Abdulrahman Al Raizah1, Fakhr Alayoubi2, Galal Hassan Abdelnaby3, Hazzaa Alzahrani4, Majid Farraj Bakheet5, Mohammed A Alskaini6, Rasha Buhumaid7, Sameer Al Awadhi8, Sara Nooruddin Kazim9, Thiagarajan Jaiganesh10, Mohamed Hamdy Hussein Naguib14 and Zohair Al Aseri11,12,13.
1 Division of
Adult Hematology, Department of Oncology, King Abdulaziz Medical City,
Ministry of National Guard Health Affairs, PO Box. 22490, 11426,
Riyadh, Saudi Arabia.
2 King Saud University, Riyadh, Saudi Arabia.
3 Alqassimi Hospital, United Arab Emirates.
4 King Faisal Specialist Hospital and Research Centre, Riyadh, Saudi Arabia.
5 Neurology Department, King Abdullah Medical City, Mecca, Saudi Arabia.
6 Department of Neurology, Prince Sultan Military Medical City, Riyadh, Saudi Arabia.
7 Mohammed Bin Rashid University of Medicine and Health Science, Dubai, United Arab Emirates.
8 Digestive Diseases Unit, Rashid Hospital, Dubai, United Arab Emirates.
9 Department of Emergency Medicine, Rashid Hospital and Trauma Centre, Dubai Health Authority, Dubai, United Arab Emirates.
10 Emergency Department, Tawam Hospital, Al Ain, United Arab Emirates.
11 Department Emergency Medicine and Critical Care, College of Medicine, King Saud University, Riyadh, Saudi Arabia.
12 Department of Clinical Sciences, College of Medicine and Riyadh Hospital, Dar Al Uloom University, Riyadh, Saudi Arabia.
13 Therapeutic Deputyship, Ministry of Health, Riyadh, Saudi Arabia.
14 AstraZeneca, Saudi Arabia.
Correspondence to:
Zohair Al Aseri, FRCPC EM & CCM. Department Emergency Medicine and
Critical Care, College of Medicine, King Saud University, Riyadh, Saudi
Arabia. Department of Clinical Sciences, College of Medicine and Riyadh
Hospital, Dar Al Uloom University, Riyadh, Saudi Arabia. Therapeutic
Deputyship, Ministry of Health, Riyadh, Saudi Arabia. E-mail:
Alaserizohair@gmail.com ORCID:
http://orcid.com/0000-0001-9869-7544
Published: May 01, 2024
Received: December 13, 2023
Accepted: April 13, 2024
Mediterr J Hematol Infect Dis 2024, 16(1): e2024038 DOI
10.4084/MJHID.2024.038
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background: The
nonvitamin K antagonist oral anticoagulants (NOACs) have become the
mainstay anticoagulation therapy for patients requiring oral
anticoagulants (OACs) in the Gulf Council Cooperation (GCC) countries.
The frequency of NOAC-associated major bleeding is expected to increase
in the Emergency Department (ED). Nonetheless, we still lack local
guidelines and recommendations for bleeding management in the region.
The present Delphi-based consensus aims to establish a standardized and
evidence-based clinical care pathway for managing NOAC-associated major
bleeding in the Kingdom of Saudi Arabia (KSA) and the United Arab
Emirates (UAE).
Methods: We
adopted a three-step modified Delphi method to develop evidence-based
recommendations through two voting rounds and an advisory meeting
between the two rounds. A panel of 11 experts from the KSA and UAE
participated in the consensus development.
Results: Twenty-eight
statements reached the consensus level. These statements addressed key
aspects of managing major bleeding events associated with NOACs,
including the increased use of NOAC in clinical practice, clinical care
pathways, and treatment options.
Conclusion: The
present Delphi consensus provides evidence-based recommendations and
protocols for the management of NOAC-associated bleeding in the region.
Patients with major DOAC-induced bleeding should be referred to a
well-equipped ED with standardized management protocols. A
multidisciplinary approach is recommended for establishing the
association between NOAC use and major bleeding. Treating physicians
should have prompt access to specific reversal agents to optimize
patient outcomes. Real-world evidence and national guidelines are
needed to aid all stakeholders involved in NOAC-induced bleeding
management.
|
Introduction
The
nonvitamin K antagonist oral anticoagulants (NOACs) have become the
mainstay anticoagulation therapy for patients requiring oral
anticoagulants (OACs).[1,2] Current guidelines
recommend the use of NOACs for a wide range of conditions, including
non-valvular atrial fibrillation (NVAF), recurrent deep vein thrombosis
(DVT), pulmonary embolism (PE), and cancer-associated thrombosis.[2,3]
Despite their advantages, NOACs are not risk-free medications. A pooled
analysis observed that the prevalence of major bleeding in patients on
NOACs ranged from 1.6 to 3.6 per 100 patient-years,[4] which can lead to massive transfusion, irreversible neurological complications, hospitalization, death,[5,6] and increased healthcare resource utilization (HCRU).[7,8]
The
management of NOAC-associated major bleeding is based on restoring
hemodynamic stability and reversing hemostasis to normal functions
before the reintroduction of NOACs.[9] Pivotal clinical trials
confirmed the effectiveness of specific reversal agents in restoring
factor Xa or direct thrombin activity in patients with life-threatening
bleeding.[10,11] Several guidelines recommend
specific reversal agents over PCC for NOAC users with life-threatening
bleeding or bleeding at critical sites.[12,13]
The
Gulf Cooperation Council (GCC) countries have experienced significant
transformations in their healthcare systems over the past few decades.
Recent reports indicated a notable increase in the use of NOACs, driven
by the increased prevalence of NVAF, DVT, and other conditions
requiring OACs.[14,15] Data from the GCC countries
showed that rivaroxaban and apixaban were the most prescribed OACs for
NVAF patients in the region.[16-19] As the use of
NOACs continues to rise in the GCC countries, the absolute number of
patients who experience major bleeding events while on these
medications is also expected to rise. Single-center reports from the
GCC countries showed that the rate of major bleeding in patients
receiving apixaban was 2.8%.[20] Despite this burden, we still lack local guidelines and recommendations for bleeding management in the GCC region.
The
present Delphi-based consensus aims to establish a standardized and
evidence-based clinical care pathway for the management of
NOAC-associated major bleeding in the Kingdom of Saudi Arabia (KSA) and
the United Arab Emirates (UAE). This consensus gathered recommendations
from regional experts to tailor the management pathway to the specific
healthcare context of KSA and UAE, considering the local clinical
practices.
Methodology
Study Design and Panel Recruitment.
We adopted a three-step modified Delphi method to recruit a panel of 11
experts from KSA and UAE. The experts were selected using a
non-probability convenient selection process to ensure a geographical
representation of major academic institutions in the GCC region. The
panel of experts comprised consultants with diverse and complementary
expertise, ensuring a comprehensive and multidisciplinary approach to
the consensus process. The expert panel included hematologists,
neurologists, emergency medicine physicians, and clinical pharmacists.
All experts participated voluntarily and were required to sign a
disclosure statement before consensus development.
Literature Review and Statements Development.
The survey development committee conducted a comprehensive search of
electronic databases, including PubMed, Embase, and Cochrane Library,
to retrieve relevant guidelines, consensus, and systematic reviews
about the management of NOAC-associated bleeding. The literature search
was performed using the following terms: ("dabigatran," "rivaroxaban,"
"apixaban," "edoxaban," OR "nonvitamin K antagonist oral anticoagulant"
OR "direct oral anticoagulant" OR "novel oral anticoagulant" OR "NOAC"
OR "DOAC" OR "Vitamin K antagonists" OR "VKA" OR "warfarin" OR
"dicoumarol" OR "acenocoumarol" OR "Coumadin") AND ("bleeding" OR
"major bleeding" OR "hemorrhage" OR "intracranial bleeding" OR "adverse
events") AND ("management" OR "prothrombin complex concentrate" OR
"Andexanet alfa" OR "Idarucizumab"). A secondary search was conducted
using the abovementioned keywords in combination with the following
GCC-related keywords to retrieve relevant citations from the GCC
region: ("Gulf Council" OR "Gulf Council Cooperation" OR "GCC" OR
"Saudi Arabia" OR "Kuwait" OR "United Arab Emirates" OR "Qatar" OR
"Bahrain" OR "Oman").
The search was supplemented by screening
relevant publications from the hematology, cardiology, or stroke
journals based in one of the GCC or Middle East regions, such as the
Saudi Heart Journal, Journal of the Saudi Heart Association, Journal of
Applied Hematology, Saudi Medical Journal, Oman Medical Journal, and
Dubai Medical Journal. There was no language, year of publications, or
country-specific restrictions on the literature search. Data were
retrieved only from level 1 quality of evidence, as classified by
Wright et al.[21]
Based on the findings of the literature review,
an initial set of draft statements was developed for the first round of
voting. These statements addressed key aspects of managing major
bleeding events associated with NOACs, including the increased use of
NOAC in clinical practice, clinical care pathways, and treatment
options.
