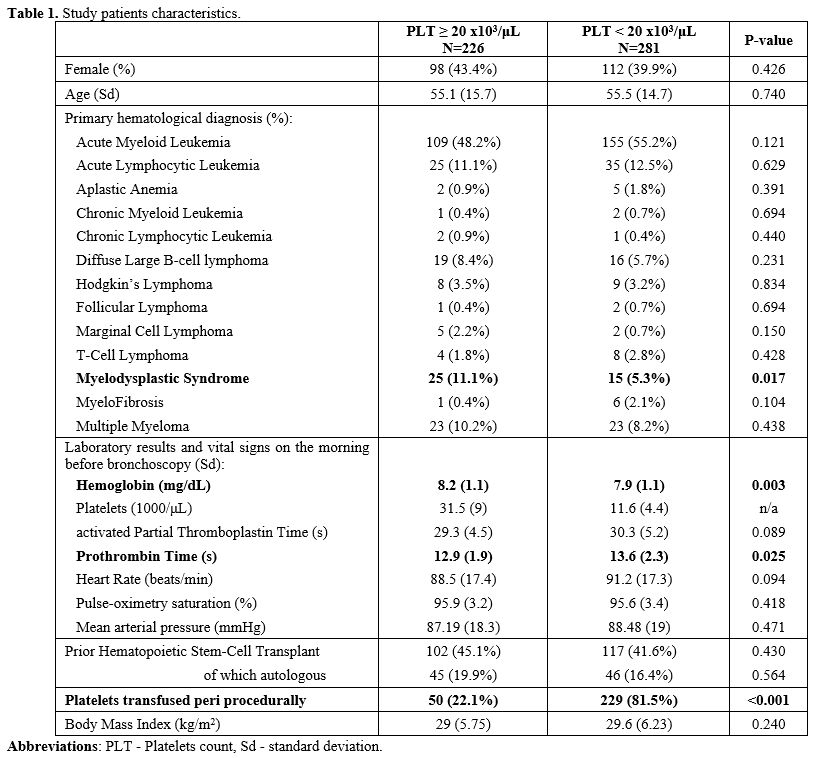
(p=0.017) associated with higher platelet counts.
When examining
the bloodwork and vital signs obtained on the morning of the procedure,
mean hemoglobin concentration was 0.3mg/dL lower (p=0.003), and mean
prothrombin time 0.7 seconds longer in patients with platelet counts
below 20x103/μL.
The mean activated partial thromboplastin time was 29.9 seconds (Sd
5.5), heart rate 89.4 bpm (18.3), SpO2 95.8% (Sd 3.4) or mean arterial
pressure 82.4 mmHg (Sd 10.4) with no significant association with
platelet count. There was no significant difference between patients
with platelet counts below and above 20x103/μL
in any of the other baseline characteristics examined, with the notable
exception of the rates of periprocedural platelet transfusion occurring
in four times as many patients with platelet counts below 20x103/μL (p<0.001). These data are summarized in Table 1.
 |
- Table
1. Study patients characteristics.
|
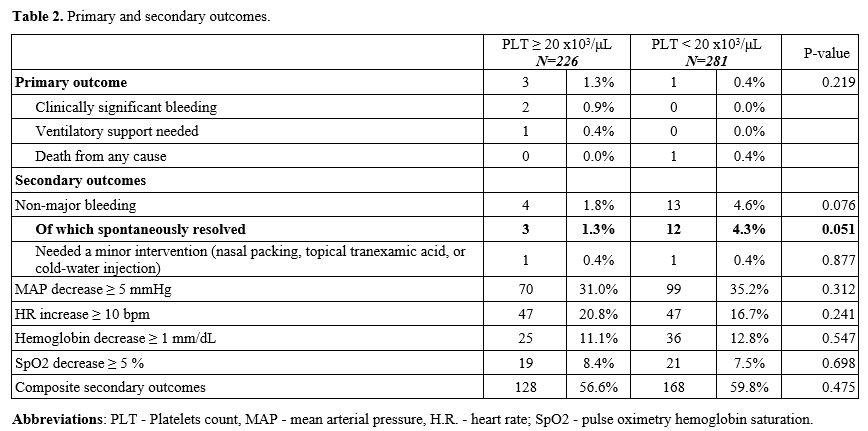
Clinically
significant bleeding (2 patients, 0.9%) and need for ventilatory
support with noninvasive bilevel positive pressure (one patient, 0.4%)
occurred exclusively in patients with platelet counts above 20x103/μL, and the only primary outcome to have been recorded in the <20x103/μL
group was one account of death due to cardiovascular collapse in a
patient with severe septic shock and multiorgan failure (manifested
prior to bronchoscopy) 21 hours after BAL (p=0.219 for the composite of
primary outcomes).
