Uğur Önal1,2, Deniz Akyol Seyhan1,9, Olcay Buse Ketenoğlu1, Merve Mert Vahabi1, Dilşah Başkol Elik1,10, Seichan Chousein Memetali1, Gamze Şanlıdağ İşbilen1, Cansu Bulut Avşar1,11, Arda Kaya1, Ayse Uyan-Önal3, Nazlıhan Yalçın1, Günel Guliyeva1,12, Şükrü Dirik1, Oğuzhan Acet1, Damla Akdağ1,13, Melike Demir Görür1, Osman Bozbıyık4, Berk Göktepe4, Tufan Gümüş4, İlkin Çankayalı5, Kubilay Demirağ5, Mehmet Uyar5, Hilal Sipahi6, Huseyin Aytac Erdem1, Meltem Işıkgöz Taşbakan1, Bilgin Arda1, Şöhret Aydemir7, Sercan Ulusoy1 and Oguz Resat Sipahi1,8.
1 Ege University, Faculty of Medicine, Department of Infectious Diseases and Clinical Microbiology, Bornova, Izmir, Turkey
2 Uludag University, Faculty of Medicine, Department of Infectious Diseases and Clinical Microbiology, Bursa, Turkey
3 Yüksek İhtisas Research and Teaching Hospital, Department of Infectious Diseases and Clinical Microbiology, Bursa, Turkey
4 Ege University, Faculty of Medicine, Department of General Surgery, Bornova, Izmir, Turkey
5 Ege University, Faculty of Medicine, Department of Anesthesiology and Intensive Care, Bornova, Izmir, Turkey
6 Bornova Directory of Health, Bornova, Izmir, Turkey
7 Ege University, Faculty of Medicine, Department of Microbiology, Bornova, Izmir, Turkey
8 King Hamad University Hospital, Bahrain Oncology Center, Department of Oncology Infectious Diseases, AlMuharraq, Bahrain.
Published: July 01, 2024
Received: January 17, 2024
Accepted: June 14, 2024
Mediterr J Hematol Infect Dis 2024, 16(1): e2024051 DOI
10.4084/MJHID.2024.051
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background: This
study aimed to evaluate the epidemiology of septic shock (SS)
associated with intraabdominal infections (IAI) as well as associated
mortality and efficacy of early source control in a tertiary-care
educational hospital.
Methods:
Patients who had SS with IAI and consulted by Infectious Diseases
consultants between December 2013 and October 2022 during night shifts
in our centre were analyzed retrospectively.
Results: A
total number of 390 patients were included. Overall, 30-day mortality
was 42.5% on day 3, while day 14 and 30 mortality rates were 63.3% and
71.3%, respectively. Source control by surgical or percutaneous
operation was performed in 123 of 390 cases (31.5%), and the mortality
rate was significantly lower in cases that were performed source
control at any time during SS (65/123-52.8% vs 213/267-79.8%,
p<0.001). In 44 of 123 cases (35.7%), source control was performed
during the first 12 hours, and mortality was significantly lower in
this group versus others (24/44-54.5% vs 254/346-73.4%, p=0.009). On
the other hand, female gender (p<0.001, odds ratio(OR)= 2.943,
95%CI=1.714-5.054), diabetes mellitus (p= 0.014, OR=2.284,
95%CI=1.179-4.424), carbapenem-resistant Gram-negative etiology
(p=0.011, OR=4.386, 95%CI=1.398-13.759), SOFA≥10 (p<0.001, OR=3.036,
95%CI=1.802-5.114), lactate >3 mg/dl (p<0.001, OR=2.764,
95%CI=1.562-4.891) and lack of source control (p=0.001, OR=2.796,
95%CI=1.523-5.133) were significantly associated with 30-day mortality
in logistic regression analysis.
Conclusion:
Source control has a vital importance in terms of mortality rates for
IAI-related septic shock patients. Our study underscores the need for
additional research, as the present analysis indicates that early
source control does not manifest as a protective factor in logistic
regression.
|
Introduction
Sepsis
is currently defined as an infection with organ dysfunction. Sepsis and
septic shock are important global health problems causing significant
mortality and morbidity rates.[1-3] Fast and proper
management of septic shock is important in terms of patient survival.
Proper management includes early initiation of the antibiotics after
the microbial cultures/testing, as well as prompt infectious source
control.[4] Infectious source control in
intra-abdominal infections comprises the rapid diagnosis of a specific
infectious site and its control via a surgical intervention such as
drainage of an abscess, necrotic tissue debridement, or removal of an
infected device. Several studies have underscored the significance of
timely source control interventions. Bloos et al. conducted a
comprehensive study demonstrating that delays in surgical source
control are significantly associated with increased mortality rates in
patients with sepsis. Their findings revealed that every hour of delay
in surgical intervention was linked to a 1% rise in 28-day mortality,
with notably higher mortality rates observed when surgical source
control was delayed beyond 6 hours.[5] Similarly,
Azuhata et al. investigated the impact of early initiation of surgical
source control in patients with gastrointestinal perforation and septic
shock. Their results emphasized the critical importance of prompt
surgical intervention, with survival rates declining substantially as
the time to initiation of surgery increased, reaching 0% for delays
exceeding 6 hours.[6] These studies collectively
highlight the imperative of promptly identifying and implementing
appropriate source control measures to improve outcomes in patients
with sepsis and septic shock. This aligns with recommendations from the
2021 Surviving Sepsis Campaign guideline, which emphasizes the need for
expedited source control interventions to rapidly identify or exclude
specific anatomical diagnoses of infection requiring emergent source
control, implementing any necessary interventions as soon as medically
and logistically practical.[7] Herein, we aimed to
evaluate the epidemiology of septic shock associated with
intra-abdominal infections (IAI) as well as associated mortality and
efficacy of early source control (during the first 12 h or afterward)
in a tertiary-care educational hospital.
