 |
|
An 84-year-old man was admitted to the hospital for melaena, causing severe anaemia, and requested a transfusion of 2 packed red blood cells (PRBC) units. He was previously transfused with 2 units of PRBC in 2003 for unknown reasons. We initially performed a complete blood test and indirect Antiglobulin test (IAT) through the microcolumn method, using the Ortho Vision instrument (Ortho Clinical Diagnostics, Raritan, New Jersey, U.S.). The IAT resulted in being negative, and the direct group determination resulted in being 0 positive, whereas, in the indirect group determination, we observed an unexpected reaction with 0 cells (score 1+). This led us to speculate about an interference given by a cold antibody, not visible at a 37°C incubation. Therefore, we repeated the IAT at room temperature (RT) using the instrument and the test tube; both resulted in positive. The Direct Antiglobulin test (DAT) and self-control test were negative. To try and identify the suspected cold antibody, we performed a C untreated (UNT) panel by hand incubating the patient's plasma, using the Neutral cards and panel cells at 4°C, which resulted being positive in 3 cells out of 11, preventing us from identifying any antibodies.
Given that the blood request was urgent, crossmatch tests were carried out on 3 fresh PRBC units: two of them proved to be compatible, so they were delivered for transfusion. The patient was transfused with both the PRBCs on the same day without any adverse reactions. The day after, we performed the B panel using Reverse cards, polyethylene glycol (PEG)-rich gel microcolumn, at 4°C and we obtained more evident reactions that allowed us to start suspecting the presence of an anti-P1 alloantibody. We also repeated the C UNT panel, and we performed a 4°C IAT using the Reverse cards. These tests confirmed our hypothesis about the presence of an anti-P1 alloantibody because the positive and negative reactions obtained matched those expected in the P1 antibody master list. Eventually, the patient was tested for the P1 antigen, and it was negative, as expected.
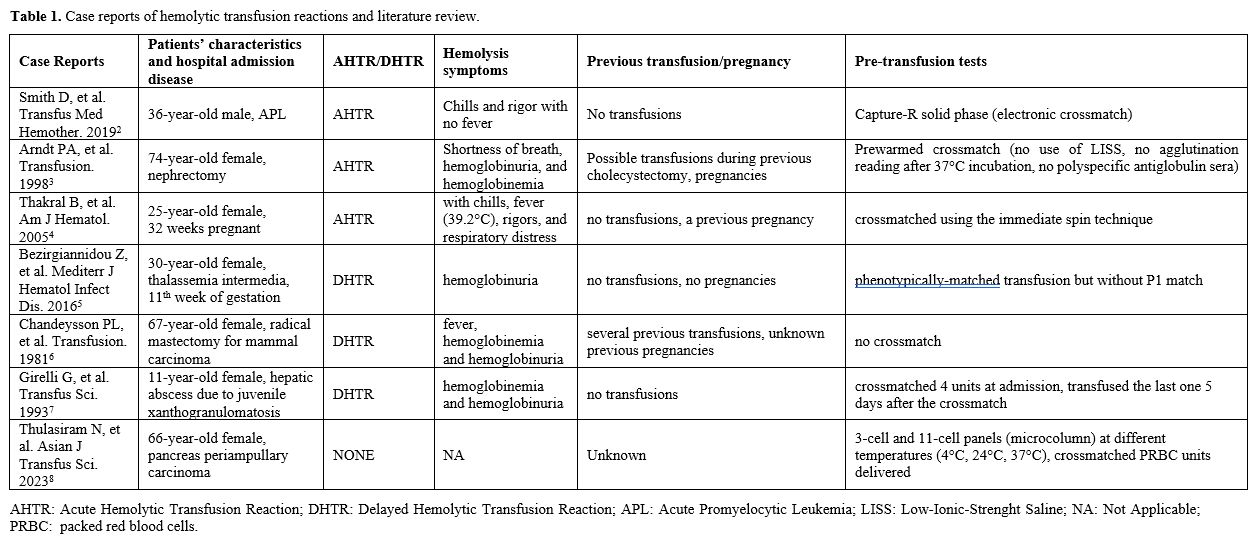
The anti-P1 antibody, often present in P2 individuals, is rarely reported as a cause of severe acute or delayed HTRs (Table 1).[2-8]
Recently, Smith et al.[2] reported a clinically mild acute HTR (AHTR) in a young man caused by an IgM anti-P1 antibody reactive at 37°C. The patient was transfused with 2 PRBC units, assigned through the capture-R solid phase (which does not detect IgM autoantibodies), and developed an AHTR. Routine antibody screening methods can lead to missing clinically significant IgM antibodies because they are designed to detect IgG antibodies.