The incidence of non-major bleeding was higher (13 (4.6%) vs 4(1.8%)) in patients with platelets below 20x103/μL
(RR 2.614, 95%CI[0.864,7.906]), a trend that approached statistical
significance (p=0.076). Further analysis demonstrated spontaneously
resolved minor bleeds to be responsible for this trend (RR 3.217,
95%CI[0.919,11.262]), with a virtual similar rate of one patient (0.4%)
requiring nasal packing with topical tranexamic acid in both groups. A
decrease in mean arterial pressure (169 patients, 33%), SpO2 (40
patients, 7.9%), hemoglobin (61 (12%)) or an increase in heart rate (94
(18%)) as defined in the secondary outcomes section above was similar
between groups, as presented in Table 2.
 |
- Table 2. Primary and secondary outcomes .
|
When
examining the microbiological diagnostic significance of these BAL
samples, overall diagnostic yield and the types and counts of major
pathogens recovered did not differ significantly between groups. These
findings are presented in Figure 2.
 |
- Figure 2.
Diagnostic Yield of Bronchoalveolar Lavage. Positive results of BAL
microbiology, either by means or direct culture or nucleic acid
amplification. Abbreviations: CMV - cytomegalovirus, GAS - group A
streptococci, HHV6 - human herpesvirus 6, HMPV - human metapneumovirus,
HSV - herpes simplex virus, NTB - nontuberculous mycobacteria, PCP -
pneumocystis Jirovecii pneumonia, RSV - respiratory syncytial virus,
S.A. - Staphylococcus aureus, T.B. - Mycobacterium tuberculosis, VZV -
varicella-zoster virus.
|
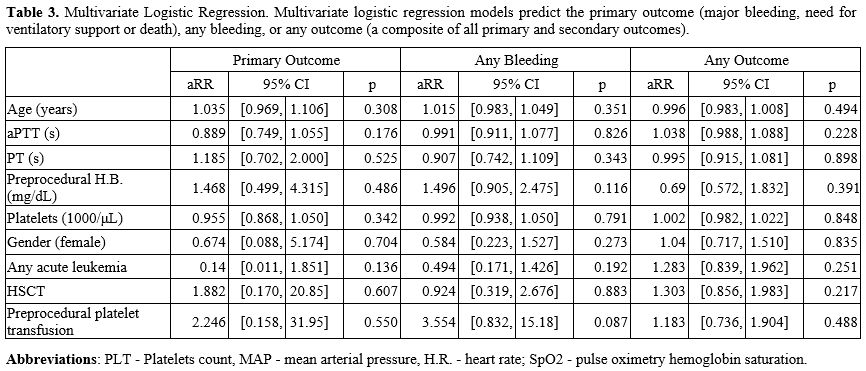
To
further illuminate the potential association between the degree of
pre-procedural thrombocytopenia and potential patient-specific
confounders, three multivariate logistic regression models were
constructed, predicting major complications (as defined by the primary
outcome), any bleeding or a composite of all recorded complications
(both primary and secondary outcomes). A trend towards increased risk
of any bleeding was observed with preprocedural platelet transfusion
(aRR 3.55, 95%CI[0.83,15.18]), although this finding did not reach the
accepted level of statistical significance (p=0.087). Age, activated
partial thromboplastin time, prothrombin time, preprocedural hemoglobin
concentration or platelet count, gender, the presence of acute
leukemia, or history of hematopoietic stem-cell transplant did not
significantly predict the development of major complications, any
bleeding, or any of the recorded complications, as detailed in Table 3.
 |
- Table
3. Multivariate Logistic Regression. Multivariate logistic regression
models predict the primary outcome (major bleeding, need for
ventilatory support or death), any bleeding, or any outcome (a
composite of all primary and secondary outcomes).