Material and Methods
This
study was performed in an 1800+-bedded tertiary-care educational
university hospital located in a city with a population of 4.3 million
in 2017.[8]
Patients with septic shock (sepsis+hypotension+adrenergic agent+arterial lactate level of >2mg/dL)[2] and IAI and consulted by Infectious Diseases consultants during night shifts between the December 1st, 2013, and October 15th,
2022, in our center were recorded prospectively and analyzed
retrospectively. The patients were evaluated following the first visit
on days 3, 14, and 30. Septic shock definition was considered to be
sepsis with hypotension requiring vasopressors to maintain a mean
arterial blood pressure above 65 mm Hg despite adequate fluid
resuscitation.[1] Systemic inflammatory response
syndrome (SIRS), Quick Sequential Organ Failure Assessment (qSOFA), and SOFA (Sequential Organ Failure Assessment)
scores with suspected infection were used for the definition of sepsis.[2,7]
SIRS was defined by several clinical variables, including temperature
>38°C or <35°C, heart rate >90 beats/min, respiratory rate
>20 breaths/min or PCO2 <32 mmHg, and WBC >12000 cells/mm3 or <4000 cells/mm3.
After that, 2 or more points increase in SOFA score or qSOFA score was used for the definition of
sepsis. For qSOFA, the following data were used: 1 point for each
systolic arterial blood pressure ≤100 mm Hg, respiratory rate above 21
breaths per minute, or altered mental status.
Case assessment
forms included data related to the demographical and clinical findings
of septic shock patients, as well as microbiological culture results,
surgical operation schedules, and mortality rates. Clinical assessment
and radiological or surgical operation reports determined underlying
intra-abdominal infectious foci.
The inclusion criteria were:
- Age ≥ 18-year-old (adult patients were included)
-
Meeting the criteria of the septic shock described above as well as a
final diagnosis of IAI, which was defined as a complicated IAI
infection that extended beyond the hollow viscus of origin into the
peritoneal space and was associated with either abscess formation or
peritonitis, whereas, uncomplicated infection involved intramural
inflammation of the gastrointestinal tract and had a substantial
probability of progressing to complicated infection if not adequately
treated.[9]
The exclusion criteria were:
- The presence of an infectious source other than IAI
-
IAI patients from other hospitals are referred to our center without
exact intervention time records, and patients are referred from our
center to other centers due to the need for more available beds in
intensive care units (ICU).
Microbiological analysis.
Peripheral/catheter blood cultures were inoculated into aerobic and
anaerobic culture bottles (BacT/ALERT, BioMérieux, Durham, USA), and an
automated microbial detection system (BacT/ALERT 3D, BioMérieux,
Durham, USA) was used. Signal-positive blood culture samples, together
with other samples like urine, intra-abdominal fluid/pus, etc, were
inoculated into 5% blood sheep agar and Eosin Methylene-blue Lactose
Sucrose (EMB) agar (BioMerieux, France). MALDI-TOF mass spectrometry
(VITEK MS, BioMérieux, France) was used for microbial identification.
Antibiotic sensitivity tests were performed using the VITEK2
(BioMérieux, France) system and evaluated according to EUCAST criteria.[10]
Carbapenem minimum inhibitory concentration (MIC) levels were
determined by gradient tests (E test, BioMérieux, France). An antimicrobial regimen
was considered to be appropriate when the isolated pathogens were found
to be sensitive to the empirically started antibiotics according to the
antibiotic susceptibility test results.
Ethics. The local Institutional Review Board approved the study (21-6.IT/63 on 25/06/2021).
Statistical analysis. SPSS
25.0 program (Statistical package for the social sciences) was used for
the statistical analysis. A comparison of categorical values between
the two groups was performed via the Chi-square test. Student t-test
was performed for the numerical values of the independent groups.
Binary
logistic regression was performed using the enter method. Day 30
mortality rate was the dependent variable, and the variables with a
p<0.05 in univariate analysis were used as covariates, i.e., source
control, source control within the first 12 hours, lactate>3 mg/dL.
A p-value of less than 0.05 was considered significant.
Results
General characteristics.
There were a total of 390 patients (mean age 67.26 ±0.73 years and
44.6% female) fulfilling the study inclusion criteria. A total of 166
(42.6%) cases were aged ≥65-year-old. Mean leucocyte, CRP,
procalcitonin, and lactate levels at the time of septic shock diagnosis
were 18621 ± 1738/mm3, 143.05 ± 6.09 mg/L, 34.47 ± 4.81 µg/L, and 11.94 ± 0.95 mg/dL, respectively.
