Urbain Tauveron--Jalenques1, Vincent Grobost2, Benoît Magnin3, Cécile Moluçon-Chabrot1, Jacques-Olivier Bay1,4, Olivier Tournilhac1,4 and Romain Guièze1,4.
1
Cellular Therapy and Clinical Haematology Department, Clermont-Ferrand University Hospital, Clermont Ferrand, France.
2 Internal Medicine Department, Clermont-Ferrand University Hospital, Clermont Ferrand, France.
3 Department of Radiology, Clermont-Ferrand University Hospital, Clermont Ferrand, France.
4 EA7453 (CHELTER), Clermont Auvergne University, Clermont-Ferrand, France.
Correspondence to:
Urbain Tauveron-Jalenques, Cellular Therapy and Clinical Haematology
Department, Clermont-Ferrand University Hospital, 1 Rue Lucie et
Raymond Aubrac, Clermont-Ferrand, 63003, France. E-mail:
utauveron-jalenques@chu-clermontferrand.fr
Published: March 01, 2024
Received: December 04, 2023
Accepted: February 09, 2024
Mediterr J Hematol Infect Dis 2024, 16(1): e2024025 DOI
10.4084/MJHID.2024.025
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
To the editor
A
42-year-old woman presented to the emergency department for a seven-day
history of lumbar pain and night fever. On admission, she was febrile
at 38.2°C. Associated symptoms included cough, dyspnoea on exertion,
and left scapular pain. Physical examination showed sinus tachycardia
and a single lingual aphtha. All peripheral pulses were well palpable
with no headache, visual loss, focal neurological deficit, signs of
polymyalgia rheumatica, history of chondritis, peripheral
lymphadenopathy, and splenomegaly. Laboratory studies showed a white
blood cell count of 46.67 x 109/L, including 66% of myeloid blasts; haemoglobin was 80 g/L, platelet count was 124 x 109/L,
and C-reactive protein was 386 mg/L. Creatinine level, electrolytes,
and bilirubin were within normal range. The thoracic-abdominopelvic CT
scan showed a wall thickening (arrows) of the aortic arch (Figure 1A), the proximal left subclavian artery (Figure 1B), and the abdominal aorta (Figure 1C) and a periaortic fat stranding (Figure 1C,
arrowhead) evocative of a panaortitis. A mild left pleural effusion was
also detected. The bone marrow aspiration showed 87% of M1 myeloid
blasts, confirming the diagnosis of acute myeloblastic leukaemia (AML)
without maturation. Further examinations revealed normal cytogenetics
(karyotype and chromosome 8 in situ hybridization) and mutations of the
NPM1, DNMT3A, IDH2 R140Q, KIT D816V, and CEBPA-bZip
genes with variant allele frequencies of 32%, 42%, 38%, 17%, and 2%
respectively. All complementary laboratory tests (blood cultures, Coxiella burnetii and Treponema pallidum,
interferon-γ release assay, antinuclear, anti-neutrophil cytoplasmic
and anti-CCP antibodies, rheumatoid factor, IgG4 antibodies) were
negative, rendering alternative aetiologies of aortitis highly
unlikely. All this led to the final diagnosis of de novo NPM1-mutated
AML associated with paraneoplastic aortitis. The patient received
induction chemotherapy with idarubicin 9mg/m²/day from day 1 to day 5
and cytarabine 200mg/m²/day from day 1 to day 7 according to the
experimental arm of the BIG-1 trial (registration number: NCT02416388).
The initial symptoms receded on day 10 of the induction therapy. A CT
scan performed on day 13 showed a complete disappearance of the
aortitis (Figure 1 D-F). A post-induction evaluation performed on day 38 demonstrated complete remission (CR) with NPM1-based minimal residual disease at 0.087%.