|
Discussion
This
study suggests a very low incidence (0.8%) of serious periprocedural
complications when performing BAL in hematological patients, even in
the presence of VST. Both cases of major bleeding (British Thoracic
Society grade 3[10] for the twain) were recorded in
patients with higher platelet counts. While the reported rates of
bronchoscopy complications range between 1 percent to 12%, various
retrospective studies have demonstrated the incidence of serious
complications (i.e., major bleeding halting procedure, need for
ventilatory support or death) to be consistently in the range of
0.7-0.9%.[4,6,7,11-13]
Interestingly, the rates of both serious complications in general and
major bleeding, in particular, are similar when examining large cohorts
of BAL in the general population and in patients with various degrees
of thrombocytopenia, which could serve as a weak, albeit ecological,
corroboration to the lack of a direct link between platelet counts and
major complications in bronchoscopy.[6,11,14]
The
rates of non-major bleeding were higher in severely thrombocytopenic
patients, approaching significance only due to very minor,
spontaneously resolving bleeding. Echoed in some previous reports[4,7,12] and considerably lower than others,[11]
this observation is consistent with our understanding of platelet
function in primary hemostasis. Namely, superficial bleeding (e.g.,
petechiae and purpura) is the major clinical manifestation of isolated
thrombocytopenia.[15]
The trend towards
increased risk of any bleeding with platelet transfusion represents
correlation rather than causation. When adjusted for other observed
confounders, such as hemoglobin concentration and hematological
history, our data showed no clear signal toward the direct benefit of
platelet transfusion in preventing BAL complications. Previous attempts
to establish a safety threshold of 30x103/μL or even 10x103/μL were hampered by their retrospective nature and high prevalence of platelet transfusions in the control group.[6,7]
Our study demonstrates the very strong predilection of clinicians to
administer platelet transfusion below the currently set threshold of
20x103/μL, which is based on a very low level of evidence.[8]
More
interestingly, there was no significant association between platelet
count or coagulation studies and any of the BAL complications examined.
Coherent with the limited role of the coagulation cascade in minor
superficial injuries, this observation is also consistent with the
mounting evidence of these limited coagulation studies' very low
predictive ability in predicting bleeding risk.[16]
To
our knowledge, this is the only study focused exclusively on BAL by
primary design. We attribute the relatively low incidence of bleeding
of all varieties observed in the study to mild tissue derangement when
bronchoscopy is confined to visualization and BAL.[17]
The shorter duration, low energy, smaller bore diameters, and minimal
tissue disruption could all explain the general safety of this
procedure, even in the sickest of patients.[18] This
is particularly important given the recent departure from tissue
sampling to diagnose infectious diseases. Particularly, most recent
guidelines[8,19] have accepted
BAL-based testing, such as galactomannan, as a preferable alternative
to diagnosing invasive pulmonary aspergillosis - by far the most
prevalent diagnosis resulting from bronchoscopy in our sample.[20]
Of
note, examining the BAL aspirate most often yielded a diagnosis of
Invasive Pulmonary Aspergillosis, followed by gram-negative Enterobacteriaceae, Herpes Simplex Virus, Cytomegalovirus, and Pneumocystis jirovecii.
These rates and relative incidence are similar to previously reported
cross-sectional studies of diagnostic BAL in hematological patients.[21]
While the degree of thrombocytopenia seems to correlate with bone
marrow dysfunction (as evidenced by the high correlation with white and
red blood cell counts), the microbiological yield is similar when
compared to patients without severe thrombocytopenia (and by
conjecture, probably a lesser degree of myelosuppression). Actually,
the underlying hematological disease, rather than peripheral counts
themselves, can explain the majority of immune dysfunction and
resultant, rather opportunistic infectious profile.[1]
Alternatively, patient selection (i.e., those who undergo BAL are those
manifesting clinical characteristics of opportunistic infections) and
characteristics (prolonged hospitalization, exposure to multiple
antimicrobial agents, etc.), rather than the degree of pancytopenia,
may be the main mechanism explaining BAL microbiology.