Comorbidities
were recorded as 122 patients (31.3%) having malignancy, 103 patients
(26.4%) having hypertension, 97 patients (24.9%) having diabetes
mellitus, 39 patients (10%) having congestive heart failure, and 27
patients (6.9%) having chronic renal failure.
Table 1 summarizes the underlying IAI foci. The most common intra-abdominal problem was peritonitis without perforation (20%).
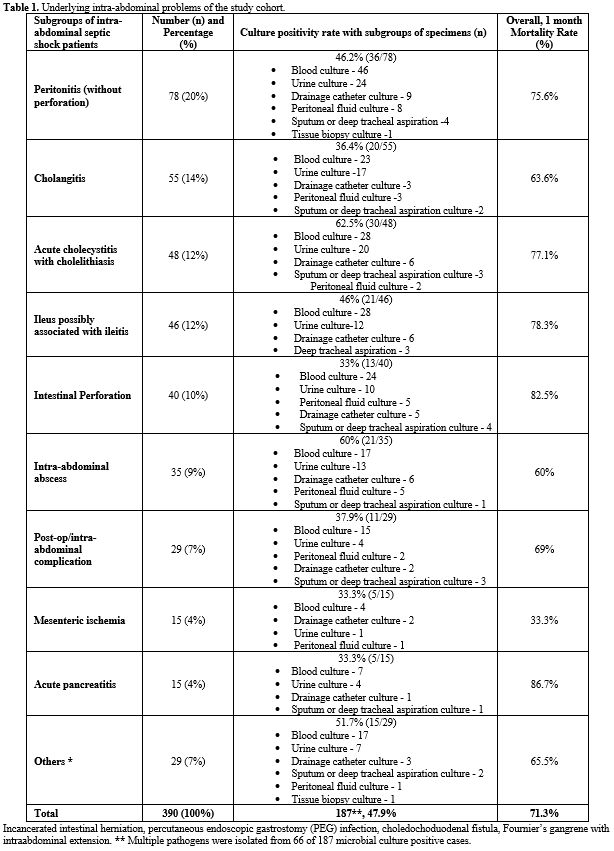 |
- Table
1. Underlying intra-abdominal problems of the study cohort.
|
Etiological agents. Microbiological etiology was elucidated in 187 (47.9%) of 143 IAI cases (Table 1, 2). The most common pathogen was E.coli (73/187, 39%, Table 2),
and multiple pathogens were isolated from 66 of 187 microbial
culture-positive cases. Carbapenem-resistance in Gram-negative
pathogens was recorded in 42 of 187 microbiologically-confirmed IAI
cases (22.5%), and 22 of the 42 (52.4%) were carbapenem-resistant
K.pneumoniae. Overall carbapenem-resistance among Gram-negative
bacteria was 31.3% (42/134), and overall extended-spectrum
beta-lactamase positivity among Gram-negative bacteria was 48.5%
(65/134). Yeasts yielded in 15% of the etiologically confirmed cases,
and they were the 4th most common etiological agent.
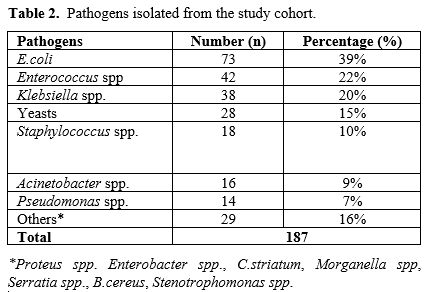 |
- Table 2. Pathogens isolated from the study cohort.
|
Mortality and associated factors.
Overall mortality was 42.5% (166/390) on day 3, while day 14 and day 30
mortality rates were 63.3% (247/390) and 71.3% (278/390), respectively.
In females, 30-day mortality was significantly higher than in males
(138/174 vs 140/216, p=0.002), but there was no significant difference
in mortality between cases aged ≥ 65 years vs. others. (Table 3).
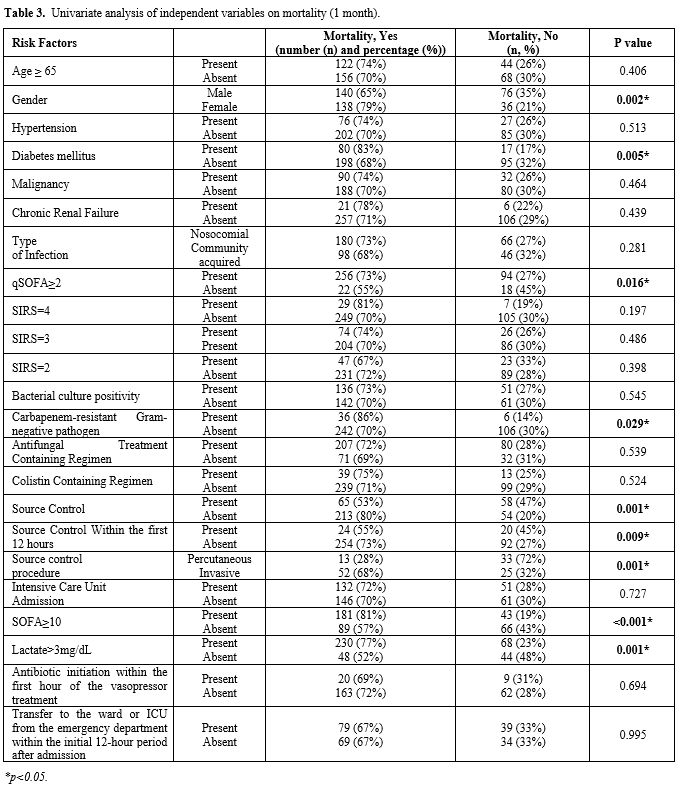 |
- Table 3. Univariate analysis of independent variables on mortality (1 month).
|
In
terms of laboratory findings during the first evaluation of the septic
shock case, 30-day mortality among the cases with lactate levels>3
mg/dL was significantly higher (230/298-77% vs 48/92-52%; p<0.001).