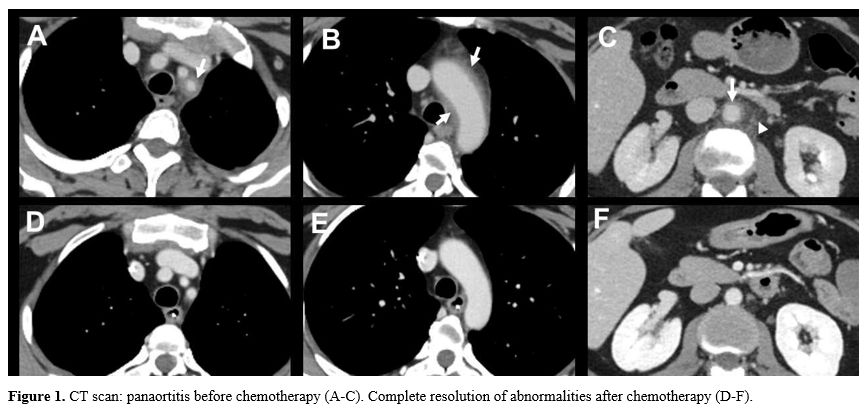 |
- Figure 1. CT scan: panaortitis before chemotherapy (A-C). Complete resolution of abnormalities after chemotherapy (D-F).
|
Discussion
The association
between aortitis and myelodysplastic neoplasms (MDS) or chronic
myelomonocytic leukaemia (CMML) is well documented,[1]
whereas the co-occurrence of aortitis and de novo AML is extremely
rare: to our knowledge, only three certain cases have been reported so
far. All three were characterized by exclusive abdominal aorta
involvement,[2,3,4]
whereas in our case, the patient presented with panaortitis. The
pathophysiology of this association is unknown. One can speculate that
abnormal expression of antigens by AML blasts could stimulate immune
dysregulation (particularly of dendritic cells and T lymphocytes),
leading to the development of vessel wall inflammation, similar to what
is described in MDS.[5]MDS
or CMML-associated large vessel vasculitis often benefits from
treatment with corticosteroids (CS); however, CS dependency or
refractoriness is a frequent eventuality.[1,6,7]
In two of the three published cases of AML-associated aortitis, precise
information about the patient's management is available. In both cases,
a treatment with CS for the inflammatory disorder was associated with
AML induction therapy. In the acute promyelocytic leukaemia (APL)
associated case, the authors indicate that treatment led to
"amelioration of the patient" and CR of APL;[3]
in the second case, evolution was characterized by progression of
inflammatory manifestations and early death due to acute coronary
syndrome.[2]
In the case of our patient, we chose the sole induction chemotherapy
regimen, which led to both early and total disappearance of the
aortitis and CR of AML, thus confirming the hypothesis of
AML-associated paraneoplastic aortitis.
References
- Roupie AL, de Boysson H, Thietart S et al.
Giant-cell arteritis associated with myelodysplastic syndrome: French
multicenter case control study and literature review. Autoimmun Rev.
2020 Feb;19(2):102446. https://doi.org/10.1016/j.autrev.2019.102446 PMid:31838164
- Attias
D, Laor R, Zuckermann E et al. Acute neutrophilic myositis in Sweet's
syndrome: late phase transformation into fibrosing myositis and
panniculitis. Hum Pathol. 1995 Jun;26(6):687-90. https://doi.org/10.1016/0046-8177(95)90177-9 PMid:7774902
- Haidar
NA, Ayvazian HJ, Farhat F. AML-214: case report: A rare case
presentation and detection of acute promyelocytic leukemia. Clinical
Lymphoma, Myeloma and Leukemia. 2021;S292. https://doi.org/10.1016/S2152-2650(21)01698-0
- Ireifej
B, Freijat M, Song D et al. Aortitis presenting as acute myeloid
leukemia - A case report. Ann Med Surg (Lond). 2022 Jul 31;80:104255. https://doi.org/10.1016/j.amsu.2022.104255 PMid:36045831 PMCid:PMC9422307
- Kouroukli
O, Symeonidis A, Foukas P et al. Bone Marrow Immune Microenvironment in
Myelodysplastic Syndromes. Cancers (Basel). 2022 Nov 17;14(22):5656. https://doi.org/10.3390/cancers14225656 PMid:36428749 PMCid:PMC9688609
- Mekinian
A, Grignano E, Braun T et al. Systemic inflammatory and autoimmune
manifestations associated with myelodysplastic syndromes and chronic
myelomonocytic leukaemia: a French multicentre retrospective study.
Rheumatology (Oxford). 2016 Feb;55(2):291-300. https://doi.org/10.1093/rheumatology/kev294 PMid:26350487
- Roupie
AL, Guedon A, Terrier B et al. Vasculitis associated with
myelodysplastic syndrome and chronic myelomonocytic leukemia: French
multicenter case-control study. Semin Arthritis Rheum. 2020
Oct;50(5):879-884. https://doi.org/10.1016/j.semarthrit.2020.07.002