Limitations
This study has several important limitations. Firstly, the
retrospective design inherently raises the risk of biases, particularly
since no randomization was performed and no strict protocol detailing
the use of platelet transfusion was followed. Secondly, despite
extensive and decade-long data collection in one of our nation's
largest hematological referral centers, the incidence of significant
adverse outcomes (defined in this study as primary outcomes) or any
bleeding complication was low. The rare event limits our ability to
fully appreciate the potentially rare complications of BAL in
thrombocytopenic patients.
Conclusions
This
observational study showed no increased risk of major or minor
complications in patients with severe thrombocytopenia due to
bronchoalveolar lavage. Consistent with previous reported evidence, our
data suggests BAL to be generally safe in hematological patients,
irrespective of platelet count. Additionally, prospectively randomized
studies are needed to validate the safe platelet count threshold prior
to BAL further and elucidate the clinical yield of periprocedural
platelet transfusion.
References
- Bitterman R, Hardak E, Raines M, Stern A, Zuckerman
T, Ofran Y, et al. Baseline Chest Computed Tomography for Early
Diagnosis of Invasive Pulmonary Aspergillosis in Hemato-oncological
Patients: A Prospective Cohort Study. Clin Infect Dis. 2019 October
30;69(10):1805-8. https://doi.org/10.1093/cid/ciz194 PMid:30855077
- Kim
SW, Rhee CK, Kang HS, Lee HY, Kang JY, Kim SJ, et al. Diagnostic value
of bronchoscopy in patients with hematologic malignancy and pulmonary
infiltrates. Ann Hematol. 2015 Jan;94(1):153-9. https://doi.org/10.1007/s00277-014-2172-3 PMid:25062720
- Choo
R, Naser NSH, Nadkarni NV, Anantham D. Utility of bronchoalveolar
lavage in the management of immunocompromised patients presenting with
lung infiltrates. BMC Pulm Med. 2019 February 26;19(1):51. https://doi.org/10.1186/s12890-019-0801-2 PMid:30808314 PMCid:PMC6390608
- Uruga
H, Sato T, Nishida A, Uchida N, Tsuji M, Moriguchi S, et al. Safety of
bronchoscopy in patients with malignant hematologic disorders. BMC Pulm
Med. 2020 September 11;20(1):243. https://doi.org/10.1186/s12890-020-01283-8 PMid:32917185 PMCid:PMC7488692
- Kaur
R, Alolaiwat AA, Ritz E, Mokhlesi B, Vines DL. A new index, Respiratory
Insufficiency index and Modified Early Warning Scores predict
extubation failure. Can J Respir Ther. 2023 May 18;59:117-22. https://doi.org/10.29390/cjrt-2023-003 PMid:37214344 PMCid:PMC10194084
- Facciolongo
N, Patelli M, Gasparini S, Lazzari Agli L, Salio M, Simonassi C, et al.
Incidence of complications in bronchoscopy. Multicentre prospective
study of 20,986 bronchoscopies. Monaldi Arch Chest Dis. 2009
Mar;71(1):8-14. https://doi.org/10.4081/monaldi.2009.370 PMid:19522159
- Faiz
SA, Jimenez CA, Fellman BM, Huk T, Jazbeh S, Haque SA, et al. Incidence
of bleeding complications with flexible bronchoscopy in cancer patients
with thrombocytopenia. J Bronchology Interv Pulmonol. 2019
Oct;26(4):280-6. https://doi.org/10.1097/LBR.0000000000000590 PMid:30973520 PMCid:PMC6768748
- Du
Rand IA, Blaikley J, Booton R, Chaudhuri N, Gupta V, Khalid S, et al.
British Thoracic Society guideline for diagnostic flexible bronchoscopy
in adults: accredited by NICE. Thorax. 2013 Aug;68 Suppl 1:i1-44. https://doi.org/10.1136/thoraxjnl-2013-203618 PMid:23860341
- Mohan
A, Madan K, Hadda V, Tiwari P, Mittal S, Guleria R, et al. Guidelines
for diagnostic flexible bronchoscopy in adults: Joint Indian Chest
Society/National College of chest physicians (I)/Indian association for
bronchology recommendations. Lung India. 2019
Jul;36(Supplement):S37-89.
- Folch
EE, Mahajan AK, Oberg CL, Maldonado F, Toloza E, Krimsky WS, et al.