There was no significant difference in the mean levels of leucocytes
(19550 ± 2331/mm3 vs. 16316 ± 1776/mm3,
p=0.401), CRP levels (146.7 ± 7.28 mg/L vs. 133.9 ± 11.14 mg/L,
p=0.342), and procalcitonin levels (33.17 ± 5.79 μg/L vs. 37.11 ± 8.77
μg/L, p=0.704) between patients who had 30-day mortality and those who
survived.
The mean SOFA score was 10.33 ± 0.16 in 374 patients,
while the median qSOFA score was 3 in 244 out of 390 patients. The mean
SOFA score among cases with 30-day mortality was 10.93 ± 0.18 versus
8.84 ± 0.28 among survived cases (p<0.001). The mortality rate among
the patients with qSOFA ≥2 was significantly higher than others (p=0.016). The overall univariate analysis for 30-day mortality is in Table 3.
Antimicrobial therapy/etiology versus mortality. 30-day mortality in cases with elucidated bacterial etiology vs. others did not change significantly (Table 3).
Comparing 30-day mortality in cases with Gram-negative etiology versus
all others, we observed rates of 98/134 vs. 180/256 (p=0.559). When we
compared, 30-day mortality in cases with carbapenem-resistant
Gram-negative etiology vs. all others was 86% (36/42 vs. 242/348,
p=0.029), and in cases with ESBL-producing Gram-negative bacteria all
others was 63.1% (41/65 vs. 237/325; p=0.109). However, 30-day
mortality was significantly higher in those with carbapenem-resistant
Gram-negative etiology vs. carbapenem-sensitive Gram-negative etiology.
In 36 of the 187 microbiological culture-positive patients, the
antimicrobial regimen did not cover the isolated pathogens at the first
visit, and 30-day mortality among them vs. others did not differ
significantly (23/36-63.9% vs 113/151-74.8%, p=0.185).
Two
hundred seven of 287 patients received antifungal-containing treatment,
and there was no significant difference in mortality rates among them
vs others (207/287-72% vs 71/103-69%, p=0.539). Furthermore, 42 cases
had microbiological culture positivity for carbapenem-resistant
Gram-negative pathogens, and 14 of them received a colistin-containing
regimen with a 30-day mortality of 85.7% (12/14 colistin-receiving
cases vs. 24/28 not colistin receiving cases, p=1.000).
When
overall 390 cases were considered, 30-day mortality was not
significantly different among cohorts in antifungal-containing regimens
vs. others (207/287-72% vs 71/103-69%, p=0.539) and in
colistin-containing regimens vs. others (39/52-75% vs 239/338-71%,
p=0.524). There was no statistically significant difference in
mortality among the patients with polymicrobial etiology versus
culture-positive cases for single pathogen (50/66-76% vs 86/121-71%,
p=0.492).
Impact of Source Control. Source
control by surgical or percutaneous operation was performed in 123 of
390 cases (31.5%), and 30-day mortality was significantly lower in
cases that were performed source control at any time during the septic
shock period vs. others (65/123-%53 vs. 213/267-80%, p<0.001, Table 3).
Additionally, in 44 out of 123 cases (35.7%), source control was
performed within the first 12 hours. The 30-day mortality rate was
significantly lower in this group compared to others (those in whom
source control was performed after the first 12 hours + source control
was not performed anytime during the septic shock period-24/44 or 55%
vs 254/346 or 73%, p=0.009, refer to Table 3).
However, 30-day mortality did not differ in those source controls
performed during the first 12 h versus any other time during the septic
shock period (24/44-55% vs. 41/79-57%, p=0.854).
In 77 of 123
cases (62.6%), invasive surgical operation/laparotomy was performed,
while percutaneous source control was performed in 46 cases (37.4%).
Mortality rates among the invasive surgical operation subgroup were
statistically higher than the percutaneous source control group
(52/77-67.5% vs 13/46-28.3%, p<0.001). The mean SOFA scores did not
change significantly (9.80±0.52) in the invasive source controlled
group vs. 10.11±0.71 in the percutaneous source controlled group
(p=0.752).
30-day mortality was found to be similar among cases
with intra-abdominal abscess vs. others (21/35-60% vs. 257/355-72.4%,
p=0.122) and among cases with organ or intestinal perforation vs.
others (33/40-82.5% vs 244/349-69.9%, p=0.096). Invasive surgical
operations were performed more commonly among cases with organ or
intestinal perforation in source controlled group (35/37-94.6% vs.
