Fan Jiang, Yuan Sun*, Zhou-Yang Liu, Shi-Fen Fan, Juan Xiao, Jiao Chen, Hong-Yan Liu, Nan-Hai Wu and Zi-Kuan Guo.
Department of Hematology and Oncology, Beijing Jingdu Children's Hospital, China
Correspondence to:
Prof Yuan Sun, Department of Hematology and Oncology, Beijing Jingdu
Children's Hospital, No.308 Huilongguan East Street, Changping
District, Beijing, 102208, People's Republic of China, Tel.:
0086-010-69787668, Email:
sy@jdetyy.com
Published: May 01, 2024
Received: December 31, 2023
Accepted: April 02, 2024
Mediterr J Hematol Infect Dis 2024, 16(1): e2024036 DOI
10.4084/MJHID.2024.036
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
The
aim of this study was to investigate the prognostic factors of haploid
hematopoietic stem cell transplantation in the treatment of X-linked
lymphoproliferative syndrome. Seven children with X-linked
lymphoproliferative syndrome diagnosed by XIAP gene analysis were
enrolled. The conditioning regimens were tolerated in all seven
patients, and the median time of neutrophil engraftment was 10 days
(8-13 days), and that of platelet engraftment was 21 days (14-24 days).
STR-PCR analysis on the peripheral blood cells showed complete donor
origins. Four cases developed Grade I acute graft versus host disease
(aGVHD), one developed Grade III aGVHD (intestinal tract), and two
cases had limited chronic GVHD. Four cases had cytomegalovirus (CMV)
reactivation, and two cases had Epstein–Barr virus (EBV) reactivation.
One case was diagnosed as pneumocystosis, and thrombotic
microangiopathy (TMA) occurred in three cases. During the follow-up
period (median time of 42 months), one patient died of TMA and six
patients survived. Statistical analysis showed that the status of
disease remission and the positive result of virus in blood before
transplantation were independent prognostic factors. Haplo-HSCT might
be a curative option for children with refractory X-linked
lymphoproliferative syndrome. Low-intensity conditioning regimens may
reduce transplant-related mortality and improve overall survival.
|
Introduction
Hemophagocytic
lymphohistiocytosis (HLH), also nominated as hemophagocytic syndrome,
includes two categories according to the pathogenesis, namely primary
HLH and secondary HLH. According to different genetic backgrounds or
acquired pathogenic factors, it is further divided into different
subtypes.[1] The optimal treatment options for HLH
depend upon the causes and progression of the disease. For the
different precipitating factors, there is one type of primary HLH that
is driven by EBV, X-linked Lymphoproliferative Syndrome (XLP). This
disorder is the most common classic HLH driven by EBV[1-4]
and includes two subtypes, XLP-1 and XLP-2 (XIAP), that correspond to
the BIRC4 gene mutation. In addition to hemophagocytic symptoms, these
patients are often associated with chronic colitis, and the minority of
them has the presentation of hypogammaglobulinemia. Lymphoma has not
been reported so far in this setting, although it is an
immunodeficiency disease.[5-7] Clinical observations
from the HLH-1994/2004 Study have shown that CD20 monoclonal antibody
and Alemtuzumab have temporary mitigation on active HLH.[8-12] Previous reports have shown that HSCT for XLP-2 had poor efficacy,[13] though it is a curative treatment for other subtypes.
Here, we summarize the therapeutic effect of Haplo-HSCT in seven
children with hemophagocytic syndrome with XIAP gene mutation. The
factors affecting the curative effect are statistically analyzed. The
results are generally acceptable as reduced-intensity conditioning
regimens were utilized.
Methods
Patients.
Seven patients with XIAP gene-positive HLH were enrolled in our
hospital from June 2015 to September 2020. The diagnosis of the disease
met the criteria of Hemophagocytic lymphohistiocytosis, which was
revised by the Histiocyte Society in 2004.[1] All children were male, and the genetic test results were positive for the XIAP gene on the X chromosome (Table 1).
The median age was 3.1 years (1.2-5.6 years), and the median time from
the onset to transplantation was 9 months (5-28 months). XIAP protein
decreased in 5 cases, while in 1 case, XIAP function was normal. XIAP
gene mutation was found in all the patients' mothers. The function of
this protein was not measured in 1 case. Assessing was made before the
start of HSCT, and 6 patients were in partial remission (PR) and one in
disease progression. Among the seven patients, in six cases at the
initial stage, the EBV-DNA copies were 103-106
copies/ml, and in 1 case, it was still positive before transplantation.