Delphi Process and Consensus Development.
The Delphi process consisted of two rounds of voting and an advisory
meeting. In the first round, the initial set of statements was
distributed to the panel of experts. Experts were asked to vote on each
statement (agree/disagree) and were encouraged to provide comments and
additional insights. A consensus was defined as an agreement level ≥
75%.[22] After the first round, the responses were collated and
analyzed. A summary of the results, along with anonymized comments from
the experts, was distributed to the panel during an advisory meeting.
Based on the feedback received during the meeting, the statements that
did not reach the consensus levels were restructured for the second
round of voting. The revised statements and questions were
redistributed to the experts in this round. They were asked to review
the changes and rate their level of agreement with the revised
statements.
All panel members reviewed and approved the final consensus statements and manuscript.
Results and Discussion
Initially,
29 statements were developed by the survey development committee and
were emailed to the experts for the first voting round. Of them, ten
statements reached the consensus level, eight were rephrased without
second-round voting, and one was removed. The remaining ten statements
were rephrased during the expert meeting and reached the consensus
level after the second round of voting. Thus, the present consensus was
composed of 28 statements.
I. Trends of NOAC utilization and incidence of major bleeding:
Three statements about the trend of NOAC use in the GCC countries reached the consensus level (Table 1).
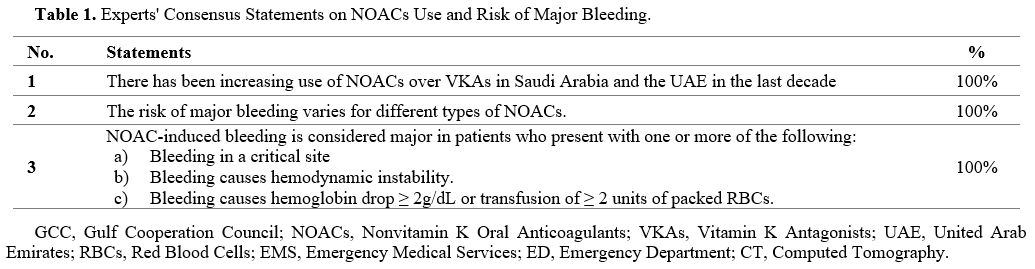 |
- Table 1. Experts' Consensus Statements on NOACs Use and Risk of Major Bleeding.
|
The
last decade has witnessed a significant shift in the OACs landscape,
characterized by a growing preference for NOACs over traditional VKAs.
The experts agreed that the utilization of NOACs over VKAs has
significantly increased in the GCC over the last decade (Statement 1).
This decision runs in line with global statistics showing a significant
increase in NOAC utilization rate from 5% to nearly 30-48% over the
past 15 years.[23,24] In the GCC region, published data indicated a dramatic increase in NOAC utilization since 2013 (Figure 1).[17-19,25-28]
 |
- Figure 1. Rates of OACs Utilization in Real-world Practice in the GCC Countries.
|
Several
reasons can explain the trends of increased NOAC use in the GCC region.
One of the primary drivers of this shift has been the pharmacological
advantages that NOACs offer over VKAs. In addition, the increasing
prevalence of conditions such as AF and VTE, which are key indications
for OACs, has also likely contributed to the growing use of NOACs.[14,29] The patient population in the GCC countries is diverse, with varying comorbidities, age groups, and risk factors,[30,31] which increase the probability of receiving prophylactic OACs.
As
the use of NOACs continues to rise in the GCC countries, emergency
departments (EDs) are anticipated to encounter cases of NOAC-associated
major bleeding increasingly. In previous retrospective studies from
Saudi Arabia, the overall rate of major bleeding ranged from 1.1% to
3.9% in NVAF and VTE patients receiving rivaroxaban or apixaban, while
the rate of fatal bleeding was 0.2%.[20,32,33]
Another study showed that the rate of intracranial hemorrhage (ICH) in
patients receiving rivaroxaban in Saudi Arabia was 0.58%.[34]
Therefore, the healthcare system must anticipate and prepare for the
increased encounters of NOAC-associated major bleeding in the GCC
region by adopting local recommendations and guidelines for clinical
care pathways and management of NOAC-associated major bleeding.
The type and indication of NOACs, as well as patient-specific factors, appear to play a role in the risk of bleeding.[35] The experts agreed that the risk of major bleeding varies for different types of NOACs in clinical practice (Statement 2).
Thus, clinicians must consider these differences, along with
patient-specific factors (e.g., renal function, concomitant
medications, and bleeding history), when selecting the most appropriate
anticoagulant for each patient.
Despite the growing number of published guidelines,[36-39]
there is no universal agreement on the definition of major bleeding.
The experts agreed on adopting the definition of the 2020 American
College of Cardiology (ACC) consensus.[12] The ACC
consensus defines NOAC-associated major bleeding as bleeding that
fulfills one or more of the following: bleeding in a critical site;
bleeding causes hemodynamic instability; and/or bleeding that causes
hemoglobin drop ≥ 2g/dL or transfusion of ≥ 2 units of packed red blood
cells (Statement 3).
II. Clinical Care Pathway:
Eight statements about the clinical care pathway for the management of NOAC-associated bleeding reached the consensus level (Table 2).
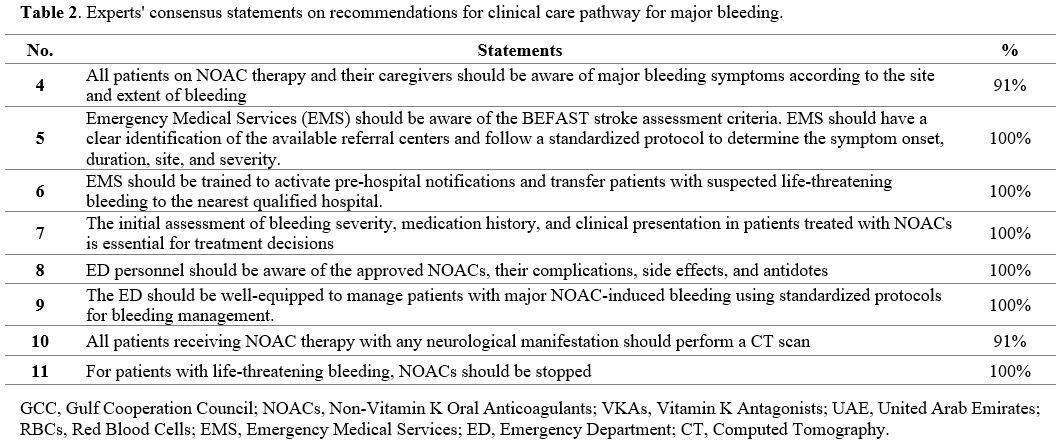 |
- Table 2. Experts' consensus statements on recommendations for clinical care pathway for major bleeding.
|
The identification of early signs of major bleeding can positively impact the clinical care pathway and patient outcome.[40,41]
Thus, educating patients and their caregivers about the signs and
symptoms of major bleeding can empower them to recognize potentially
life-threatening events early and seek timely medical intervention (Statement 4).
Several
simple and easy-to-use scores have been developed to identify early
signs of stroke and ensure rapid access to medical care.[42]
The BEFAST (Balance, Eyes, Face, Arm, Speech, Time) criteria were
developed to improve the diagnostic accuracy of the original FAST tool
in patients with signs of a stroke.[43] In the GCC
setting, when paramedics recognize stroke or major bleeding using
BEFAST criteria, they are required to activate pre-hospital
notifications and transfer patients to the nearest qualified hospital.
The decision to use the specific reversal agents then depends on the
presence of an emergency medicine consultant or a hematologist (Figure 2).
