Matteo
Chinello, Olivia Chapin Arnone, Silvia Artusa, Giorgia Mazzuca, Elisa
Bonetti, Virginia Vitale, Ada Zaccaron, Dario Raniero and Simone Cesaro.
Azienda Ospedaliera Universitaria Integrata Verona. Piazzale Aristide Stefani, 1. 37126 Verona. Italy.
Published: March 01, 2024
Received: January 05, 2024
Accepted: February 12, 2024
Mediterr J Hematol Infect Dis 2024, 16(1): e2024028 DOI
10.4084/MJHID.2024.028
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
To the editor
A
Caucasian 6-year-old girl was admitted to our Hospital in July 2017
because of T-cell acute lymphoblastic leukemia (T-ALL) with central
nervous system (CNS) involvement, diagnosed at 4 years of age. The
patient underwent BFM-backbone induction chemotherapy for T-ALL
patients, which included dexamethasone, cyclophosphamide, vincristine,
daunorubicin, and intrathecal therapy with methotrexate, prednisone,
and cytarabine and achieving complete remission in both bone marrow and
CNS. The chemotherapy was continued with the consolidation phase, based
on high-dose methotrexate, with plans to perform cranial radiotherapy.
However, a CNS relapse was detected; therefore, a protocol for HR
patients was administered with the indication to proceed with the HCT.
The therapy includes three blocks of chemotherapy (HR1, HR2, HR3) and
intrathecal therapy with methotrexate, prednisone, and cytarabine. She
continued reinduction therapy with protocol III. Moreover, the patient
was a candidate for an allogeneic HCT, and a donor search was started.
After the reinduction protocol, due to the persistence of CNS
involvement, the patient underwent rescue chemotherapy treatment with a
FLAG-Myocet regimen followed by HCT. The patient underwent matched
unrelated (MUD) HSCT in April 2018. The conditioning regimen consisted
of total body irradiation (fractionated TBI, 2 Gy x 2/day for 3 days),
Rabbit anti-thymocyte globulin (ATG) 240 mg/m2/day for 2 days, and cyclophosphamide 60 mg/m2/day
for 2 days. The graft source was bone marrow, and 4.45 total nucleated
cells x 10^8/kg and 5.02 CD34+ cells x 10^6/kg were infused. GVHD
prophylaxis with cyclosporine and short-term methotrexate was
administered. Neutrophil engraftment was observed on day +24, and
platelet engraftment on day +29. On day +18, the patient developed a
grade II cutaneous GVHD, which was managed with topic and systemic
prednisone (1 mg/kg/day). On day +28, she showed signs of grade I
intestinal GVHD, which was treated with beclomethasone dipropionate.
Coproculture was negative for bacterial, viral and fungal infections.
The post-transplant period was complicated by Escherichia Coli sepsis
with concomitant posterior reversible encephalopathy syndrome (PRES),
requiring hospitalization in the pediatric intensive care unit. Three
months after HSCT, an intestinal infection by norovirus was detected,
and the patient presented signs of pulmonary and hepatic GVHD.
Intestinal GVHD worsened over several months, requiring
hospitalizations. Seven months after HCT, she presented persistent
diarrhea, vomiting, abdominal pain, and weight loss, and high doses of
steroids were administered. Specifically, beclomethasone dipropionate
(maximum dosage of 4 mg/Kg/die), prednisone (maximum dosage of 5
mg/Kg/die), and methylprednisolone (bolus of 0.5 mg/Kg of
methylprednisolone twice a week) were used. Coproculture was negative
for bacterial, viral and fungal infections. Abdominal ultrasound showed
an intestinal atony without thickening of the loops. In the following
weeks, the immunosuppressive therapy was modulated, introducing
mycophenolate mofetil and tacrolimus with a partial clinical response.
Eight
months after HCT, while she was playing with other children at home,
she was pushed to the ground, hitting the left side of her body. After
the fall, the child experienced abdominal pain, and tramadol was
administered by her mother. The girl then manifested seizures and
trismus. She was promptly taken to the emergency room, where she
arrived in cardio-circulatory arrest. Resuscitation maneuvers were
performed unsuccessfully. The body autopsy showed epicardial petechiae,
some yellowish myocardial discolorations in the interventricular
septum, hypochromic areas in the lungs and kidneys, pulmonary edema,
and bowel distension with multiple submucosal bubbles in the colon and
cecum. The microscopic examination revealed multiorgan blood stasis,
submucosal gas-filled cysts within the colon, and round air bubbles in
the renal and pulmonary basal sections. Toxicological analysis was
negative.