42/86-48.8%, p<0.001), and percutaneous source control was performed
more commonly among cases with intra-abdominal abscess (19/22-86.4% vs
27/101-26.7%, p<0.001) in source-controlled group. Univariate
analyses of independent variables on 30-day mortality are shown in Table 3.
Multivariate analysis.
In logistic regression analysis, female gender (p<0.001, odds ratio
(OR)=2.943, 95%CI=1.714-5.054), diabetes mellitus (p=0.014,
OR=2.284, 95%CI=1.179-4.424), carbapenem-resistant Gram-negative
etiology (p=0.011, OR=4.386, 95%CI=1.398-13.759), SOFA≥10 (p<0.001,
OR=3.036, 95%CI=1.802-5.114), lactate >3 mg/dl (p<0.001,
OR=2.764, 95%CI=1.562-4.891) and lack of source control (p=0.001,
OR=2.796, 95% CI =1.523-5.133) were significantly associated with
30-day mortality (Table 4).
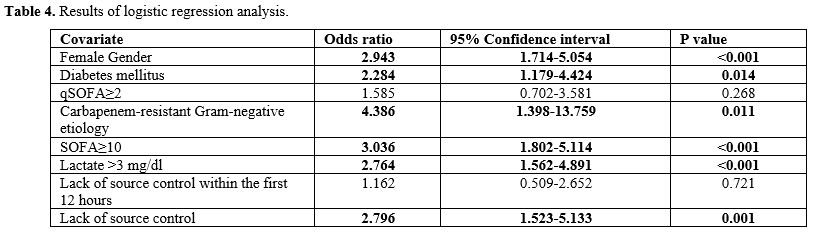 |
- Table 4. Results of logistic regression analysis.
|
Discussion
Herein,
we analyzed the outcomes of 390 SS with IAI. The 30-day mortality rate
was 71.3%. Source control by surgical or percutaneous operation was
performed in 31.5%, and in 35.7% of them, source control was performed
during the first 12 hours. On the other hand, female gender, diabetes
mellitus, carbapenem-resistant Gram-negative etiology, SOFA≥10, lactate
>3 mg/dl, and lack of source control were significantly associated
with 30-day mortality in logistic regression analysis.
Source
control in patients with septic shock is vital and recommended by the
guidelines. However, the evidence regarding optimal timing for these
interventional approaches is relatively limited.[5-7]
Thus, this paper is one of the unique studies that emphasizes the
impact of source control together with the optimal duration of surgical
intervention, especially for the subgroup of septic shock patients with
IAI.
In 2003, the Turkish Ministry of Finance, which is
responsible for the payback of over 90% of the population's health
expenditures, eased a new budget application instruction for regulating
the usage of parenteral antibiotics inside and outside of the
hospitals. The instruction took effect on March 1st,
2003. According to this instruction, the payback of extended-spectrum
antimicrobials (vancomycin, teicoplanin, meropenem, imipenem,
antifungals, etc.) has been restricted without prior approval of
infectious diseases specialist (IDS). Hence, all septic shock cases
that were considered to be starting extended-spectrum antibiotics or
consulted to Infectious Diseases Consultants during night shifts were
included in the study.[11-13]
Martinez et al.[14]
performed a multicenter observational study in Spain with 3663 severe
sepsis or septic shock patients. Of these, 1234 cases had the abdominal
site of infection source, with 788 (67.2%) patients requiring source
control included. They reported that the crude ICU mortality rate was lower for those who underwent source control
(21.2% vs 25.1%; p=0.010), but the source control after 12 hours was
not found statistically significant in terms of mortality (27.6% vs
26.8%; p=0.789).[14] Kim et al. also performed a
multicenter observational study with a total number of 2250 septic
shock patients visiting 11 different Emergency Departments. They
reported that 28-day mortality was significantly lower in patients who
underwent source control (p<0.001). However, no significant
association was noted between the duration of source control after 6
hours or 12 hours and mortality.
Additionally, out of the 2,250
patients, 46.6% met the criteria for Sepsis-3 septic shock, and 26.8%
underwent source control; nevertheless, source control conducted after
6 or 12 hours showed no significant association with 28-day mortality
in Sepsis-3 septic shock, with adjusted hazard ratios of 1.309 (95% CI:
0.612–2.797, p=0.488) and 1.344 (95% CI: 0.612–2.951, p=0.462).[15]
The guidelines of the Infectious Diseases Society of America (IDSA)
about the diagnosis and management of complicated IAI in adults and
children recommends (B-II) an appropriate source control procedure for
nearly all patients with IAI.[16] This guideline also
recommends (B-II) an urgent approach for hemodynamically stable
patients without acute organ failure. Intervention may be delayed for
as long as 24 hours with appropriate antimicrobial therapy plus close
clinical monitoring. In contrast, it recommends (B-II) that patients
with diffuse peritonitis should undergo an emergency surgical procedure
as soon as possible.[16] Furthermore, the
guideline recommends (B-II) that a percutaneous approach of abscesses
and other well-localized fluid collections, if feasible, is preferable
to surgical drainage.[16] The percutaneous approach
of selected cases may cause fewer physiologic alterations with more
acceptable mortality and morbidity rates and may eliminate or reduce
the need for open techniques.[17,18] Marshall et al.
also reviewed that source control represents a key component of success
in the therapy of sepsis, which includes drainage of infected fluids,
debridement of infected soft tissues, removal of infected devices or
foreign bodies, and definite measures to correct anatomic derangement.