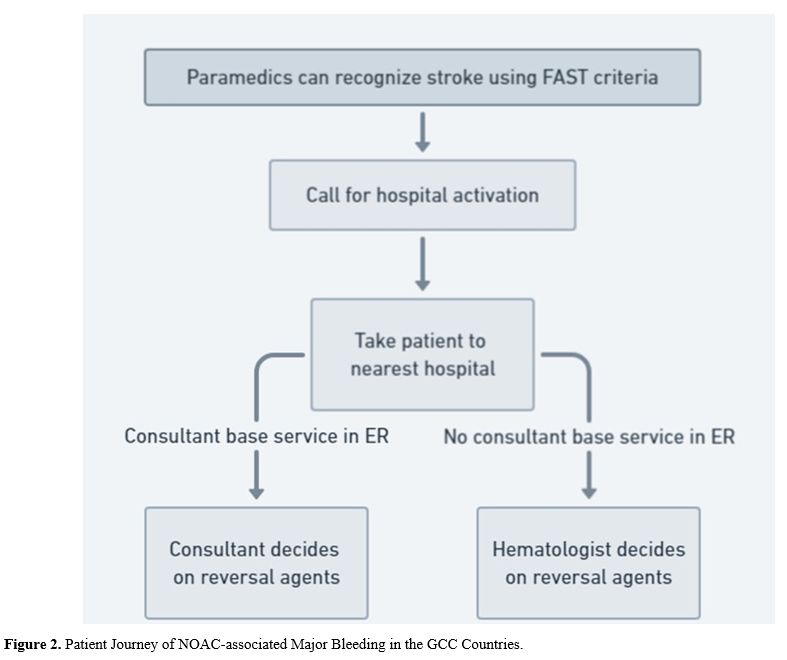 |
- Figure 2. Patient Journey of NOAC-associated Major Bleeding in the GCC Countries.
|
Thus,
the experts agreed that the Emergency Medical Services (EMS) staff
should be aware of the BEFAST stroke assessment criteria. The EMS
should Identify the available referral centers clearly and follow a
standardized protocol to determine the symptom onset, duration, site,
and severity (Statement 5).
The EMS staff should be trained to activate pre-hospital notifications
and transfer patients with suspected life-threatening bleeding to the
nearest qualified hospital (Statement 6).
The
initial assessment of bleeding severity, medication history, and
clinical presentation in patients treated with NOACs is essential for
treatment decisions (Statement 7).
The current international guidelines indicate that the management of
bleeding complications depends primarily on the severity and location
of the bleeding.[12,13] Thus, the ED personnel should be aware of the approved NOACs, their complications, side effects, and antidotes (Statement 8).
Alongside
the initial assessment, several laboratory measures have been
recommended to assess patients with suspected NOAC-associated bleeding,
mainly the coagulation profile, including prothrombin time (PT),
activated partial thromboplastin time (aPTT), and fibrinogen level.[44] However, the PT and aPTT are limited when used for qualitative assessment of NOAC activities.[45]
Previous reports suggested that PT and aPPT prolongation is not
exclusively associated with NOAC over-dosage and may lead to misleading
results.[46-48] In contrast, certain conditions may be associated with normal limits despite elevated NOAC concentrations.[49] Thus, it has been previously advocated that PT and aPTT should not be used alone.[50]
Quantitative
assessment of NOAC levels is crucial to determine the serum
concentration of the NOAC. Specialized assays, such as the anti-factor
Xa assay (for rivaroxaban and apixaban) and the diluted thrombin time
(dTT) or ecarin clotting time (ECT) for dabigatran, can be used to
measure the specific NOAC levels.[51-53] One of the
critical advantages of measuring NOAC plasma concentrations is the
ability to ascertain whether bleeding events are attributable to
over-dosage, and that is particularly relevant in acute settings where
rapid decision-making is crucial. Establishing the presence of
excessive anticoagulation can guide the administration of specific
reversal agents, ensuring targeted and efficient management of bleeding
complications.[49,54] Moreover,
patients experiencing recurrent thrombosis while on NOAC therapy may
benefit from plasma concentration testing to evaluate under-dosage or
inadequate anticoagulation.[55] While not routinely
recommended, adjusting the dosage based on plasma levels could be
considered in such cases to optimize anticoagulation and prevent
further thrombotic events. Additional clinical scenarios where NOAC
plasma concentration testing is valuable include before initiating
treatment, in preparation for surgical or invasive procedures, and
before thrombolytic therapy for acute ischemic stroke.[50] Qualitative assays can also provide information on the NOAC activity in the case of normal PT or aPTT.[56] Plasma NOAC assays are also commercially available and easy to operate, with comparable results between laboratories.[50]
Despite
the benefits and availability of NOAC plasma concentration testing, a
notable barrier to its widespread adoption is physician reluctance.
This hesitance may stem from a lack of familiarity with the tests,
uncertainty about interpreting results, or concerns over the impact of
testing on clinical workflow.[12] Addressing these
concerns through education and evidence-based guidelines could enhance
the utilization of NOAC testing in appropriate clinical scenarios.
Currently,
there is significant variability in the laboratory assessment of
patients with NOAC-associated major bleeding. Different institutions
may have different protocols, and not all laboratories have the
capacity to perform specialized NOAC assays. This lack of
standardization can lead to variability in patient care and may affect
outcomes. Therefore, the ED should be well-equipped with standardized
protocols to manage patients with major NOAC-induced bleeding (Statement 9).
All patients receiving NOAC therapy with any neurological manifestation should perform a computed tomography (CT) scan (Statement 10). For patients with life-threatening bleeding, NOACs should be stopped (Statement 11).
III. Management of NOAC-associated Major Bleeding:
In
the present consensus, 13 recommendations were developed regarding
management approaches for patients with NOAC-associated major bleeding (Table 3).
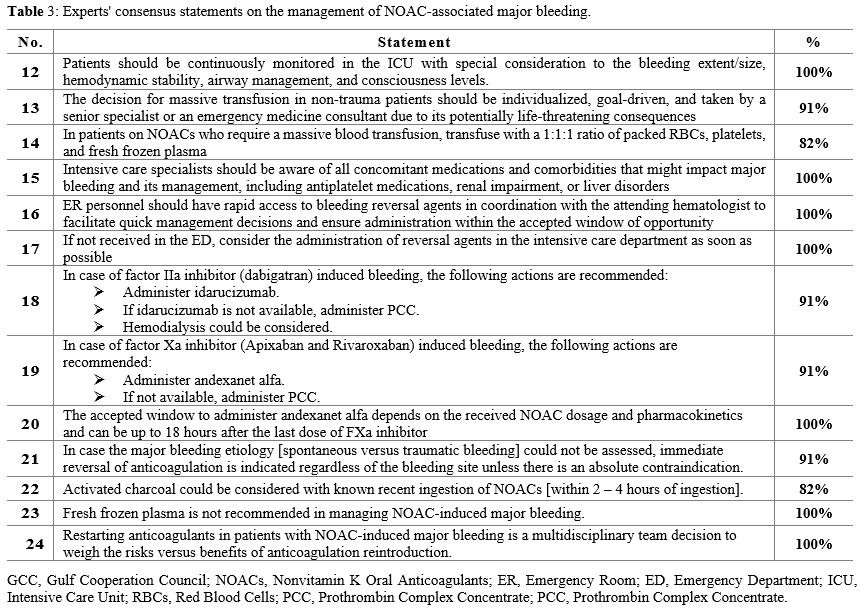 |
- Table 3. Experts' consensus statements on the management of NOAC-associated major bleeding.
|
The
current guidelines emphasize the importance of a structured and
systematic approach to the management of NOAC-associated bleeding. The
initial management step is to stabilize the patient's hemodynamics in
case of hemodynamic instability.[57-60] The experts
agreed that patients with NOAC-associated bleeding should be
continuously monitored in the intensive care unit (ICU) with special
consideration to the bleeding extent/size, hemodynamic stability,
airway management, and consciousness level (Statement 12).
For patients with severe or life-threatening bleeding, activation of a massive transfusion protocol may be necessary.[61]
However, previous trials demonstrated better survival and lower risk of
recurrent bleeding with restrictive rather than massive transfusion.[62]
Additionally, massive transfusion can be associated with
life-threatening consequences, such as coagulopathy,
transfusion-related acute lung injury, and transfusion-associated
circulatory overload.[63] The decision for massive
transfusion in NOAC-associated major bleeding should be individualized
in patients on NOACs who require a massive blood transfusion with a
1:1:1 ratio of packed RBCs, platelets, and fresh frozen plasma (FFP) (Statements 13 and 14).
In
the setting of NOAC-associated bleeding, a comprehensive understanding
of concomitant medications and comorbidities is essential. Concurrent
use of antiplatelet medications with DOACs can significantly increase
the risk of bleeding events.[64] Renal impairment is
another critical consideration, as most NOACs, particularly dabigatran,
are partially excreted by the kidneys; thus, renal dysfunction can lead
to longer half-lives and an increased risk of bleeding.[65]
In case of renal impairment, desmopressin acetate or cryoprecipitate
may be beneficial in correcting uremia-associated platelet dysfunction.[66,67]
Liver disorders, often associated with coagulopathy due to decreased
synthesis of clotting factors, can further complicate the bleeding risk
and the approach to reversal and hemostasis.[12] Therefore, intensive care specialists must thoroughly know the patient's medication profile and comorbid conditions (Statement 15).
