We conducted a single-centre analysis on 79 patients with NDTE MM managed at the Department of Translational and Precision Medicine of Sapienza University in Rome between 2020 and 2023. Data regarding baseline characteristics, induction therapy, response after induction according to the International Myeloma Working Group (IMWG),[8] mobilization regimen, stem cell collection parameters and outcomes of transplant were collected. Patients were divided in two groups: Arm A with 36 patients treated with D-VTD since January 2022, analysed prospectively; Arm B with 43 patients treated with VTD between 2020 and 2021 before daratumumab approval, analysed retrospectively. All subjects gave informed consent for the use of clinical data for research purposes, in accordance with the Declaration of Helsinki. Patients in Arm A received four 28-days cycles of D-VTd (thalidomide 100 mg/die), while patients in Arm B received 4/6 21-days cycles of VTD (thalidomide 200 mg/die). The aim of this study was to identify the possible impact of daratumumab-based induction therapy on stem cell mobilization and collection, engraftment kinetics and transplantation complications. Furthermore, we evaluated the effectiveness of different mobilization strategies.
Based on our institutional practice, all patients received mobilization in day service with intermediate dose cyclophosphamide (ID-CTX) (2.4 g/m2 in divided doses) plus G-CSF 48 MU BID. Optimal stem cell collection target was set at 9.0 x 106 CD34+/kg. Apheresis were performed with Spectra Optia ® for one to three days until a minimum collection goal of 2.0-2.5 × 106 CD34+ cells/kg for one ASCT and 4.0-4.5 × 106 CD34+ cells/kg for tandem ASCT. Based on the evidence of poor harvesting, the dose of CTX was increased to 3.0 g/m2 in Arm A since October 2022. Plerixafor was