The main symptoms before transplantation were intestinal and pulmonary
infections.
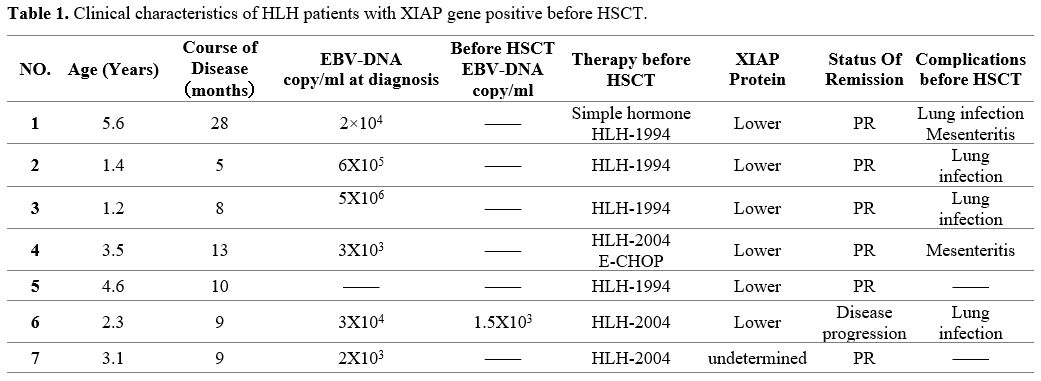 |
- Table 1. Clinical characteristics of HLH patients with XIAP gene positive before HSCT.
|
The modes of
transplantation. Five patients received paternal grafts and 2 cases
received hematopoietic grafts from mothers. Graft failure occurred in
these two cases, and they received secondary transplantation with their
mothers as the donors.
The methods of transplantation.
Conditioning regimen.
A conditioning regimen consisting of Etoposide (VP-16), Fludarabine
(Flu), Busulfan (BU), Anti-thymocyte globulin (ATG), and
cyclophosphamide (CTX) was performed before the transplantation as
previously reported, according to the conditioning regimen.[20] The doses were as follows: VP16, 600mg/m2 from days -11 to -9; Bu, 9.6-14.4mg/kg from days -8 to -6; Flu, 30mg/m2
from days -5 to -3; ATG, 8.5mg/kg within 4 days, from days -5 to -2,
and CTX, 10mg/Kg from days -4 to -3. For the two cases that had
received maternal grafts and engraftment failure had occurred,
Melphalan (MEL) at a total dose of 130mg/m2 injected from days -6 to -5 was added to the regimen described above.
Mobilization and Collection of Hematopoietic Stem Cells. Hematopoietic mobilization and collection of the grafts were performed as previously described.[19]
Briefly, the related donors received recombinant human granulocyte
colony-stimulating factor (G-CSF) at a dose of 5-10ug/kg/d for 5
continuous days. On the fourth day, the bone marrow was collected under
continuous epidural anesthesia, and on the 5th day, peripheral stem
cells were collected by a cell separator. The median count of bone
marrow plus peripheral stem cells was 9.07 (8.45-9.98)×108/kg, and CD34+ was 6.45 (4.67-8.53) ×106/kg.
The Criteria of Hematopoietic Reconstruction or Stem Cell Engraftment.
DNA fingerprinting was used to determine donor origins, and blood type
identification was performed if the donor and the recipient had
different blood types. Myeloid reconstruction was identified if the
absolute peripheral blood neutrophil count was above 0.5×109/L without injection of G-CSF and the platelet above 20×109/L without platelet transfusion for more than two weeks.
Prevention of Complications.
Graft Versus Host Disease (GVHD).
The prophylactic was Cyclosporin or Tacrolimus (FK506), Mycophenolate
Mofetil, and Anti-CD25 monoclonal antibody. The Intravenous dosage of
Cyclosporin was 2.5mg/kg/d from -10 days, and the dosage was adjusted
according to the blood concentration; Tacrolimus was taken orally at
-10 days and reduced by half after stem cell transplantation, then
stopped till the 28th day; all patients' therapy contains Anti-CD25
monoclonal antibody (from +1day and 10mg once time) and infusing
Disease monitoring. Post-HSCT, Bone marrow morphology, chimerism, XIAP gene mutation, and protein function were monitored regularly.
Results
Stem cell Engraftment and the Toxicity of conditioning Regimen.