The
introduction of specific reversal agents has significantly improved the
outcomes of bleeding complications of NOACs and provided a new horizon
for better utilization of NOACs in clinical practice. Idarucizumab is a
humanized monoclonal antibody fragment (Fab) that binds specifically
and with high affinity to dabigatran, neutralizing its anticoagulant
effect.[68,69] The pivotal REVERSE AD trial showed
that idarucizumab effectively restored normal hemostatic activity in 88
to 98% of the patients with life-threatening/uncontrolled bleeding due
to dabigatran. Similar findings were in patients who needed urgent
surgery.[70] In 2018, the FDA fully approved
idarucizumab as the first reversal agent for restoring hemostasis in
dabigatran users with life-threatening/uncontrolled bleeding or needing
urgent surgery.[71]
Andexanet alfa is a
recombinant modified human factor Xa protein that acts as a decoy
receptor for factor Xa inhibitors. It binds to these drugs, reducing
their ability to inhibit endogenous factor Xa and reversing their
anticoagulant effects.[72] In the ANNEXA-A and
ANNEXA-R studies, which evaluated and examined alfa in healthy
volunteers, anti-factor Xa activity was reduced by 92-94%, and the
thrombin generation was restored in 100% of the patients.[73]
In the pivotal phase III/IV ANNEXA-4 trial, which recruited patients
with acute major bleeding due to apixaban or rivaroxaban, Overall, 82%
of the patients had adequate restoration of homeostasis within 12
hours, while the median reduction in the anti-factor Xa activity was
92% within 18 hours. Within 30 days of follow-up, 10% of the patients
had thromboembolic events. The overall mortality rate was 14%.[11]
Based on these findings, andexanet alfa was granted accelerated
approval by the FDA in 2018 as the first specific reversal agent for
apixaban and rivaroxaban-treated patients.[74]
The
experts agreed that, in the case of NOAC-induced life-threatening
bleeding, specific reversal agents are recommended. Thus, ER personnel
should have rapid access to these agents in coordination with the
attending hematologist to facilitate quick management decisions and
ensure administration within the accepted window of opportunity. If not
received in the ED, reversal agents should be administered in the ICU
as soon as possible (Statements 16 and 17).
Idarucizumab and andexanet alfa are recommended in patients with
life-threatening bleeding due to factor IIa and Xa inhibitors,
respectively (Statements 18 and 19).
The accepted window to administer and examine alfa depends on the NOAC
dosage received and pharmacokinetics and can be up to 18 hours after
the last dose of the FXa inhibitor (Statement 20). Patients on dabigatran, especially those with renal insufficiency, may benefit from hemodialysis;[12] thus, hemodialysis could be considered (Statement 18).
In case the major bleeding etiology [spontaneous versus traumatic
bleeding] could not be assessed, immediate reversal of anticoagulation
is indicated regardless of the bleeding site unless there is an
absolute contraindication (Statement 21).
Measuring
plasma concentrations of NOAC before the administration of a specific
reversal agent should be considered whenever possible.[50]
Although the pivotal clinical trials evaluating the reversal agents did
not include the measurement of NOAC concentrations before
administrating the reversal agents, post-hoc analyses of registration
trials for idarucizumab and andexanet alfa revealed that approximately
30% of patients treated had relatively low NOAC concentrations at the
time of antidote administration.[70,75]
These findings raise important questions about the necessity and
efficacy of administering antidotes without significant NOAC levels,
suggesting that some patients may receive treatment without a clear
pharmacological need. Measuring NOAC levels before administering
antidotes could help identify patients who would most benefit from
reversal agents, thereby enhancing the clinical value of antidote
administration and preventing unnecessary use.[50]
However, in the case of life-threatening bleeding, point-of-care NOAC
assessment can be used. The NOAC assays require standardization and
calibration for specific NOACs, and the performance and interpretation
of these tests require a specialist.[76] Hence, most
of the guidelines highlighted the importance of the clinical history of
the patient and only using the test of NOACs if the test is available
and the results will be ready within less than 20 minutes.[77]
When
specific reversal agents are not available, 4-factor PCC, which
contains the vitamin K-dependent coagulation factors II, VII, IX, and
X, can be used to reverse the effects of NOACs (Statements 18 and 19).
Observation studies showed that 4-factor PCC restored the hemostatic
efficacy in nearly two-thirds of the patients with major bleeding due
to apixaban or rivaroxaban.[78,79] In a recent
meta-analysis, it was concluded that it is difficult to determine
whether 4F-PCC, in addition to cessation of direct oral FXa inhibitor,
is more effective than cessation of direct oral FXa inhibitor alone in
patients with direct FXa inhibitor-related major bleeding.[80]
Activated
charcoal is a highly porous substance with a large surface area that
can bind to various drugs and toxins, thereby reducing systemic
absorption. In the context of NOAC-associated bleeding, activated
charcoal is considered a potential intervention to limit further drug
absorption, especially when the NOAC has been recently ingested.[81] Activated charcoal could be considered with known recent (2-4 hours) ingestion of NOACs (Statement 22). On the other hand, FFP is not recommended due to the lack of supporting evidence and the potential risk of transfusion (Statement 23).
A
growing number of studies indicated that NOAC stoppage increased the
risk of ischemic events in NVAF patients who experienced major
bleeding.[82] Restarting NOAC demonstrated safety and feasibility without increasing the risk of future bleeding.[83] Notably, restarting NOAC was found to be associated with a reduced risk of long-term disability.[84]
Nonetheless, limited evidence is available regarding the factors that
guide NOAC restarting decision. For instance, conflicting results exist
regarding the impact of ICH location (lobar versus subarachnoid), the
presence of aneurysm or hematoma, and patient-specific factors (e.g.,
age, history of thromboembolic events) on the recurrence risk after
resuming NOACs.[85-88] The experts agreed that
restarting anticoagulants in patients with NOAC-induced major bleeding
requires a multidisciplinary team to weigh the risks versus benefits of
anticoagulation reintroduction (Statement 24).
IV. Unmet Medical Needs in the GCC Countries:
Four statements about the recommendations to address the current gaps in NOAC-associated bleeding reached the consensus level (Table 4).
 |
- Table 4. Experts' consensus statements on the unmet needs for the management of NOAC-associated major bleeding.
|
The
experts agreed on the importance of conducting real-world studies in
the GCC to understand the characteristics and outcomes of
NOAC-associated major bleeding in the region (Statement 25).
Local real-world evidence is vital in the GCC countries, where
healthcare systems and patient demographics may differ significantly
from those in Western countries.[43] Such evidence
can provide insights into patient adherence to NOACs, the incidence and
management of major bleeding events, and patient outcomes following
such events. It can also help identify potential gaps in healthcare
services, such as the need for more widespread availability of reversal
agents or more extensive education on NOAC use and bleeding management.[89,90]
The
experts agreed on the need for standardized definitions of major and
life-threatening bleeding in the GCC setting to allow proper assessment
and standardized treatment plans (Statement 26).
The lack of standardized definitions for major and life-threatening
bleeding can lead to variability in clinical practice, making it
challenging to compare data across different studies or healthcare
settings.[91] A standardized definition would enable
clinicians in the GCC countries to assess the severity of bleeding
events uniformly, guide treatment decisions, and allow for more
meaningful data comparisons across different healthcare institutions.
This standardization could, in turn, lead to more standardized and
evidence-based treatment plans for patients experiencing
NOAC-associated major bleeding.[92]
In addition,
national and institutional guidelines are needed to aid all
stakeholders involved in NOAC-associated bleeding management (Statement 27).
The development of national and institutional guidelines in the GCC
countries can provide an evidence-based and standardized approach to
managing NOAC-associated bleeding.
The experts also agreed that
cross-specialty collaboration can improve communication, decisions, and
outcomes of patients with NOAC-associated bleeding (Statement 28).
The management of NOAC-associated major bleeding is complex and often
requires the involvement of multiple healthcare professionals,
including emergency physicians, hematologists, intensivists, and
pharmacists. In the GCC context, fostering cross-specialty
collaboration is essential. Such collaboration can facilitate more
effective communication among healthcare professionals, leading to
quicker and more informed decision-making. It can also promote a more
holistic approach to patient care, considering all aspects of a
patient's condition and treatment needs.[93,94]
Conclusions
In
conclusion, the use of NOACs continues to rise in the GCC countries,
and EDs are anticipated to encounter cases of NOAC-associated major
bleeding increasingly. Therefore, it is imperative for the healthcare
system to anticipate and prepare for the increased encounters of
NOAC-associated major bleeding in the GCC region by adopting local
recommendations and guidelines for clinical care pathways and
management of NOAC-associated major bleeding. The present Delphi
consensus provided evidence-based recommendations and protocols for the
management of NOAC-associated bleeding in the region. Patients with
major DOAC-induced bleeding should be referred to well-equipped EDs
with standardized management protocols. A multidisciplinary approach is
recommended for establishing the association between NOAC use and major
bleeding. Treating physicians should have rapid access to specific
reversal agents to optimize patient outcomes. Real-world evidence and
national guidelines are needed to aid all stakeholders involved in
NOAC-associated bleeding management.