used on demand in case of low peripheral blood CD34+ on the day of planned apheresis, according to the local practice. A maximum of three mobilization attempts were performed in case of poor mobilization, defined as the inability to collect a minimum of 2.0 x 106/kg stem cells despite adequate stimulation. The primary study objective was the difference in terms of rate of successful PBSC collections at the first mobilization attempt between the two arms. The secondary study objectives were the differences in terms of median number of apheresis at the first mobilization attempt and rates of poor mobilizers; time of engraftment in neutrophils (PMNs) and platelets (PLTs), rate of infectious complications, transfusions, and duration of hospitalization after ASCT. At the first mobilization attempt, median absolute value and timing of peripheral blood leukocytes and CD34+ nadir and azimuth (lowest and highest number/mm3 reached during stimulation, respectively) and median total collection volumes (millilitres, ml) were compared between the two groups. In Arm A, the difference in collection efficacy with two different doses of CTX (2.4 g/m2 and 3.0 g/m2) was evaluated.
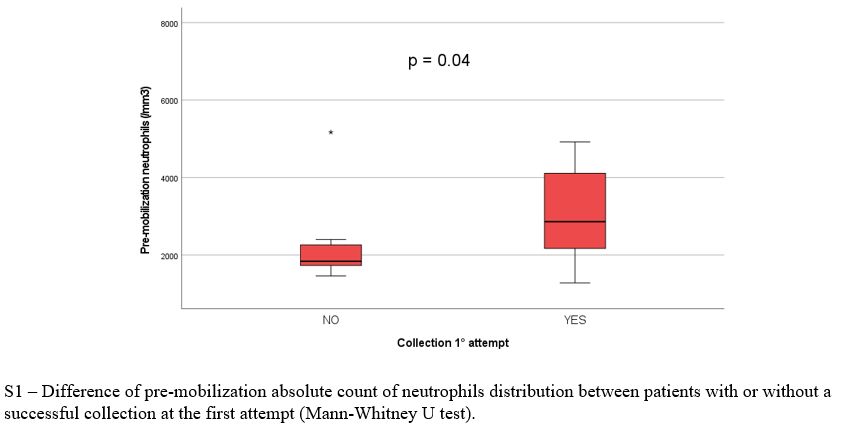
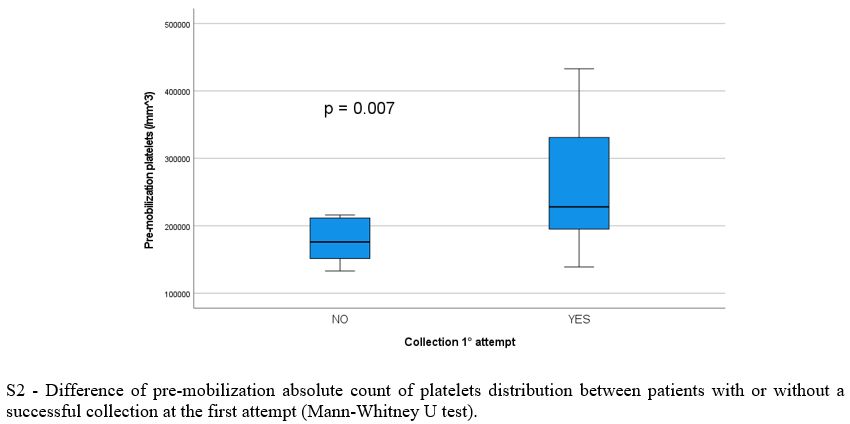
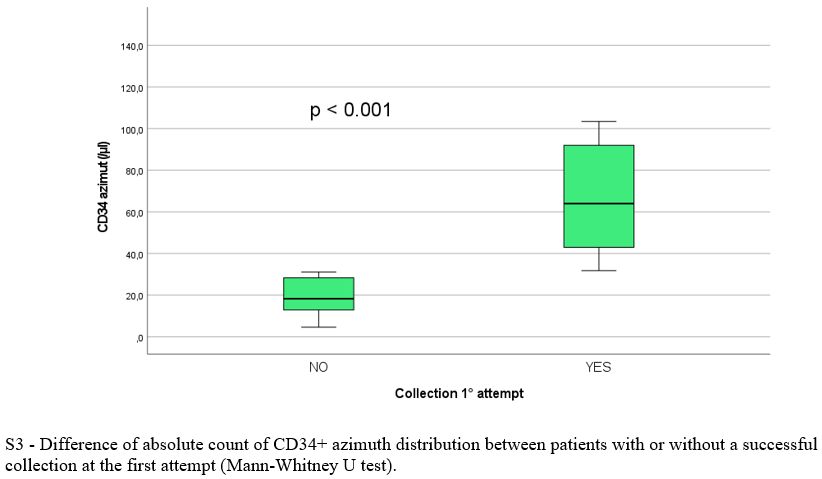
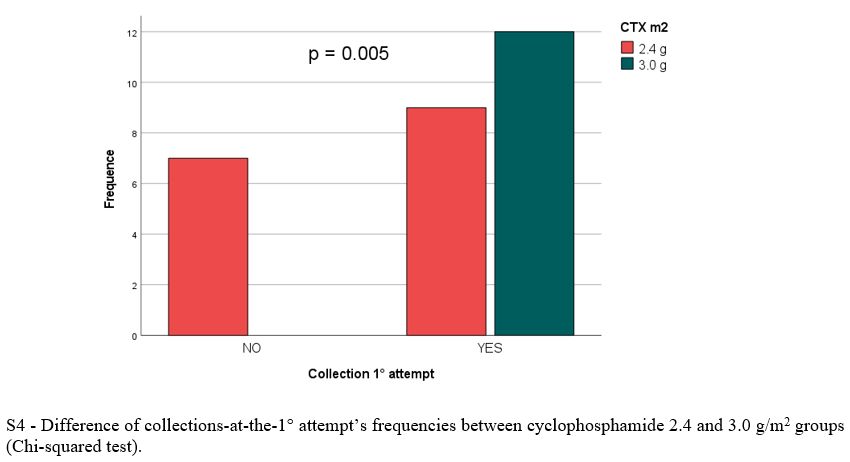
Baseline population, treatment and mobilization characteristics are reported in Table 1 and 2, respectively. There was no significant difference between the two arms regarding clinical and biological variables evaluated at baseline, during and after induction, except for significantly lower pre-mobilization PMN (p = 0.016) and PLT counts (p = 0.007) and longer median time between the end of induction and mobilization (30.0 vs 21 days, p = 0.009) in Arm A. Overall, 69/79 