Thus, they emphasized that appropriate source control should be part of
the systematic checklist in sepsis with Grade E recommendation.[19]
Hecker et al. reviewed that an IAI source could be detected in 66% of
all surgical patients with sepsis, and the 28-day mortality rate
increased from 26.7% to 42.9% in patients with inadequate initial
source control.[20] Unlikely the literature, 30-day
mortality was found to be as high as 71.3% (53% in the
source-controlled group versus 80% in the non-source-controlled group)
in our study. In comparison to a multicenter study in our country
reporting 6.9% of patients meeting SEPSIS-III criteria for septic shock
with a 75.9% mortality rate, our study found a similar 30-day mortality
(71.3%) with a focus on Sepsis-3 septic shock patients, suggesting the
potential influence of patient selection on the elevated mortality.[21]
Van
de Groep et al. performed a retrospective study with a total number of
353 critically ill patients with IAI, and they concluded that the
persistence of organ failure on day 14 was associated with inadequacy
of source control while the clinical outcomes were similar between the
patients having a surgical versus percutaneous initial approach to
source containment.[22] In our study, the mortality
was significantly lower in the group source control was performed
(65/123 vs 213/267, p<0.001). These data suggest that the source of
infection is one of the most important factors in septic shock patients
in terms of management and prognosis. Aligned with existing literature,
effective source control, encompassing interventions such as surgical
procedures or percutaneous drainage, is essential for eradicating the
infectious focus and decreasing mortality rates. We believe that source
control plays a pivotal role in managing intraabdominal septic shock,
highlighting the critical need to promptly address the infection's
source for effective management. Despite its significance in the larger
picture, source control executed within the initial 12 hours did not
emerge as a protective factor against mortality. This nuanced result
sheds light on the complexities of timing and effectiveness in source
control, highlighting that achieving source control within a specific
time frame may not necessarily confer a survival advantage in septic
shock patients with intraabdominal infection. Further investigation and
analysis are imperative to understand the intricacies of this
relationship and to refine our approach to source control in septic
shock scenarios.
Jung et al. reported that procalcitonin kinetics
failed to predict the outcome in 101 consecutive cases that had
perioperative abdominal infection with septic shock.[23]
Wang et al. showed that the most accurate index was the SOFA score for
predicting the outcome of patients with sepsis caused by abdominal
cavity infection when the threshold value was 9.50. They found that
sensitivity and specificity were 81.2% and 83.5%, respectively.[24]
In our study, we also found that there was no statistically significant
difference in the mean values of CRP, procalcitonin, and leucocyte
levels among the mortal cases vs. others. On the other hand, SOFA score
≥10 (OR=3.036, 95%CI=1.802-5.114, p<0.001) was significantly
associated with 30-day mortality in logistic regression analysis.
Conversely, we believe that additional data are necessary to
demonstrate the prognostic value of these biomarkers and scoring
systems.
The results of the multicentered cohort study by Arvaniti et al.[25]
involving 2337 critically ill elderly adults with intra-abdominal
infection shed light on crucial risk factors impacting mortality such
as late-onset hospital-acquired intra-abdominal infection, diffuse
peritonitis, sepsis/septic shock, source control failure, and
underlying conditions like liver disease, congestive heart failure,
diabetes mellitus, and malnutrition. Our study also revealed distinctive
correlations, underscoring that factors like female gender, diabetes
mellitus, carbapenem-resistant Gram-negative etiology, SOFA score
(≥10), elevated lactate levels (>3 mg/dl), and inadequate source
control were notably associated with poorer outcomes. These
observations stress the necessity for customized interventions
targeting these specific risk elements to enhance patient outcomes in
IAI cases.
Our study has several limitations, such as the
mortality rates were recorded as all-cause mortality (autopsy could not
been performed), repeated source control procedures could not be
investigated, and timing of "12 hours" was considered to be a cut-off
time for source control. However, to our knowledge, this is one of the
unique and the largest studies performed solely in septic shock cases
and concluding that surgery performed after 12 h may also contribute to
survival and management of these groups of patients and that showed no
difference between early source control vs. source control anytime
during the septic shock episode. In addition, this is the first study
from our country regarding the source control in septic shock patients
with IAI.
Conclusions
These
data suggest that source control has major and vital importance in
terms of mortality rates for IAI-related septic shock patients. This
effect was regardless of the timing (< or >12 h) in our study
sample. The more favorable outcomes observed during the source control
via percutaneous procedures in our cohort need to be confirmed in
further studies.
References
- Cecconi M, Evans L, Levy M, Rhodes A. Sepsis and septic shock. Lancet. 2018;392:75-87 https://doi.org/10.1016/S0140-6736(18)30696-2 PMid:29937192
- Singer
M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M,
Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy
MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T,
Vincent JL, Angus DC. The Third International Consensus Definitions for
Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:801-10 https://doi.org/10.1001/jama.2016.0287 PMid:26903338 PMCid:PMC4968574
- Akın
A, Alp E, Altındiş M, Azak E, Batırel A, Çağ Y, Durmuş G, Kepenek Kurt
E, Sağıroğlu P, Türe Z, Candevir Ulu A, Ekmud Sepsis Working Group.