References
- Vinogradova Y, Coupland C, Hill T, Hippisley-Cox J.
Risks and benefits of direct oral anticoagulants versus warfarin in a
real world setting: cohort study in primary care. BMJ. 2018;362:k2505. https://doi.org/10.1136/bmj.k2505 PMid:29973392 PMCid:PMC6031213
- Paul
C, Baby M, Anthraper AR, K K. NOACs: an emerging class of oral
anticoagulants-a review article. Futur J Pharm Sci 2020 61 [Internet].
2020 Nov 24 [cited 2022 Dec 4];6(1):1-7. https://doi.org/10.1186/s43094-020-00114-1
- Laslo
CL, Bacalbasa N, Stanescu AMA, Carsote M, Bungau S, Rus M, et al. New
oral anticoagulants ‑ possible extension to other indications (Review).
Exp Ther Med [Internet]. 2020 Sep 1 [cited 2022 Dec 4];20(3):2401-5.
Available from: http://www.spandidos-publications.com/10.3892/etm.2020.8713/abstract
- Niessner
A, Tamargo J, Morais J, Koller L, Wassmann S, Husted SE, et al.
Reversal strategies for non-vitamin K antagonist oral anticoagulants: a
critical appraisal of available evidence and recommendations for
clinical management-a joint position paper of the European Society of
Cardiology Working Group on Cardiovascular Pharmacotherapy and European
Society of Cardiology Working Group on Thrombosis. Eur Heart J
[Internet]. 2017 Jun 7 [cited 2022 Dec 5];38(22):1710-6. Available
from: https://pubmed.ncbi.nlm.nih.gov/26705385/
- Undas
A, Drabik L, Potpara T. Bleeding in anticoagulated patients with atrial
fibrillation: Practical considerations. Vol. 130, Polish Archives of
Internal Medicine. 2020. p. 47-58. https://doi.org/10.20452/pamw.15136
- Kawada
T. Vitamin K and direct oral anticoagulants in patients with major
bleeding: Risk assessment. Vol. 235, International Journal of
Cardiology. 2017. p. 199. https://doi.org/10.1016/j.ijcard.2017.01.029 PMid:28342498
- Deitelzweig
SB, Lovelace B, Christoph M, Lingohr-Smith M, Lin J, Fermann GJ.
Evaluation of the Incremental Healthcare Economic Burden of Patients
with Atrial Fibrillation Treated with Direct-Acting Oral Anticoagulants
and Hospitalized for Major Bleeds in the USA. Adv Ther [Internet]. 2020
Sep 1 [cited 2022 Dec 5];37(9):3942-53. https://doi.org/10.1007/s12325-020-01440-9 PMid:32699994
- Deitelzweig
S, Neuman WR, Lingohr-Smith M, Menges B, Lin J. Incremental economic
burden associated with major bleeding among atrial fibrillation
patients treated with factor Xa inhibitors. J Med Econ [Internet]. 2017
Dec 2 [cited 2022 Dec 5];20(12):1217-23. https://doi.org/10.1080/13696998.2017.1362412 PMid:28760063
- Escobar
A, Salem AM, Dickson K, Johnson TN, Burk KJ, Bashoura L, et al.
Anticoagulation and bleeding in the cancer patient. Support Care
Cancer. 2022;30(10):8547-57. https://doi.org/10.1007/s00520-022-07136-w PMid:35579752 PMCid:PMC9529787
- Pollack
C V., Reilly PA, van Ryn J, Eikelboom JW, Glund S, Bernstein RA, et al.
Idarucizumab for Dabigatran Reversal - Full Cohort Analysis. N Engl J
Med [Internet]. 2017 Aug 3 [cited 2023 Jan 23];377(5):431-41. https://doi.org/10.1056/NEJMoa1707278 PMid:28693366
- Connolly
SJ, Crowther M, Eikelboom JW, Gibson CM, Curnutte JT, Lawrence JH, et
al. Full Study Report of Andexanet Alfa for Bleeding Associated with
Factor Xa Inhibitors. N Engl J Med. 2019;380(14):1326-35. https://doi.org/10.1056/NEJMoa1814051 PMid:30730782 PMCid:PMC6699827
- Tomaselli
GF, Mahaffey KW, Cuker A, Dobesh PP, Doherty JU, Eikelboom JW, et al.
2020 ACC Expert Consensus Decision Pathway on Management of Bleeding in
Patients on Oral Anticoagulants: A Report of the American College of
Cardiology Solution Set Oversight Committee. J Am Coll Cardiol.
2020;76(5):594-622. https://doi.org/10.1016/j.jacc.2020.04.053 PMid:32680646
- Singh
B, Pai P, Kumar H, George S, Mahapatra S, Garg V, et al. Expert
Recommendations on the Usage of Non-vitamin K Antagonist Oral
Anticoagulants (NOACs) from India: Current Perspective and Future
Direction. Vol. 11, Cardiology and Therapy. 2022. p. 49-79. https://doi.org/10.1007/s40119-022-00254-w PMid:35137335 PMCid:PMC8933593
- Al-Shamkhani
W, Ayetey H, Lip GYH. Atrial fibrillation in the Middle East: unmapped,
underdiagnosed, undertreated.
https://doi.org/101080/1477907220181457953 [Internet].
2018 May 4 [cited 2022
Dec 4];16(5):341-8.
Available from:
https://www.tandfonline.com/doi/abs/10.1080/14779072.2018.1457953 https://doi.org/10.1080/14779072.2018.1457953 PMid:29575965
- Aljefree
N, Ahmed F. Prevalence of Cardiovascular Disease and Associated Risk
Factors among Adult Population in the Gulf Region: A Systematic Review.
Adv Public Heal. 2015;2015:1-23. https://doi.org/10.1155/2015/235101
- Hersi
A, Abdul-Moneim M, Almous'ad A, Al-Samadi F, AlFagih A, Sweidan R.
Saudi Atrial Fibrillation Survey: national, observational,
cross-sectional survey evaluating atrial fibrillation management and
the cardiovascular risk profile of patients with atrial fibrillation.
Angiology [Internet]. 2015 Mar 1 [cited 2022 Dec 4];66(3):244-8. https://doi.org/10.1177/0003319714529180 PMid:24687415
- Kadri
M El, Ghorab A, Joury J, Farghaly M, Awad N, Ramachandrachar BC, et al.
Patient characteristics, adherence, and costs of oral anticoagulation
therapy in non-valvular atrial fibrillation using the Dubai Real-World
Claims Database. Avicenna J Med [Internet]. 2021 Apr [cited 2022 Dec
4];11(2):93. https://doi.org/10.4103/ajm.ajm_228_20 PMid:33996647 PMCid:PMC8101642
- Alajami
HN, Alshammari SA, Al-Dossari DS, Alajmi AN, Alsaikhan AS, Alessa MS,
et al. Knowledge of Anticoagulation Among Saudi Patients With Atrial
Fibrillation: A Cross-Sectional Study. Cureus [Internet]. 2021 Nov 3
[cited 2022 Dec 4];13(11). https://doi.org/10.7759/cureus.19237
- AlRuthia
Y, AlOtaibi BQ, AlOtaibi RM, AlOtaibi NQ, Alanazi M, Asaad Assiri G.
Cost effectiveness of rivaroxaban versus warfarin among nonvalvular
atrial fibrillation patients in Saudi Arabia: A Single-Center
retrospective cohort study. Saudi Pharm J [Internet]. 2022 Nov 18
[cited 2022 Dec 4]; Available from: https://linkinghub.elsevier.com/retrieve/pii/S1319016422002791
- Al
Sulaiman K, Badreldin HA, Korayem GB, Alenazi AA, Alsuwayyid F,
Alrashidi A, et al. Evaluation of Apixaban safety and effectiveness in
morbidly obese patients with atrial fibrillation: a retrospective
cohort study. Thromb J [Internet]. 2022 Dec 1 [cited 2022 Dec
4];20(1):1-9. https://doi.org/10.1186/s12959-022-00379-x PMid:35501916 PMCid:PMC9063081
- Wright
JG, Swiontkowski MF, Heckman JD. Introducing levels of evidence to the
journal. Vol. 85, Journal of Bone and Joint Surgery - Series A. 2003.
p. 1-3. https://doi.org/10.2106/00004623-200301000-00001
- Lynn MR. Determination and quantification of content validity. Nurs Res. 1986;35(6):382-6. https://doi.org/10.1097/00006199-198611000-00017 PMid:3640358
- Bezabhe
WM, Bereznicki LR, Radford J, Wimmer BC, Curtain C, Salahudeen MS, et
al. Ten-Year Trends in the Use of Oral Anticoagulants in Australian
General Practice Patients With Atrial Fibrillation. Front Pharmacol.