Five of the seven cases were successfully implanted, and the other two
cases failed in the primary engraftment, but all were successfully
implanted after secondary transplantation. The chimerism rate of 7
patients was 100%, and the median time of neutrophils above 0.5×109/Kg was 10 (8-13) days. The median time of platelets above 20×109/L
without platelet transfusion was 21 (14-24) days. All the patients
tolerated the conditioning regimen well. Among them, five patients had
no toxicity, but two cases had Toxicity associated with the
conditioning regiments. It should be manifested as fever, diarrhea,
reactions of the digestive tract, etc., without complications of major
organ bleeding, severe infection, organ failure, and so on.
GVHD.
Five cases of GVHD were reported, including four cases of Grade I GVHD,
mainly involving the liver and skin, and one case of Grade III aGVHD in
the intestinal tract. Two cases developed into limited cGVHD, mainly
involving the liver. After adjustment of immunosuppressive agents, the
symptoms were alleviated.
Viruses and Other Complications.
CMV reactivation occurred in four cases and EBV reactivation in two
cases, while none of them developed viral infections. No patient was
associated with viral diseases. One case (No.3) developed PCP months
post-transplantation, and the condition was controlled after TMP-SMZ
treatment. Three cases developed TMA; remission occurred in two cases
after treatment. One case with TMA died of Grade III aGVHD (Table 2).
Clinical outcome. Six cases have survived disease-free. The overall median survival time was 42 (21-63) months.
 |
- Table 2. Outcome of the patients post-transplantation.Baseline . *It applies only to the AML
patients.Outcome of the patients post-transplantation.Baseline . *It applies only to the AML
patients.Baseline . *It applies only to the AML
patients.
|
Discussion
Hemophagocytic
syndrome (HLH) with XIAP gene mutation, caused by mutations in the
BIRC4 gene, is a rare congenital immunodeficiency disease. XLP2 gene,
located in the 25th region of the long arm of the X chromosome, encodes
the X-linked inhibitor of apoptosis protein (XIAP), which is an
apoptotic protein. It is expressed in virtually all normal cells and
can inhibit the process of cell apoptosis. In addition to its
anti-apoptotic effect, it is also involved in multiple signal pathways.[2-5]
The mechanisms underlying XIAP-linked HLH remain elusive until now. It
has been reported that increased sensitivity of lymphocytes to
undefined apoptotic signals causes damage to NK and T cells and limits
the cytotoxic function of lymphocytes that remain during viral
infection. The ineffectiveness of lymphocytes in lysing pathogenic
microorganisms leads to the long-term persistence of these pathogenic
agents, which constantly stimulate and activate macrophages and T
lymphocytes. The activated immune cells may produce a large number of
inflammatory factors, resulting in the occurrence of the
life-threatening hyperinflammatory syndrome, HLH.[6-9]
Lack of XIAP protein expression detected by flow cytometry and BIRC4
mutation in gene sequencing are utilized as the gold standards for
diagnosis of XIAP.[10] Glucocorticoids and etoposide
regimens are commonly used in the induction of remission for HLH. In
addition, it has been reported that Alemtuzumab and CD20 monoclonal
antibodies are also effective for HLH induced by EBV infection.[13-15] HSCT can be performed in refractory cases, and usually acceptable outcomes have been achieved.
In the present study, Haplo-identical HSCT was performed in seven cases
with HLH, all of which were in incomplete remission after routine
therapy. All the patients had lost the option of accepting
HLA-identical HSCT. The overall outcome was generally acceptable, in
contrast to the results reported previously. Clinical reports have
shown that HSCT early after the induction of remission by traditional
therapeutic strategies is recommended for a curative goal.[7] Empirically, for XIAP-positive HLH, the HLH-1994 regimen is commonly used to induce remission.[1]
Meanwhile, transplantation as soon as remission has been achieved might
be the key to success. For the conditioning, we recommend a
reduced-intensity strategy in order to reduce transplant-related
mortalities. The doses used in this report had not elicited fatal
toxicities, though primary engraftment failed in two cases, who had
experienced successful transplantation when more intense
preconditioning was utilized. Analyze the reasons for engraftment,
considering that it is highly likely to be associated with
hemophagocytic syndrome and lymphocyte activation.[16-17]
Most of the patients in this group were found positive for EBV-DNA in
plasma in the early stage of the disease, and two cases had EBV viremia
post-HSCT. Therefore, virus load before transplantation might not be
associated with viremia after transplantation. The conditions of the
case who died after transplantation were complex, having experienced a
variety of deteriorations that included long-term course of the
disease, sustained application of glucocorticoids, severe intestinal
symptoms caused by Hemophagocytic syndrome before transplantation,
repeated diarrhea and gastrointestinal bleeding, and disorders in the
functions of the liver and the kidneys. Despite the successful
engraftment, this case had intestinal grade Ⅲ aGVHD after
transplantation.