Current Diagnosis and Treatment Approach to Sepsis. Mediterr J Infect
Microb Antimicrob. 2018;7:17 https://doi.org/10.4274/mjima.2018.17
- Howell MD, Davis AM. Management of Sepsis and Septic Shock. JAMA. 2017;317:847-8 https://doi.org/10.1001/jama.2017.0131 PMid:28114603
- Bloos
F, Rüddel H, Thomas-Rüddel D, Schwarzkopf D, Pausch C, Harbarth S,
Schreiber T, Gründling M, Marshall J, Simon P, Levy MM, Weiss M,
Weyland A, Gerlach H, Schürholz T, Engel C, Matthäus-Krämer C, Scheer
C, Bach F, Riessen R, Poidinger B, Dey K, Weiler N, Meier-Hellmann A,
Häberle HH, Wöbker G, Kaisers UX, Reinhart K; MEDUSA study group.
Effect of a multifaceted educational intervention for anti-infectious
measures on sepsis mortality: a cluster randomized trial. Intensive
Care Med. 2017;43:1602-12 https://doi.org/10.1007/s00134-017-4782-4 PMid:28466151
- Azuhata
T, Kinoshita K, Kawano D, Komatsu T, Sakurai A, Chiba Y, Tanjho K. Time
from admission to initiation of surgery for source control is a
critical determinant of survival in patients with gastrointestinal
perforation with associated septic shock. Crit Care. 2014;18:R87 https://doi.org/10.1186/cc13854 PMid:24886954 PMCid:PMC4057117
- Evans
L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C,
Machado FR, Mcintyre L, Ostermann M, Prescott HC, Schorr C, Simpson S,
Wiersinga WJ, Alshamsi F, Angus DC, Arabi Y, Azevedo L, Beale R,
Beilman G, Belley-Cote E, Burry L, Cecconi M, Centofanti J, Coz Yataco
A, De Waele J, Dellinger RP, Doi K, Du B, Estenssoro E, Ferrer R,
Gomersall C, Hodgson C, Hylander Møller M, Iwashyna T, Jacob S,
Kleinpell R, Klompas M, Koh Y, Kumar A, Kwizera A, Lobo S, Masur H,
McGloughlin S, Mehta S, Mehta Y, Mer M, Nunnally M, Oczkowski S, Osborn
T, Papathanassoglou E, Perner A, Puskarich M, Roberts J, Schweickert W,
Seckel M, Sevransky J, Sprung CL, Welte T, Zimmerman J, Levy M.
Surviving Sepsis Campaign: International Guidelines for Management of
Sepsis and Septic Shock 2021. Crit Care Med. 2021;49:e1063-e1143 https://doi.org/10.1097/CCM.0000000000005337 PMid:34605781
- Oner
AC, Durmaz-Drinkwater B, Grant RJ. Precarity of refugees: the case of
Basmane-İzmir, Turkey. Journal of Ethnic and Migration Studies.
2020;47:4651-70 https://doi.org/10.1080/1369183X.2020.1732591
- Solomkin
JS, Mazuski JE, Bradley JS, Rodvold KA, Goldstein EJ, Baron EJ, O'Neill
PJ, Chow AW, Dellinger EP, Eachempati SR, Gorbach S, Hilfiker M, May
AK, Nathens AB, Sawyer RG, Bartlett JG. Diagnosis and management of
complicated intra-abdominal infection in adults and children:
guidelines by the Surgical Infection Society and the Infectious
Diseases Society of America. Surg Infect (Larchmt). 2010;11:79-109 https://doi.org/10.1089/sur.2009.9930 PMid:20163262
- Brown
DF, Wootton M, Howe RA. Antimicrobial susceptibility testing
breakpoints and methods from BSAC to EUCAST. J Antimicrob Chemother.
2016;71:3-5 https://doi.org/10.1093/jac/dkv287 PMid:26377864
- Sipahi OR. Economics of antibiotic resistance. Expert Rev Anti Infect Ther. 2008;6:523-39 https://doi.org/10.1586/14787210.6.4.523 PMid:18662118
- Arda
B, Sipahi OR, Yamazhan T, Tasbakan M, Pullukcu H, Tunger A, Buke C,
Ulusoy S. Short-term effect of antibiotic control policy on the usage
patterns and cost of antimicrobials, mortality, nosocomial infection
rates and antibacterial resistance. J Infect. 2007;55:41-8 https://doi.org/10.1016/j.jinf.2007.02.014 PMid:17512598
- Önal
U, Akyol D, Mert M, Başkol D, Memetali SC, Şanlıdağ G, Kenanoğlu B,
Uyan-Önal A, Quliyeva G, Avşar CB, Akdağ D, Demir M, Erdem HA, Kahraman
Ü, Bozbıyık O, Özgiray E, Bozkurt D, Akarca FK, Demirağ K, Çankayalı İ,
Uyar M, Çilli F, Arda B, Yamazhan T, Pullukçu H, Taşbakan MI, Sipahi H,
Ulusoy S, Sipahi OR. Carbapenem-resistant Gram-negative pathogens
associated with septic shock: a review of 120 cases. J Chemother.