2021 Mar 23;12:441. https://doi.org/10.3389/fphar.2021.586370 PMid:33867975 PMCid:PMC8044929
- Navar
AM, Kolkailah AA, Overton R, Shah NP, Rousseau JF, Flaker GC, et al.
Trends in Oral Anticoagulant Use Among 436 864 Patients With Atrial
Fibrillation in Community Practice, 2011 to 2020. J Am Heart Assoc
[Internet]. 2022 Nov 15 [cited 2022 Dec 4];11:26723. https://doi.org/10.1161/JAHA.122.026723 PMid:36346063 PMCid:PMC9750070
- Johnston
KM, Osenenko KM, Qatami L, Donato BMK, Alsheikh-ali AA, Binbrek AS, et
al. Health Care Resource Utilization and Costs in Individuals with
Atrial Fibrillation in United Arab Emirates and Kingdom of Saudi
Arabia : A Retrospective Cohort Study. Int J Intern Med.
2015;4(2):17-25. https://doi.org/10.1016/j.jval.2014.08.1527 PMid:27201532
- El
Kadri M, Bazargani N, Farghaly M, Mohamed R, Awad N, Natarajan A, et
al. Profiling Clinical Characteristics and Treatment Patterns Among
Non-Valvular Atrial Fibrillation Patients: A Real-World Analysis in
Dubai, United Arab Emirates. Open Med J. 2019 Jun 11;6(1):33-41. https://doi.org/10.2174/1874220301906010033
- Ali
MD, Ahmad A, Banu N, Patel M, Ghosn SA, Eltrafi Z. Anticoagulant drug
utilization pattern and their cost analysis: a retrospective study from
Saudi Arabia. J Pharm Heal Serv Res [Internet]. 2020 Nov 9 [cited 2022
Dec 4];11(4):411-4. https://doi.org/10.1111/jphs.12382
- Elewa
H, Alhaddad A, Al-Rawi S, Nounou A, Mahmoud H, Singh R. Trends in oral
anticoagulant use in Qatar: a 5-year experience. J Thromb Thrombolysis
2017 433 [Internet]. 2017 Jan 30 [cited 2022 Dec 4];43(3):411-6.
Available from:
https://link.springer.com/article/10.1007/s11239-017-1474-4 https://doi.org/10.1007/s11239-017-1474-4 PMid:28138812
- AlAmri
A, AlShehri A, AlShammari R, AlAmri R, AlMulhim L, AlGhamdi M.
Prevalence of atrial fibrillation among Saudi patients who had stroke:
a retrospective cross-sectional study at a university hospital. Int J
Med Dev Ctries. 2022;333-9. https://doi.org/10.24911/IJMDC.51-1639698513
- Zubaid
M, Rashed WA, Alsheikh-Ali AA, AlMahmeed W, Shehab A, Sulaiman K, et
al. Gulf survey of atrial fibrillation events (Gulf SAFE) design and
baseline characteristics of patients with atrial fibrillation in the
arab middle East. Circ Cardiovasc Qual Outcomes. 2011;4(4):477-82. https://doi.org/10.1161/CIRCOUTCOMES.110.959700 PMid:21772004
- Ajlan
M, Almazroa L, AlHabib KF, Elasfar AA, Alfaleh H, Albackr H, et al.
Atrial Fibrillation in Patients Hospitalized With Heart Failure:
Patient Characteristics and Outcomes From the HEARTS Registry.
Angiology [Internet]. 2018 Feb 1 [cited 2022 Dec 4];69(2):151-7. https://doi.org/10.1177/0003319717711764 PMid:28592150
- Alosaimi
HM, Alqahtani S, Balkhi B, Alqahtani M, Alzamil F, Alhossan A, et al. A
retrospective study of real-world effectiveness and safety of
rivaroxaban in patients with non-valvular atrial fibrillation and
venous thromboembolism in Saudi Arabia. PeerJ [Internet]. 2022 Sep 9
[cited 2022 Dec 5];10. https://doi.org/10.7717/peerj.13974 PMid:36105646 PMCid:PMC9466595
- Riazuddin
M, Mpharm MA, Butt MI, Abufarhaneh M, Khan SM, Emadi O, et al.
Rivaroxaban Experience at Tertiary Care Centre in Saudi Arabia:A
Retrospective Observational Study. Galen Med J [Internet]. 2020 Dec 26
[cited 2022 Dec 5];9:e1882. https://doi.org/10.31661/gmj.v9i0.1882 PMid:34466605 PMCid:PMC8343821
- Alkhotani
A, Alrishi N, Alharthi M, Alzahrani W. Rivaroxaban-associated
intracranial hemorrhage in Saudi atrial fibrillation patients. Medicine
(Baltimore) [Internet]. 2020 Nov 25 [cited 2022 Dec 5];99(48):e23316. https://doi.org/10.1097/MD.0000000000023316 PMid:33235091 PMCid:PMC7710243
- Gieling
EM, van den Ham HA, van Onzenoort H, Bos J, Kramers C, de Boer A, et
al. Risk of major bleeding and stroke associated with the use of
vitamin K antagonists, nonvitamin K antagonist oral anticoagulants and
aspirin in patients with atrial fibrillation: a cohort study. Br J Clin
Pharmacol [Internet]. 2017 [cited 2022 Dec 5];83(8):1844-59. https://doi.org/10.1111/bcp.13265 PMid:28205318 PMCid:PMC5510083
- Burnett
AE, Mahan CE, Vazquez SR, Oertel LB, Garcia DA, Ansell J. Guidance for
the practical management of the direct oral anticoagulants (DOACs) in
VTE treatment. J Thromb Thrombolysis. 2016;41(1):206-32. https://doi.org/10.1007/s11239-015-1310-7 PMid:26780747 PMCid:PMC4715848
- Frontera
JA, Lewin JJ, Rabinstein AA, Aisiku IP, Alexandrov AW, Cook AM, et al.
Guideline for Reversal of Antithrombotics in Intracranial Hemorrhage: A
Statement for Healthcare Professionals from the Neurocritical Care
Society and Society of Critical Care Medicine. Neurocrit Care
[Internet]. 2016 Feb 1 [cited 2023 Jan 23];24(1):6-46. Available from:
https://pubmed.ncbi.nlm.nih.gov/26714677/ https://doi.org/10.1007/s12028-015-0222-x PMid:26714677
- Heidbuchel
H, Verhamme P, Alings M, Antz M, Diener HC, Hacke W, et al. Updated
European Heart Rhythm Association practical guide on the use of
non-vitamin-K antagonist anticoagulants in patients with non-valvular
atrial fibrillation: Executive summary. Eur Heart J.
2017;38(27):2137-49. https://doi.org/10.1093/eurheartj/ehw058 PMid:27282612 PMCid:PMC5837231
- Cuker
A, Burnett A, Triller D, Crowther M, Ansell J, Van Cott EM, et al.
Reversal of direct oral anticoagulants: Guidance from the
Anticoagulation Forum. Am J Hematol [Internet]. 2019 Jun 1 [cited 2023
Jan 23];94(6):697-709. https://doi.org/10.1002/ajh.25475 PMid:30916798
- Spahn
DR, Bouillon B, Cerny V, Duranteau J, Filipescu D, Hunt BJ, et al. The
European guideline on management of major bleeding and coagulopathy
following trauma: fifth edition. Crit Care. 2019;23(1):98. https://doi.org/10.1186/s13054-019-2347-3 PMid:30917843 PMCid:PMC6436241
- Witt
DM, Nieuwlaat R, Clark NP, Ansell J, Holbrook A, Skov J, et al.
American Society of Hematology 2018 guidelines for management of venous
thromboembolism: Optimal management of anticoagulation therapy. Vol. 2,
Blood Advances. 2018. p. 3257-91. https://doi.org/10.1182/bloodadvances.2018024893 PMid:30482765 PMCid:PMC6258922
- Huwez
F, Casswell EJ. FAST-AV or FAST-AB tool improves the sensitivity of
FAST screening for detection of posterior circulation strokes. Vol. 8,
International Journal of Stroke. 2013. https://doi.org/10.1111/ijs.12008 PMid:23489668
- Chen
X, Zhao X, Xu F, Guo M, Yang Y, Zhong L, et al. A Systematic Review and
Meta-Analysis Comparing FAST and BEFAST in Acute Stroke Patients. Vol.