A fully HLA-matched sibling donor is the primary choice for allogeneic
HSCT. However, HLH patients who are prepared for HSCT generally have
genetic factors leading to immune deficiency, so HLA-related donors
might be excluded from the same genes or immune deficiency due to the
fact that some of the primary HLH cannot be diagnosed by existing
technical means clearly. Patients with refractory or recurrent HLH
cannot exclude the genetic background or immunodeficiency, so it should
be considered that the sibling donors may have the same genetic
background. Therefore, the advantages and disadvantages should be fully
evaluated. For the above reasons, international reports also suggested
that the efficacy of non-related all-matched HLA donors was
significantly better than related fully matched donors.[7]
When the fully matched HLA is not available, HLA-haploidentical
transplantation becomes a suitable alternative. Because for primary
HLH, the majority of HLA-haploidentical donors are gene carriers, the
donor needs to be tested for cellular function. Only the donors without
obvious functional abnormalities might be chosen.[18]
In summary, HSCT is an available curative treatment for HLH patients
who are fit for the transplant indications. HLH patients within a
remission stage provide the best condition for HSCT. The effect of
transplantation in the remission stage was significantly better, so it
is recommended that HLH patients with XIAP undergo allo-HSCT as early
as possible in remission. The status of disease remission before HSCT
and the virus presence are independent prognostic factors for the
efficacy of transplantation. Virus reactivation after transplantation
is a transplant-related complication and should be treated with early
intervention. For patients who have already had the disease, timely,
effective treatment can alleviate the symptoms as soon as possible,
which is helpful in reducing the incidence of complications. It can
provide the opportunity for HSCT and improve the overall survival rate.
Acknowledgments
Grateful
acknowledgement is made to my supervisor, Prof. Yuan Sun, who gave me
considerable help through suggestions, comments, and criticism. His
encouragement and unwavering support have sustained me through
frustration and depression. Without his pushing me ahead, the
completion of this thesis would be impossible. In addition, I deeply
appreciate the contribution to this thesis made in various ways by my
friends and colleagues.
References
- Henter JI, Horne A, Arico M, et al. HLH-2004:
Diagnostic and therapeutic guidelines for hemophagocytic
lymphohistiocytosis. Pediatr Blood Cancer, 2007; 48(2): 124-131. https://doi.org/10.1002/pbc.21039 PMid:16937360
- Bryceson
YT, Pende D, Maul-Pavicic A, et al. A prospective evaluation of
degranulation assays in the rapid diagnosis of familial hemophagocytic
syndromes[J]. Blood, 2012; 119(12): 2754-2763. https://doi.org/10.1182/blood-2011-08-374199 PMid:22294731
- Trottestam
H, Beutel K, Meeths M, et al. Treatment of the X-linked
lymphoproliferative, Griscelli and Chediak-higashi syndrome by HLH
Directed Therapy. Pediatr Blood Cancer, 2009; 52(2): 268-272. https://doi.org/10.1002/pbc.21790 PMid:18937330
- Marsh
RA, Jordan MB, Allen CE, et al. Salvage therapy of refractory
hemophagocytic lymphohistiocytosis with Alemtuzumab. Pediatr Blood
Cancer, 2013; 60: 101-109. https://doi.org/10.1002/pbc.24188 PMid:22522603 PMCid:PMC3410971
- Masri
A, Bakri FG, Al-Hussaini M, et al. Griscelli syndrome type 2: a rare
and lethal disorder. J Child NeuroI 2008; 23(8): 964-967. https://doi.org/10.1177/0883073808315409 PMid:18403584
- Mallcini
AJ, Chan LS, Pallet AS. Partial albinism with immunodeficiency:
Griscelli syndrome: report of a case and review of the literature. J Am
Acad Dermatol 1998; 38(2 Pt 2): 295-300. https://doi.org/10.1016/S0190-9622(98)70568-7 PMid:9486701
- Pachlopnik
Schmid J, Moshous D, Boddaea N, et al. Hematopoietic stem cell
transplantation in Griscelli syndrome type 2: a single-center report on
10 patients. Blood, 2009; 114(1): 211-218. https://doi.org/10.1182/blood-2009-02-207845 PMid:19403888
- Marsh
RA, Villanueva J, Kim MO, et al. Patients with X-linked
lymphoproliferative disease due to BIRC4 mutation have normal invariant
natural killer T-cell populations. Clin Immunol, 2009; 132(1): 116-123.