Discussion
Pneumatosis
cystoides intestinalis (PCI) is a relatively rare clinical condition in
which gas accumulates in the gastrointestinal tract lining, forming
cysts, specifically in the submucosal and subserosal bowel wall.[1]
PCI can be asymptomatic or present with very mild and unspecific
symptoms, including abdominal pain, diarrhea, flatulence, nausea,
obstruction, and hematochezia. The severity of clinical presentation
and outcome depends on the triggering pathologies, which could be
numerous: mechanical and traumatic factors, autoimmune and inflammatory
disease, infections, cardio-respiratory conditions, surgeries, and use
of drugs.[2,3] PCI in the non-neonatal period can be
generally considered a benign condition with spontaneous resolution in
about 80% of cases.[2] In certain specific cases, it
can become a serious and life-threatening condition. GVHD, bowel
ischemia, presence of portal venous gas, and acidosis are correlated
with poor prognosis.[2] The estimated incidence of PCI in the pediatric oncology population is 1%.[4]
The use of chemotherapeutic agents (i.e., cyclophosphamide, cytarabine,
vincristine, doxorubicin, daunorubicin, etoposide, docetaxel,
irinotecan, and cisplatin), immunosuppressive drugs (i.e.,
corticosteroids), infectious colitis, septic shock, and GVHD emerge as
important causes of PCI in the pediatric population in the non-neonatal
period.[3,5] Several
pathophysiological mechanisms leading to PCI have been described.
According to the immunosuppression theory, chemotherapeutic use and
steroid administration determine a rapid constriction of lymphatic
nodules and, as a consequence, mucosal damage and aspiration of air
from the bowel lumen.[1,5]
Furthermore, a gastrointestinal form of chronic GVHD (cGVHD) leads to
intestinal mucosal damage with the development of atrophic mucositis,
which leads to ulcers, infections, and fibrosis. This condition, along
with the concomitant use of steroid therapy, predisposes to PCI. The
development of asymptomatic PCI is a benign condition following HCT.[4]
The risk of PCI is increased in patients with gastrointestinal GVHD, in
patients receiving steroid therapy, and in those relying on
supplemental nasogastric tube feeds for at least one-half of their
total daily nutrition.[6] Cases of PCI in patients
with cGVHD described in the literature occurred 2–8 months after bone
marrow transplantation and were usually mild.[1,5,7]
In our case, the development of PCI cannot be dated exactly.
During the hospitalization, one month prior to the event, the patient
presented with persistent diarrhea, abdominal pain, weight loss, and
vomits, and such symptoms could be consistent with PCI.
The
increase in steroid therapy can certainly play a decisive role. Autopsy
and histological examination showed a thickened and emphysematous colic
wall, as well as the presence of multiple gas cysts, confirming PCI (Figure 1 and 2).
In addition, the microscopic examination also showed multiple optically
empty circular areas within the vascular sections of the lung and
kidney, consistent with gaseous bubbles. This evidence leads to the
assumption that the contents of the intestinal intraparietal cysts
embolized, then collected in the intestinal venous drainage system
pertaining to the portal system, passed through the hepatic filter, and
from there reached the heart (which had an obliterated foramen ovale),
and finally the pulmonary circulation. The diffuse pulmonary embolism
found on histological examination leads to the conclusion that a
cardio-respiratory failure was the pathophysiological process that led
to the death of the patient.
 |
Figure 1. Macroscopic appearance of the bowel during autopsy. Macroscopic appearance of open bowel during autopsy, with evidence of wall thickening and emphysema. |
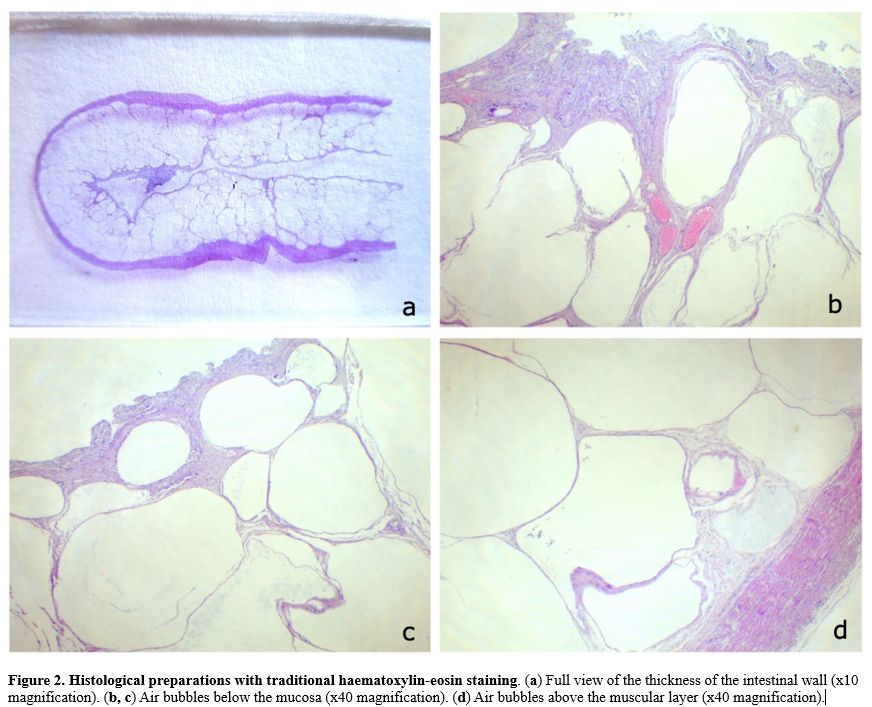 |
Figure
2. Histological preparations with traditional haematoxylin-eosin staining. (a) Full view of the thickness of the intestinal wall (x10 magnification). (b, c) Air bubbles below the mucosa (x40 magnification). (d) Air bubbles above the muscular layer (x40 magnification).