12, Frontiers in Neurology. 2022. https://doi.org/10.3389/fneur.2021.765069 PMid:35153975 PMCid:PMC8837419
- Dager
W, Hellwig T. Current knowledge on assessing the effects of and
managing bleeding and urgent procedures with direct oral
anticoagulants. Am J Heal Pharm. 2016;73(10):S14-26. https://doi.org/10.2146/ajhp150960 PMid:27147455
- Samuelson
BT, Cuker A, Siegal DM, Crowther M, Garcia DA. Laboratory assessment of
the anticoagulant activity of direct oral anticoagulants: a systematic
review. Chest. 2017;151(1):127-38. https://doi.org/10.1016/j.chest.2016.08.1462 PMid:27637548 PMCid:PMC5310120
- Lindahl
TL, Baghaei F, Blixter IF, Gustafsson KM, Stigendal L, Sten-Linder M,
et al. Effects of the oral, direct thrombin inhibitor dabigatran on
five common coagulation assays. Thromb Haemost. 2011;105(2):371-8. https://doi.org/10.1160/TH10-06-0342 PMid:21103660
- Tripodi
A, Chantarangkul V, Guinet C, Samama MM. The International Normalized
Ratio calibrated for rivaroxaban has the potential to normalize
prothrombin time results for rivaroxaban-treated patients: Results of
an in vitro study. Vol. 9, Journal of Thrombosis and Haemostasis. 2011.
p. 226-8. https://doi.org/10.1111/j.1538-7836.2010.04106.x PMid:20942848
- Samama
MM, Martinoli JL, LeFlem L, Guinet C, Plu-Bureau G, Depasse F, et al.
Assessment of laboratory assays to measure rivaroxaban - An oral,
direct factor Xa inhibitor. Thromb Haemost. 2010;103(4):815-25. https://doi.org/10.1160/TH09-03-0176 PMid:20135059
- Testa
S, Legnani C, Tripodi A, Paoletti O, Pengo V, Abbate R, et al. Poor
comparability of coagulation screening test with specific measurement
in patients receiving direct oral anticoagulants: results from a
multicenter/multiplatform study. J Thromb Haemost.
2016;14(11):2194-201. https://doi.org/10.1111/jth.13486 PMid:27566988
- Tripodi
A, Ageno W, Ciaccio M, Legnani C, Lippi G, Manotti C, et al. Position
paper on laboratory testing for patients on direct oral anticoagulants.
A Consensus Document from the SISET, FCSA, SIBioC and SIPMeL. Blood
Transfus. 2018;16(5):462-70.
- Nowak
G. The ecarin clotting time, a universal method to quantify direct
thrombin inhibitors. Vol. 33, Pathophysiology of Haemostasis and
Thrombosis. 2003. p. 173-83. https://doi.org/10.1159/000081505 PMid:15583446
- Samama
MM, Contant G, Spiro TE, Perzborn E, Guinet C, Gourmelin Y, et al.
Evaluation of the anti-factor Xa chromogenic assay for the measurement
of rivaroxaban plasma concentrations using calibrators and controls.
Thromb Haemost. 2012;107(2):379-87. https://doi.org/10.1160/TH11-06-0391 PMid:22187012
- Gosselin
RC, Adcock DM, Bates SM, Douxfils J, Favaloro EJ, Gouin-Thibault I, et
al. International Council for Standardization in Haematology (ICSH)
Recommendations for Laboratory Measurement of Direct Oral
Anticoagulants. Thromb Haemost. 2018;118(3):437-50. https://doi.org/10.1055/s-0038-1627480 PMid:29433148
- Bauer KA. Targeted Anti-Anticoagulants. N Engl J Med. 2015;373(6):569-71. https://doi.org/10.1056/NEJMe1506600 PMid:26095632
- Mazahreh F, Habash F, López-Candales A. Venous Thromboembolism While on Anticoagulation With Apixaban. Cureus. 2021; https://doi.org/10.7759/cureus.15189 PMid:34178510 PMCid:PMC8218250
- Kaide
CG, Gulseth MP. Current Strategies for the Management of Bleeding
Associated with Direct Oral Anticoagulants and a Review of
Investigational Reversal Agents. J Emerg Med [Internet]. 2020 Feb 1
[cited 2023 Jan 23];58(2):217-33. https://doi.org/10.1016/j.jemermed.2019.10.011 PMid:31831187
- HEUBNER
L, VICENT O, BEYER-WESTENDORF J, SPIETH PM. Bleeding management in
patients with direct oral anticoagulants. Minerva Anestesiol.
2023;89(7-8). https://doi.org/10.23736/S0375-9393.23.17230-0
- Baradarian
R, Ramdhaney S, Chapalamadugu R, Skoczylas L, Wang K, Rivilis S, et al.
Early Intensive Resuscitation of Patients with Upper Gastrointestinal
Bleeding Decreases Mortality. Am J Gastroenterol. 2004;99(4):619-22. https://doi.org/10.1111/j.1572-0241.2004.04073.x PMid:15089891
- Spoerke
N, Michalek J, Schreiber M. Crystalloid resuscitation improves survival
in trauma patients receiving low ratios of fresh frozen plasma to
packed red blood cells. Vol. 71, Journal of Trauma - Injury, Infection
and Critical Care. 2011. https://doi.org/10.1097/TA.0b013e318227f1c5 PMid:21814108
- Perel
P, Roberts I, Ker K. Colloids versus crystalloids for fluid
resuscitation in critically ill patients. Vol. 2013, Cochrane Database
of Systematic Reviews. 2013. https://doi.org/10.1002/14651858.CD000567.pub6 PMCid:PMC8925276
- Holcomb
JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, et al.
Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a
1:1:2 ratio and mortality in patients with severe trauma: The PROPPR
randomized clinical trial. JAMA - J Am Med Assoc. 2015;313(5):471-82. https://doi.org/10.1001/jama.2015.12 PMid:25647203 PMCid:PMC4374744
- Villanueva
C, Colomo A, Bosch A, Concepción M, Hernandez-Gea V, Aracil C, et al.
Transfusion Strategies for Acute Upper Gastrointestinal Bleeding. N
Engl J Med. 2013;368(1):11-21. https://doi.org/10.1056/NEJMoa1211801
PMid:23281973
- Pham HP, Shaz BH. Update on massive transfusion. Br J Anaesth. 2013;111(SUPPL.1). https://doi.org/10.1093/bja/aet376 PMid:24335401
- Zhang
Y, Souverein PC, Gardarsdottir H, van den Ham HA, Maitland-van der Zee
AH, de Boer A. Risk of major bleeding among users of direct oral
anticoagulants combined with interacting drugs: A population-based
nested case-control study. Br J Clin Pharmacol. 2020;86(6):1150-64. https://doi.org/10.1111/bcp.14227 PMid:32022295 PMCid:PMC7256117
- January
CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, et al. 2019
AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the
Management of Patients With Atrial Fibrillation: A Report of the
American College of Cardiology/American Heart Association Task Force on
Clinical Practice Guidelines and the Heart R. Vol. 140, Circulation.
2019. p. e125-51. https://doi.org/10.1161/CIR.0000000000000665 PMid:30686041
- Janson
PA, Jubelirer SJ, Weinstein MJ, Deykin D. Treatment of the Bleeding
Tendency in Uremia with Cryoprecipitate. N Engl J Med.
1980;303(23):1318-22. https://doi.org/10.1056/NEJM198012043032302 PMid:6776402
- Hedges
SJ, Dehoney SB, Hooper JS, Amanzadeh J, Busti AJ. Evidence-based
treatment recommendations for uremic bleeding. Vol. 3, Nature Clinical
Practice Nephrology. 2007. p. 138-53. https://doi.org/10.1038/ncpneph0421 PMid:17322926
- Schiele
F, Van Ryn J, Canada K, Newsome C, Sepulveda E, Park J, et al. A
specific antidote for dabigatran: functional and structural
characterization. Blood [Internet]. 2013 May 2 [cited 2022 Dec
6];121(18):3554-62. https://doi.org/10.1182/blood-2012-11-468207 PMid:23476049
- Pollack
C V., Reilly PA, Bernstein R, Dubiel R, Eikelboom J, Glund S, et al.
Design and rationale for RE-VERSE AD: A phase 3 study of idarucizumab,
a specific reversal agent for dabigatran. Thromb Haemost.