https://doi.org/10.1016/j.clim.2009.03.517 PMid:19398375 PMCid:PMC2729708
- Gochuico
BR, Huizing M, Golas GA, et al. Interstitial lung disease and pulmonary
fibrosis in Hermansky-Pudlak syndrome type 2, an adaptor protein-3
complex disease. Mol Med, 2012; 18(1): 56-64. https://doi.org/10.2119/molmed.2011.00198 PMid:22009278 PMCid:PMC3269640
- Wenham
M, Grieve S, Cummins M, et al. Two patients with Hermansky Pudlak
syndrome type 2 and hovel mutations in AP3Bl. Haematologica, 2010;
95(2): 333-337. https://doi.org/10.3324/haematol.2009.012286 PMid:19679886 PMCid:PMC2817039
- Fontana
S, Parolini S, Vitali W, et al. Innate immunity defects in
Hermansky-Pudlak type 2 syndrome. Blood, 2006; 107(12): 4857-4864. https://doi.org/10.1182/blood-2005-11-4398 PMid:16507770
- Pachlopnik
Schmid J, Canioni D, Moshous D, et al. Clinical similarities and
differences of patients with X-linked lymphoproliferative syndrome type
1(XLP-l/SAP deficiency) versus type 2(XLP-2/XIAP deficiency). Blood,
2011; 117(5): 1522-1529. https://doi.org/10.1182/blood-2010-07-298372 PMid:21119115
- Rebecca
Marsh, Kanchan Rao, Filipovich AH, et al. Allogeneic hematopoietic cell
transplantation for XIAP deficiency: an international survey reveals
poor outcomes. Blood, 2013; 121(6): 877-883. https://doi.org/10.1182/blood-2012-06-432500 PMid:23131490 PMCid:PMC5162550
- Sharifi
R, Sinclair JC, Gilmour KC, et al. SAP mediates specific cytotoxic
T-cell functions in X-linked lymphoproliferative disease. Blood, 2004;
103(10): 3821-3827. https://doi.org/10.1182/blood-2003-09-3359 PMid:14726378
- Filipovich
AH. Hemophagocytic lymphohistiocytosis(HLH) and related disorders.
Hematology Am Soc Hematol Educ Program, 2009; 127-131. https://doi.org/10.1182/asheducation-2009.1.127 PMid:20008190
- Filipovich
AH, Zhang K, Snow AL, et al. X-linked lymphoproliferative syndromes:
brothers or distant cousins. Blood, 2010; 116(18): 3398-3408. https://doi.org/10.1182/blood-2010-03-275909 PMid:20660790 PMCid:PMC2981470
- Enders
A, Zieger B, Schwarz K, et al. Lethal hemophagocytic
lymphohistiocytosis in Hermansky-Pudlak syndrome type II. Blood, 2006;
108(1): 81-87. https://doi.org/10.1182/blood-2005-11-4413 PMid:16551969
- Marsh
RA, Madden L, Kitchen BJ, et al. XIAP deficiency: a unique primary
immunodeficiency best classified as X-linked familial hemophagocytic
lymphohistiocytosis and not as X-linked lymphoproliferative disease.
Blood, 2010; 116(7): 1079-1082. https://doi.org/10.1182/blood-2010-01-256099 PMid:20489057 PMCid:PMC2938130
- Yuhong
Liu, Xiaojun Huang, Daopei Lu, et al. A pilot study of G-CSF mobilized
allogeneic bone marrow cells plus peripheral blood stem cells
transplantation for malignant hematological diseases. National Medical
Journal of China, 2002; 82(19): 1306-1309.
- Lanping
Xu, Hu Chen, Jing Chen, et al. The consensus on indications,
conditioning regimen, and donor selection of allogeneic hematopoietic
cell transplantation for hematological diseases in China
recommendations from the Chinese Society of Hematology. Journal of
Hematology & Oncology. 2018, 11(1):33. https://doi.org/10.1186/s13045-018-0564-x PMid:29495966 PMCid:PMC5833104

