A 31-year-old woman was admitted to our center with a recent history of symptomatic SARS CoV-2 infection, which occurred one week before, and fatigue. Initial laboratory tests revealed: white blood cells 61.10 × 109/L, hemoglobin level 7.4 g/dl, platelets 12 × 109/L, increased levels of serum ferritin (519 ng/mL; normal range, 8-252 ng/mL), lactate dehydrogenase (LDH; 1,107 U/l; normal range, 125–220 U/l), triglycerides (178 mg/dL; normal range, <150 md/dL), reduced level of fibrinogen (< 70 mg/dL) and increased INR (1.4).
Bone marrow aspirate at onset revealed a hypercellular marrow with 51% myeloid blasts. Karyotype analysis showed 45XX, monosomy 7, and ins (3;14), while FISH showed ins (3;14) and monosomy 7 in 90.5% of examined metaphases. On next-generation sequencing (NGS), KRAS mutation was detected in 51% of cells. NMP1, FLT3 ITD, BCR-ABL, RUNX/RUNX1T1, CBFB/MYH11, and PML RAR-alpha mutations were absent.
A diagnosis of AML with myelodysplasia-related cytogenetic abnormalities (WHO 2022) was made, and the patient was initially treated with daunorubicin (60 mg/m2 i.v, days 1-3) and cytosine arabinoside (200mg/m2, days 1–7). The main complications after induction were pneumonia, typhlitis, and subacute polyneuropathy. Bone marrow evaluation showed 8% of myeloid blasts, besides negativization of KRAS mutation on NGS.
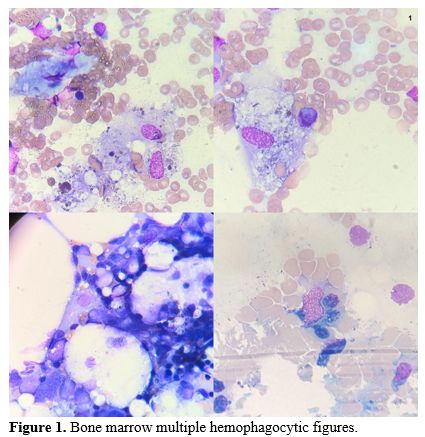
One month after induction started, the patient developed a progressive cognitive impairment. Noteworthy, concomitant laboratory examinations showed triglycerides 415 mg/dL, lactate dehydrogenase 239 U/L, serum ferritin 4009 ng/mL, and fibrinogen 74 mg/dL. Bone marrow examination performed due to persistent cytopenia (Hb 9.5 g/dL, WBC 200/MMC, neutrophils 0/mmc, platelets 15000/MMC) showed multiple hemophagocytic figures (Figure 1). Considering the clinical and laboratory findings, the patient was diagnosed with HLH according to the 2004 diagnostic guidelines for HLH (HScore 80-88% probability of hemophagocytic syndrome). Treatment with dexamethasone (40 mg/day) and etoposide was immediately started according to the HLH-2004 scheme with clinical and laboratory partial improvement. Infusion of immunoglobulins at a dose of 0.4 g/kg for five days was started due to concomitant acute/subacute polyneuropathy.
 |
|
After one further month, the patient once again developed progressive cognitive decline, with amnesia, slowed speech, and psychomotor agitation. Brain MRI showed increased T2/FLAIR signal intensity with associated restricted diffusion involving dorsomedial thalami, highly suggestive of Wernicke's encephalopathy. A high dose of thiamine (800 mg/day for two weeks) was promptly started with rapid improvement.
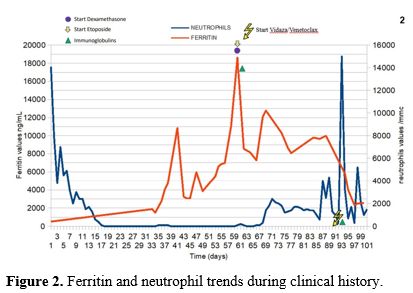
After two months and a half of medullary aplasia, a further bone marrow evaluation with osteomedullary biopsy (punctio sicca on bone marrow aspiration) showed 60% of CD34+ and MPO+ blasts (Karyotype: 46XX, FISH unchanged except for the absence of monosomy 7). Considering an ECOG Performance Status of 2-3 and the multiple therapy-related complications, second-line treatment with a hypomethylating agent and BCL-2 inhibitor (azacytidine 75 mg/m2 days 1-7 plus venetoclax 400 mg day 1-28) was started. After three days of therapy, a sudden improvement in blood values was observed with WBC > 2300/MMC (neutrophils count 1400/MMC), hemoglobin 11.4 g/dL, and platelets >20.000/MMC. Four weeks later, bone marrow evaluation showed complete remission and, above all, no figures of medullary HLH. At the same time, a progressive reduction of ferritin level was observed in the following months (Figure 2). The patient maintained the CR after four cycles of therapy and underwent haploidentical allogeneic hematopoietic stem cell transplantation after achieving minimal residual disease negativity. Unfortunately, an early FLT3 ITD-positive relapse was documented three months after transplantation, and she has recently failed salvage therapy with Gilteritinib.
 |
|
Azacitidine is known to have a variety of actions, such as direct cytotoxicity due to incorporation into DNA, silenced tumor suppressor genes re-activation by inhibition of DNA methyltransferase, and immunomodulation. Furthermore, the restoration of aberrant T cell receptor repertoire pattern has been described in AML and MDS patients.[3] Azacitidine was also found to restore the impaired cytotoxic activity of NK cells in MDS patients.[3] In the present case, after therapy with azacitidine and venetoclax, we observed not only a rapid response of AML but, above all, a dramatic clinical and laboratory resolution of HLH. Such a clinical pattern would suggest that this therapy could have been therapeutically effective on HLH not only due to its direct cytotoxic effects against AML clones but also indirectly in the context of an overall immune restoration.[4]
We also highlight that the patient presented with SARS CoV-2 infection almost concomitantly with AML diagnosis, and it may be interesting to speculate about a possible influence of the infection on the occurrence of HLH. In fact, secondary HLH has been described in SARS-CoV-2 recovered patients, in which dysregulation of the immune system driven by the infection may be a possible cause of HLH, furthermore linked with significant mortality.[5,6] A latent state and a subclinical persistent inflammation related to macrophage activation and modulation, with the presence in medullary samples of polyclonal CD3+, CD20+, plasmacytes, and histiocytes, may be implicated in this clinical association.[7,8]
This case suggests that a therapeutic approach based on azacitidine plus venetoclax can be effective against AML-associated HLH, likely by acting directly on leukemia clones and also by remodeling the immune system as well as the intramedullary inflammatory process. Further studies are necessary to confirm this clinical suggestion and elucidate the molecular and immune pathways underlying such a therapeutic efficacy.