patients (87%) had ≥ 3.0 x 106 CD34+/kg stem cell collection, including 28/36 in arm A (78%) and 41/43 (95%) in arm B (p = 0.07). Successful stem cell collections were performed at the first mobilization attempt in 21/28 patients in Arm A (75%) and 39/41 in Arm B (95%) (p 0.026). At the first attempt, the median number of CD34+ cells x 106/kg collected was not significantly different between the two groups (8.6 vs 9.1 x 106/kg in arm A and B respectively, p = 0.4) as well as the median number of apheresis performed (p = 0.3). In arm A 8/36 patients (22%) required rescue plerixafor vs 6/43 patients (14%) in arm B (p = 0.2). Median total collection volumes at the first mobilization attempt were significantly higher in Arm A (median 402 vs 301 ml, p = 0.016). The median peripheral blood leukocytes azimuth value was not different in the two groups (39.500 vs 37.400/mm3, p = 0.2) but it was reached after a significantly longer time in arm A (11.0 vs 8.0 days, p= 0.003). In addition, the median peripheral blood CD34+ azimuth value was significantly lower in arm A (44.0 vs 98.0 CD34+/μl, p< 0.001) and it was reached after a median of 9.0 and 8.0 days in the two arms respectively (p= 0.017). Among the clinical and biological characteristics evaluated, the only variables significantly associated with the inability to perform a minimum PBSC collection in Arm A were lower pre-mobilization counts of PMNs (p = 0.043), PLTs (p= 0.009) and peripheral blood CD34+/μl azimuth (p< 0.001) and delay in CD34+ azimuth (p= 0.05). Response to induction treatment (at least a partial response / PR) was not associated with an increased likelihood of collection (p= 0.7). Use of a higher 3.0 g/m2 dose of CTX was significantly associated with the probability of collection (p= 0.005), but not with the use of rescue plerixafor (p= 