Standardized definitions of bleeding after transbronchial lung biopsy:
A delphi consensus statement from the nashville working group. Chest.
2020 Jul;158(1):393-400. https://doi.org/10.1016/j.chest.2020.01.036 PMid:32067944 PMCid:PMC7403751
- Bo
L, Shi L, Jin F, Li C. The hemorrhage risk of patients undergoing
bronchoscopic examinations or treatments. Am J Transl Res. 2021 August
15;13(8):9175-81.
- Cefalo
M, Puxeddu E, Sarmati L, Paterno G, Fontana C, Nasso D, et al.
Diagnostic performance and safety of bronchoalveolar lavage in
thrombocytopenic haematological patients for invasive fungal infections
diagnosis: A monocentric, retrospective experience. Mediterr J Hematol
Infect Dis. 2019 November 1;11(1):e2019065. https://doi.org/10.4084/mjhid.2019.065 PMid:31700590 PMCid:PMC6827601
- Jacomelli
M, Margotto SS, Demarzo SE, Scordamaglio PR, Cardoso PFG, Palomino ALM,
et al. Early complications in flexible bronchoscopy at a university
hospital. J Bras Pneumol. 2020 Jun 1;46(4):e20180125. https://doi.org/10.36416/1806-3756/e20180125 PMid:32490906 PMCid:PMC7567622
- Nandagopal
L, Veeraputhiran M, Jain T, Soubani AO, Schiffer CA. Bronchoscopy can
be done safely in patients with thrombocytopenia. Transfusion. 2016
Feb;56(2):344-8. https://doi.org/10.1111/trf.13348 PMid:26446048
- Schiffer
CA, Bohlke K, Delaney M, Hume H, Magdalinski AJ, McCullough JJ, et al.
Platelet transfusion for patients with cancer: american society of
clinical oncology clinical practice guideline update. J Clin Oncol.
2018 Jan 20;36(3):283-99. https://doi.org/10.1200/JCO.2017.76.1734 PMid:29182495
- Shi
Y, Zhang G, Ma C, Xu J, Xu K, Zhang W, et al. Machine learning
algorithms to predict intraoperative hemorrhage in surgical patients: a
modeling study of real-world data in Shanghai, China. BMC Med Inform
Decis Mak. 2023 Aug 10;23(1):156. https://doi.org/10.1186/s12911-023-02253-w PMid:37563676 PMCid:PMC10416513
- Zhou
G-W, Zhang W, Dong Y-C, Huang H-D, Hu C, Sun J, et al. Flexible
bronchoscopy-induced massive bleeding: A 12-year multicentre
retrospective cohort study. Respirology. 2016 Jul;21(5):927-31. https://doi.org/10.1111/resp.12784 PMid:27061330
- Costa
A da S, Scordamaglio PR, Suzuki I, Palomino ALM, Jacomelli M.
Indications, clinical outcomes and complications of 1,949 flexible
bronchoscopies. Einstein (Sao Paulo). 2018 Nov 8;16(4):eAO4380. https://doi.org/10.31744/einstein_journal/2018AO4380 PMid:30427487 PMCid:PMC6223942
- Hardak
E, Fuchs E, Leskes H, Geffen Y, Zuckerman T, Oren I. Diagnostic role of
polymerase chain reaction in bronchoalveolar lavage fluid for invasive
pulmonary aspergillosis in immunocompromised patients - A retrospective
cohort study. Int J Infect Dis. 2019 Jun;83:20-5. https://doi.org/10.1016/j.ijid.2019.03.025 PMid:30926540
- Shimoda
M, Tanaka Y, Morimoto K, Abe T, Asaga R, Nakajima K, et al. Analysis of
predicted factors for bronchoalveolar lavage recovery failure: An
observational study. PLoS ONE. 2022 Sep 30;17(9):e0275377. https://doi.org/10.1371/journal.pone.0275377 PMid:36178919 PMCid:PMC9524652
- Gur
I, Giladi A, Isenberg YN, Neuberger A, Stern A. COVID-19 in Patients
with Hematologic Malignancies: Clinical Manifestations, Persistence,
and Immune Response. Acta Haematol. 2022 Mar 2;145(3):297-309. https://doi.org/10.1159/000523872 PMid:35235928 PMCid:PMC9254311