2022;34:436-45 https://doi.org/10.1080/1120009X.2022.2064703 PMid:35446235
- Martínez
ML, Ferrer R, Torrents E, Guillamat-Prats R, Gomà G, Suárez D,
Álvarez-Rocha L, Pozo Laderas JC, Martín-Loeches I, Levy MM, Artigas A;
Edusepsis Study Group. Impact of Source Control in Patients With Severe
Sepsis and Septic Shock. Crit Care Med. 2017;45:11-9 https://doi.org/10.1097/CCM.0000000000002011 PMid:27611975
- Kim
H, Chung SP, Choi SH, Kang GH, Shin TG, Kim K, Park YS, Han KS, Choi
HS, Suh GJ, Kim WY, Lim TH, Ko BS; Korean Shock Society (KoSS)
Investigators. Impact of timing to source control in patients with
septic shock: A prospective multicenter observational study. J Crit
Care. 2019;53:176-82 https://doi.org/10.1016/j.jcrc.2019.06.012 PMid:31247517
- Solomkin
JS, Mazuski JE, Bradley JS, Rodvold KA, Goldstein EJ, Baron EJ, O'Neill
PJ, Chow AW, Dellinger EP, Eachempati SR, Gorbach S, Hilfiker M, May
AK, Nathens AB, Sawyer RG, Bartlett JG. Diagnosis and management of
complicated intra-abdominal infection in adults and children:
guidelines by the Surgical Infection Society and the Infectious
Diseases Society of America. Clin Infect Dis. 2010;50:133-64 https://doi.org/10.1086/649554 PMid:20034345
- Akinci
D, Akhan O, Ozmen MN, Karabulut N, Ozkan O, Cil BE, Karcaaltincaba M.
Percutaneous drainage of 300 intraperitoneal abscesses with long-term
follow-up. Cardiovasc Intervent Radiol. 2005;28:744-50 https://doi.org/10.1007/s00270-004-0281-4 PMid:16091990
- Theisen
J, Bartels H, Weiss W, Berger H, Stein HJ, Siewert JR. Current concepts
of percutaneous abscess drainage in postoperative retention. J
Gastrointest Surg. 2005;9:280-3 https://doi.org/10.1016/j.gassur.2004.04.008 PMid:15694825
- Marshall
JC, Maier RV, Jimenez M, Dellinger EP. Source control in the management
of severe sepsis and septic shock: an evidence-based review. Crit Care
Med. 2004;32:S513-26 https://doi.org/10.1097/01.CCM.0000143119.41916.5D PMid:15542959
- Hecker
A, Reichert M, Reuß CJ, Schmoch T, Riedel JG, Schneck E, Padberg W,
Weigand MA, Hecker M. Intra-abdominal sepsis: new definitions and
current clinical standards. Langenbecks Arch Surg. 2019;404:257-71 https://doi.org/10.1007/s00423-019-01752-7 PMid:30685836
- Baykara
N, Akalın H, Arslantaş MK, Hancı V, Çağlayan Ç, Kahveci F, Demirağ K,
Baydemir C, Ünal N; Sepsis Study Group. Epidemiology of sepsis in
intensive care units in Turkey: a multicenter, point-prevalence study.
Crit Care. 2018;22:93 https://doi.org/10.1186/s13054-018-2013-1 PMid:29656714 PMCid:PMC5901868
- van
de Groep K, Verhoeff TL, Verboom DM, Bos LD, Schultz MJ, Bonten MJM,
Cremer OL; MARS consortium. Epidemiology and outcomes of source control
procedures in critically ill patients with intra-abdominal infection. J
Crit Care. 2019;52:258-64 https://doi.org/10.1016/j.jcrc.2019.02.029 PMid:31054787
- Jung
B, Molinari N, Nasri M, Hajjej Z, Chanques G, Jean-Pierre H, Panaro F,
Jaber S. Procalcitonin biomarker kinetics fails to predict treatment
response in perioperative abdominal infection with septic shock. Crit
Care. 2013;17:R255 https://doi.org/10.1186/cc13082 PMid:24156734 PMCid:PMC4056026
- Wang
Y, Wang D, Fu J, Liu Y. [Predictive value of SOFA, qSOFA score and
traditional evaluation index on sepsis prognosis]. Zhonghua Wei Zhong
Bing Ji Jiu Yi Xue. 2017;29:700-4
- Arvaniti
K, Dimopoulos G, Antonelli M, Blot K, Creagh-Brown B, Deschepper M, de
Lange D, De Waele J, Dikmen Y, Eckmann C, Einav S, Francois G,
Fjeldsoee-Nielsen H, Girardis M, Jovanovic B, Lindner M, Koulenti D,
Labeau S, Lipman J, Lipovestky F, Makikado LDU, Maseda E, Mikstacki A,
Montravers P, Paiva JA, Pereyra C, Rello J, Timsit JF, Tomescu D,
Vogelaers D, Blot S; Abdominal Sepsis Study (AbSeS) Group on behalf of
the Trials Group of the European Society of Intensive Care Medicine.
Epidemiology and age-related mortality in critically ill patients with
intra-abdominal infection or sepsis: an international cohort study. Int
J Antimicrob Agents. 2022;60:106591 https://doi.org/10.1016/j.ijantimicag.2022.106591 PMid:35460850