0.9). All the patients mobilized after ≥ 6 weeks since the last administration of daratumumab and after a total dose of 3.0 g/m2 of CTX (12/36, 33%) achieved stem cell harvest at the first attempt, without the need for plerixafor. The results of multivariate logit regression analysis are reported in Supplementary materials.
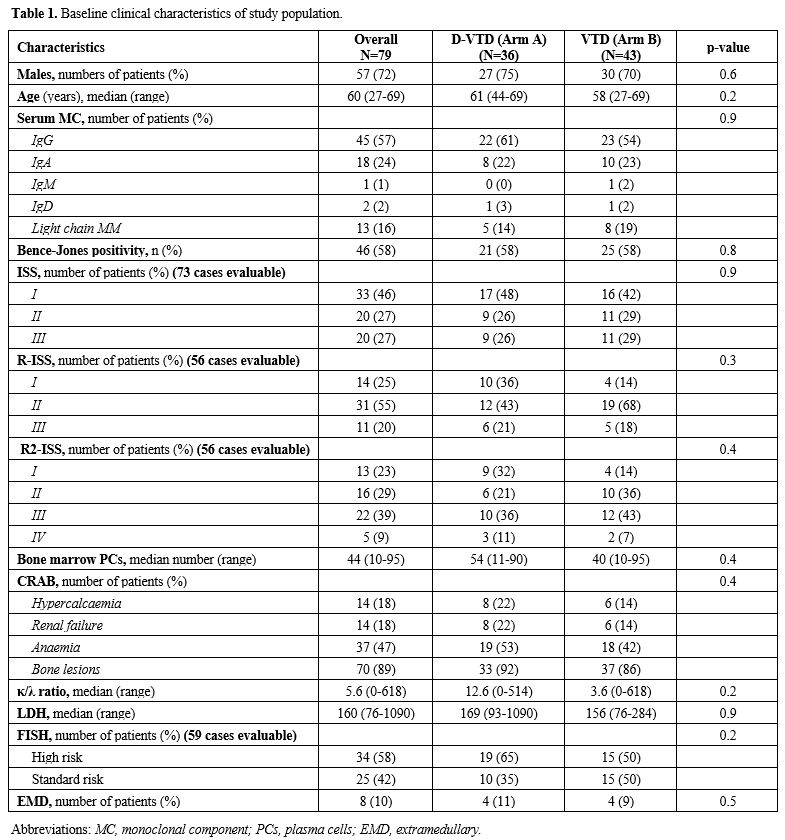 |
Table 1. Baseline clinical characteristics of study population. |
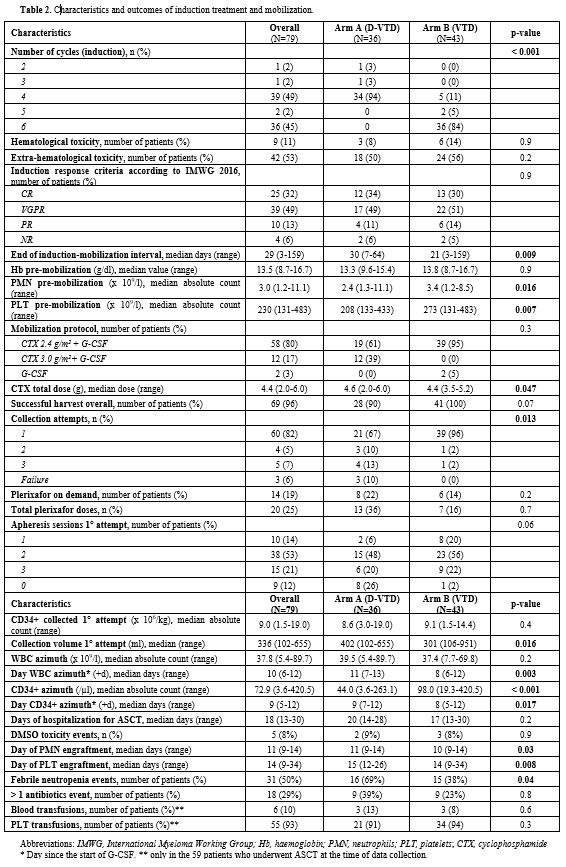 |
Table 2. Characteristics and outcomes of induction treatment and mobilization. |
After a median follow-up of 21.2 months for the entire cohort (IQR 14.8 – 27.5), 62 patients have already received at least one ASCT (64% in Arm A vs 91% in arm B). After ASCT, median time of engraftment was significantly longer in arm A both for PMNs (11.0 vs 10.0 days, p= 0.03) and PLTs (15.0 vs 14.0 days, p= 0.008). There was no significant difference between the two arms in terms of events of dimethyl sulfoxide (DMSO) toxicity (p= 0.9), infections requiring more than 1 antibiotic (p = 0.8), erythrocyte (p= 0.6) and PLT transfusions (p= 0.3) and median days of hospitalization (p = 0.2). Events of neutropenic fever were significantly more frequent in Arm A (p= 0.04).
In our analysis, despite significantly lower median values of PMN and PLT at the end of induction therapy in arm A, no additional events of haematological and even extra-haematological toxicity were observed with the addition of daratumumab, confirming the safety of combining anti-CD38 monoclonal antibodies with other novel drugs.[9] Slightly delayed engraftment and higher frequency of febrile neutropenia after ASCT were manageable and did not result in prolonged hospitalization or in the use of multiple antibiotics, differently from what was recently reported by other authors.[6,7]