2015;114(1):198-205. https://doi.org/10.1160/TH15-03-0192 PMid:26020620
- Garrett AD. Idarucizumab for dabigatran reversal. Drug Topics [Internet]. 2015 Aug 1 [cited 2022 Dec 6];159(8). Available from: https://www.nejm.org/doi/full/10.1056/nejmoa1502000
- Idarucizumab receives full FDA approval | MDedge Hematology and Oncology [Internet]. [cited 2022 Dec 6]. Available from: https://www.mdedge.com/hematology-oncology/article/185007/thrombosis/idarucizumab-receives-full-fda-approval
- Kaatz
S, Bhansali H, Gibbs J, Lavender R, Mahan CE, Paje DG. Reversing factor
Xa inhibitors - Clinical utility of andexanet alfa [Internet]. Vol. 8,
Journal of Blood Medicine. Dove Press; 2017 [cited 2022 Dec 6]. p.
141-9. https://doi.org/10.2147/JBM.S121550 PMid:28979172 PMCid:PMC5602457
- Siegal
DM, Curnutte JT, Connolly SJ, Lu G, Conley PB, Wiens BL, et al.
Andexanet Alfa for the Reversal of Factor Xa Inhibitor Activity. N Engl
J Med [Internet]. 2015 Dec 17 [cited 2022 Dec 6];373(25):2413-24. https://doi.org/10.1056/NEJMoa1510991 PMid:26559317
- Andexanet Becomes First FDA-Approved Antidote for Factor Xa Inhibitors. 2021.
- Connolly
SJ, Milling TJ, Eikelboom JW, Gibson CM, Curnutte JT, Gold A, et al.
Andexanet Alfa for Acute Major Bleeding Associated with Factor Xa
Inhibitors. N Engl J Med. 2016;375(12):1131-41. https://doi.org/10.1056/NEJMoa1607887 PMid:27573206 PMCid:PMC5568772
- Walenga
JM. Can We Improve on the Rapid Assessment of Clinically Relevant
Levels of Direct Acting Oral Anticoagulants (DOAC)? Vol. 28, Clinical
and Applied Thrombosis/Hemostasis. 2022. https://doi.org/10.1177/10760296221096422 PMid:35473406 PMCid:PMC9099059
- Christensen
H, Cordonnier C, Kõrv J, Lal A, Ovesen C, Purrucker JC, et al. European
Stroke Organisation Guideline on Reversal of Oral Anticoagulants in
Acute Intracerebral Haemorrhage. Eur Stroke J. 2019;4(4):294-306. https://doi.org/10.1177/2396987319849763 PMid:31903428 PMCid:PMC6921939
- Majeed
A, Ågren A, Holmström M, Bruzelius M, Chaireti R, Odeberg J, et al.
Management of rivaroxaban- or apixaban-associated major bleeding with
prothrombin complex concentrates: a cohort study. Blood [Internet].
2017 Oct 12 [cited 2023 Jan 23];130(15):1706-12. https://doi.org/10.1182/blood-2017-05-782060 PMid:28835439
- Schulman
S, Gross PL, Ritchie B, Nahirniak S, Lin Y, Lieberman L, et al.
Prothrombin Complex Concentrate for Major Bleeding on Factor Xa
Inhibitors: A Prospective Cohort Study. Thromb Haemost [Internet]. 2018
May 1 [cited 2023 Jan 23];118(5):842-51. https://doi.org/10.1055/s-0038-1636541 PMid:29564837
- Piran
S, Khatib R, Schulman S, Majeed A, Holbrook A, Witt DM, et al.
Management of direct factor Xa inhibitor-related major bleeding with
prothrombin complex concentrate: A meta-analysis. Blood Adv.
2019;3(2):158-67. https://doi.org/10.1182/bloodadvances.2018024133 PMid:30658963 PMCid:PMC6341194
- Van
Ryn J, Stangier J, Haertter S, Liesenfeld KH, Wienen W, Feuring M, et
al. Dabigatran etexilate - A novel, reversible, oral direct thrombin
inhibitor: Interpretation of coagulation assays and reversal of
anticoagulant activity. Vol. 103, Thrombosis and Haemostasis. 2010. p.
1116-27. https://doi.org/10.1160/TH09-11-0758 PMid:20352166
- Nielsen
PB, Larsen TB, Gorst-Rasmussen A, Skjøth F, Rasmussen LH, Lip GYH.
Intracranial hemorrhage and subsequent ischemic stroke in patients with
atrial fibrillation: a nationwide cohort study. Chest [Internet]. 2015
Jun 1 [cited 2023 Jan 24];147(6):1651-8. https://doi.org/10.1378/chest.14-2099 PMid:25412369
- Li
Y guang, Lip GYH. Anticoagulation Resumption After Intracerebral
Hemorrhage. Curr Atheroscler Rep [Internet]. 2018 Jul 1 [cited 2023 Jan
24];20(7). https://doi.org/10.1007/s11883-018-0733-y PMid:29781063 PMCid:PMC5960649
- Murphy
MP, Kuramatsu JB, Leasure A, Falcone GJ, Kamel H, Sansing LH, et al.
Cardioembolic Stroke Risk and Recovery after Anticoagulation-Related
Intracerebral Hemorrhage. Stroke [Internet]. 2018 [cited 2023 Jan
24];49(11):2652. https://doi.org/10.1161/STROKEAHA.118.021799 PMid:30355194 PMCid:PMC6211810
- Hemphill
JC, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, et al.
Guidelines for the Management of Spontaneous Intracerebral Hemorrhage:
A Guideline for Healthcare Professionals From the American Heart
Association/American Stroke Association. Stroke [Internet]. 2015 Jul 4
[cited 2023 Jan 24];46(7):2032-60. https://doi.org/10.1161/STR.0000000000000069 PMid:26022637
- Biffi
A, Kuramatsu JB, Leasure A, Kamel H, Kourkoulis C, Schwab K, et al.
Oral Anticoagulation and Functional Outcome after Intracerebral
Hemorrhage. Ann Neurol [Internet]. 2017 Nov 1 [cited 2023 Jan
24];82(5):755. https://doi.org/10.1002/ana.25079 PMid:29028130 PMCid:PMC5730065
- Mujer
MTP, Rai MP, Atti V, Dimaandal IL, Chan AS, Shrotriya S, et al. An
Update on the Reversal of Non-Vitamin K Antagonist Oral Anticoagulants.
Adv Hematol [Internet]. 2020 [cited 2023 Jan 24];2020. https://doi.org/10.1155/2020/7636104 PMid:32231703 PMCid:PMC7097770
- Steffel
J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe L, et al. The
2018 European Heart Rhythm Association Practical Guide on the use of
non-vitamin K antagonist oral anticoagulants in patients with atrial
fibrillation. Eur Heart J [Internet]. 2018 Apr 21 [cited 2023 Jan
24];39(16):1330-93. Available from: https://pubmed.ncbi.nlm.nih.gov/29562325/
- Liu
F, Demosthenes P. Real-world data: a brief review of the methods,
applications, challenges and opportunities. Vol. 22, BMC Medical
Research Methodology. 2022. https://doi.org/10.1186/s12874-022-01768-6 PMid:36335315 PMCid:PMC9636688
- Roberti
R, Iannone LF, Palleria C, Curcio A, Rossi M, Sciacqua A, et al. Direct
Oral Anticoagulants: From Randomized Clinical Trials to Real-World
Clinical Practice. Vol. 12, Frontiers in Pharmacology. 2021. https://doi.org/10.3389/fphar.2021.684638 PMid:34122113 PMCid:PMC8188985
- Mehran
R, Rao S V, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, et al.
Standardized bleeding definitions for cardiovascular clinical trials: a
consensus report from the Bleeding Academic Research Consortium.
Circulation. 2011 Jun;123(23):2736-47. https://doi.org/10.1161/CIRCULATIONAHA.110.009449 PMid:21670242
- Kaatz
S, Ahmad D, Spyropoulos AC, Schulman S. Definition of clinically
relevant non-major bleeding in studies of anticoagulants in atrial
fibrillation and venous thromboembolic disease in non-surgical
patients: Communication from the SSC of the ISTH. J Thromb Haemost.
2015;13(11):2119-26. https://doi.org/10.1111/jth.13140 PMid:26764429
- Von
Heymann C, Rosenthal C, Kaufner L, Sander M. Management of direct oral
anticoagulants-associated bleeding in the trauma patient. Curr Opin
Anaesthesiol [Internet]. 2016 Mar 23 [cited 2023 Apr 2];29(2):220-8. https://doi.org/10.1097/ACO.0000000000000294 PMid:26934279
- Moia
M, Squizzato A. Reversal agents for oral anticoagulant-associated major
or life-threatening bleeding. Vol. 14, Internal and Emergency Medicine.
2019. p. 1233-9. https://doi.org/10.1007/s11739-019-02177-2 PMid:31446606 PMCid:PMC6881427