|
It
should be noted that the mild abdominal trauma that the patient
suffered from could represent a contributing factor towards the
initiation of the overall process. Portosystemic air emboli are a rare
but mortal complication of PCI described only in a few pediatric cases.[8]
PCI can be detected through an X-ray of the digestive tract; however,
computed tomography (CT) and endoscopy are the methods of choice for
the diagnosis.[5,7] There is no standardized treatment for PCI; it is usually managed conservatively, while complications can require surgery.[1] To our knowledge, this is the first case of death from portosystemic embolism in a child with intestinal GVHD and PCI.
References
- Di Pietropaolo M, Trinci M, Giangregorio C,
Galluzzo M, Miele V. Pneumatosis cystoides intestinalis: case report
and review of literature. Clin J Gastroenterol. 2020 Feb;13(1):31-36. https://doi.org/10.1007/s12328-019-00999-3 PMid:31161540
- Kurbegov AC, Sondheimer JM. Pneumatosis intestinalis in non-neonatal pediatric patients. Pediatrics. 2001 Aug;108(2):402-6. https://doi.org/10.1542/peds.108.2.402 PMid:11483806
- Shulman
SC, Chiang F, Haight AE, Steelman CK, Chiang KY, Gow K, Shehata BM.
Pneumatosis intestinalis in pediatric hematopoietic stem cell
transplantation patients: an uncommon complication. Fetal Pediatr
Pathol. 2012 Oct;31(5):309-14. https://doi.org/10.3109/15513815.2012.659389 PMid:22432915
- Bailey
KA, Kodikara H, Mauguen A, Price A, LaQuaglia M, Boulad F. Pneumatosis
intestinalis in the pediatric oncology population: An 11-year
retrospective review at Memorial Sloan Kettering Cancer Center. Pediatr
Blood Cancer. 2022 Jul;69(7):e29539. doi: 10.1002/pbc.29539. Epub 2021
Dec 28. PMID: 34962703. https://doi.org/10.1002/pbc.29539 PMid:34962703 PMCid:PMC10499335
- Laskowska
K, Burzyńska-Makuch M, Krenska A, Kołtan S, Chrupek M, Nawrocka E,
Lasek W, Serafin Z. Pneumatosis cystoides interstitialis: A
complication of graft-versus-host disease. A report of two cases. Pol J
Radiol. 2012 Apr;77(2):60-3. https://doi.org/10.12659/PJR.882972 PMid:22844311 PMCid:PMC3403803
- Frolova
Gregory P, Angus J, Brothers AW, Gray AN, Skeen K, Gooley T, Davis C,
Kim HHR, Weissman SJ, Zheng HB, Mallhi K, Baker KS. Risk Factors for
Development of Pneumatosis Intestinalis after Pediatric Hematopoietic
Stem Cell Transplantation: A Single-Center Case-Control Study.
Transplant Cell Ther. 2022 Nov;28(11):785.e1-785.e7. https://doi.org/10.1016/j.jtct.2022.08.023 PMid:36038104
- McCarville
MB, Whittle SB, Goodin GS, Li CS, Smeltzer MP, Hale GA, Kaufman RA.
Clinical and CT features of benign pneumatosis intestinalis in
pediatric hematopoietic stem cell transplant and oncology patients.
Pediatr Radiol. 2008 Oct;38(10):1074-83. https://doi.org/10.1007/s00247-008-0944-4 PMid:18665358 PMCid:PMC3612433
- Yalιndağ
Öztürk MN, Tüney D. Pneumatosis intestinalis and fatal portosystemic
air emboli. Intensive Care Med. 2019 Dec;45(12):1827-1828. https://doi.org/10.1007/s00134-019-05712-z PMid:31359079