Arndt et al.[3] reported an AHTR in a woman caused by an anti-P1 that reacted at 37°C. In this case, the antibody was not found before transfusion when a prewarmed crossmatch was done on 2 PRBC units. In fact, a low-ionic-strength saline (LISS) additive was not used, and a polyspecific antiglobulin serum was not added.
Thakral et al.[4] reported a case of a pregnant woman who developed an AHTR straight after the start of the transfusion of a PRBC unit. The Anti-P1 antibody was missed on immediate spin crossmatch and was then detected on extended incubation for half an hour.
The most recent delayed HTR (DHTR) was reported in 2016 by Bezirgiannidou et al.,[5] describing a case of a pregnant woman suffering from thalassemia intermedia in need of a transfusion of PRBC units. The patient was typed P1 negative, while her husband was P1 positive. She received a phenotypically matched transfusion (but not matched for P1 antigen). On day 19, during pre-transfusion tests, DAT resulted positive against anti-C3d, and IAT resulted positive against P1+ erythrocytes at 37°C, 22°C and 4°C showing the presence of an anti-P1 alloantibody (gel microcolumn tests). On day 29, she developed a DHTR. The extensive screening revealed the presence of an IgM autoantibody with anti-I specificity, demonstrating that the patient suffered from autoimmune hemolysis, manifesting as a DHTR followed by a cold agglutinin syndrome. Therefore, in thalassemic pregnant women, it would be useful to avoid PRBC units with antigens absent on maternal erythrocytes and present on paternal ones.
Two others severe DHTRs due to anti-P1 have been reported. The first was described in 1981 regarding a woman who developed a DHTR 48 hours after several PRBC unit transfusions, with evidence of an anti-P1 IgM with a wide thermal range. In this case, the PRBC units were delivered with no cross-matching,[6] and the second, reported by Girelli et al.,[7] regarded a child who underwent surgery. In this case, the anti-P1 antibody was detected at admission, reacting only at 22°C, and 4 PRBC units were crossmatched and resulted in compatibility. However, after 5 days, the patient needed a PRBC unit, and one of the units crossmatched five days before was transfused. Forty-eight hours after transfusion, she developed a DHTR, and an anti-P1 antibody, detected at room temperature and 37°C, was demonstrated. In this case, probably crossmatching the last unit before the second operation could have demonstrated incompatibility at 37°C.
A case of successful anti-P1 identification was reported by Thulasiram and colleagues[8] in a patient suffering from a pancreas periampullary carcinoma. Similarly to our case, they started investigating the presence of a cold alloantibody due to a reaction in the O cells of the indirect grouping. Therefore, they performed 3-cell and 11-cell panels (microcolumn) at different temperatures (4°C, 24°C, 37°C), having a strong reaction at 4 °C which would fade gradually while raising the temperature, confirming the presence of an IgM antibody. The patient proved to be phenotypically negative for the presence of P1 antigen, so they crossmatched P1 negative PRBC units, which were eventually transfused without any transfusion reactions.
In conclusion, we presented a case report in which we successfully prevented a possible HTR due to the antibody screening and the crossmatch, which allowed us to suspect the presence of a cold antibody interfering with the indirect group determination. From this observation, we performed an antibody screening and, subsequently, the identification panels at LT and 4°C. Moreover, the use of Reverse cards, which are richer in PEG gel compared to the Neutral cards (3% versus 1% respectively), permitted us to detect the cold alloantibody due to the enhancement of the reaction.
Based on our experience and analysis of methods used by several authors,[2-8] we would suggest suspecting the presence of a cold antibody, which in some cases could be clinically significant, every time there is an unexpected reaction in the control column of the indirect group and crossmatching PRBC units for such patients. We would additionally recommend that tests performed to identify the cold antibody be carried out also at LT and 4°C, preferring the use of Reverse cards; these are richer in PEG gel compared to the Neutral cards and, therefore, more sensitive in detecting the alloantibody. Furthermore, in the case of patients who require PRBC units to be matched phenotypically, such as individuals affected by hemoglobinopathies, we suggest studying P antigens and selecting P1-compatible PRBC units. Finally, in the case of pregnant patients, it would be convenient to avoid PRBC units with antigens absent on maternal erythrocytes and which are present on paternal ones.