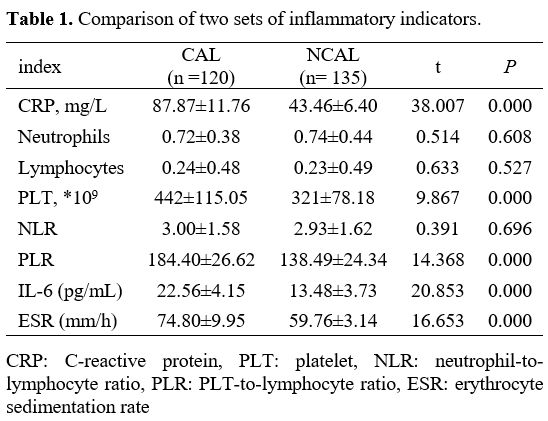
Comparison of Two Sets of Inflammatory Indicators. As shown in Table 1, the levels of CRP, PLT, PLR, IL-6, and ESR in the CAL group were prominently more than that in the NCAL group. Interestingly, there is no difference in neutrophils, lymphocytes, and NLR between the two groups.
 |
|
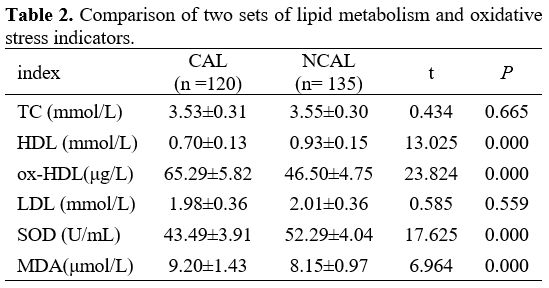
Comparison of Two Sets of Lipid Metabolism and Oxidative Stress Indicators. As shown in Table 2, the levels of ox-HDL and MDA in the CAL group were dramatically more than that in the NCAL group; interestingly, the content of HDL and SOD in the CAL group was prominently low. There is no difference in TC and LDL between the two groups.
 |
|
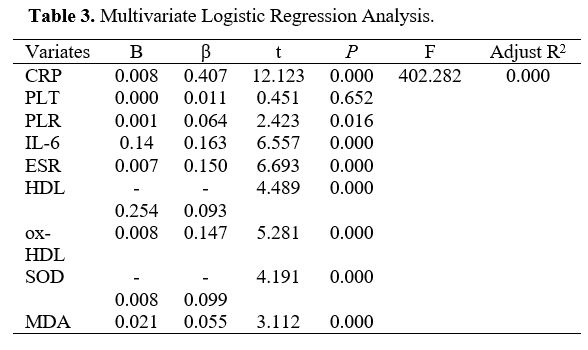
Multivariate Logistic Regression Analysis (MLRA). Statistically significant variables, including CRP, PLT, HDL, IL-6, ox-HDL, SOD, MDA, PLR, and ESR, were enrolled in the logistic regression analysis. Higher CRP, PLR, IL-6, MDA, ox-HDL, ESR, lower HDL, and SOD were independent factors for CALs with KD, whereas PLT failed to reach significant differences (Table 3).
 |
|
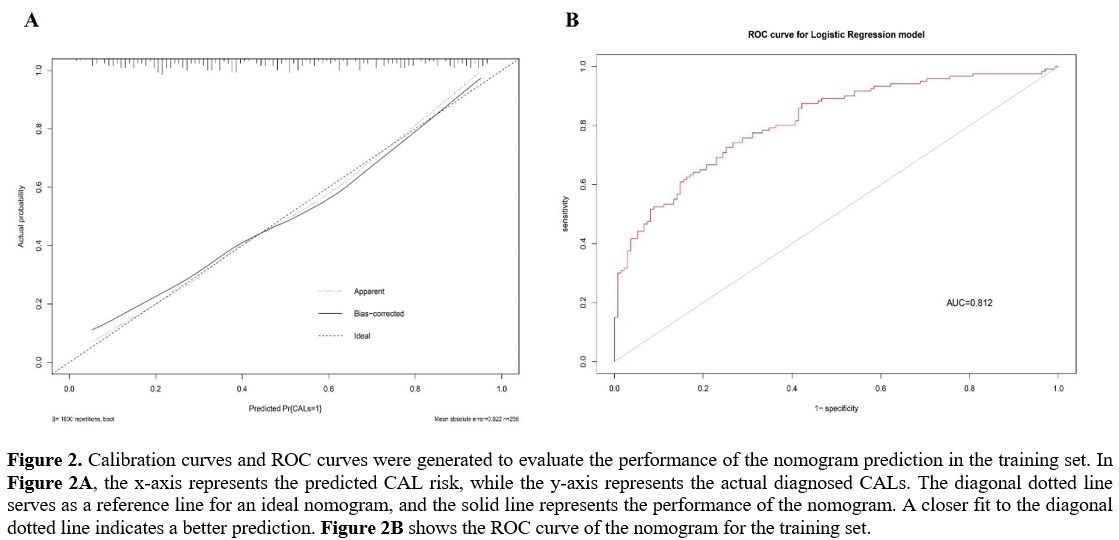
Development and Evaluation of the Nomogram for Predicting CALs in KD. The nomogram was constructed using the eight independent risk factors identified through MLRA. (Figure 1). The calibration curve of the nomogram demonstrated good agreement in the CALs (Figure 2A). The area under the ROC curve (AUC) was 0.812 (95% CI, 0.76-0.865), and the sensitivity and specificity were 0.73 and 0.74, respectively (Figure 2B).
We further used the verification set to verify the model, which showed adequate discrimination (AUC, 0.799; 95% CI, 0.742-0.856). The sensitivity and specificity were 0.85 and 0.68, respectively (Figure 3).
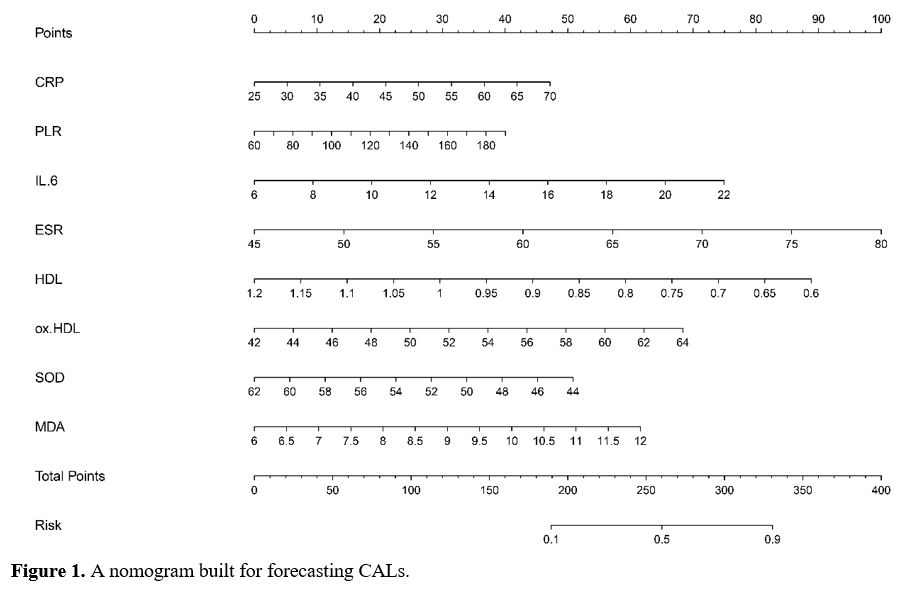 |
Figure 1. A nomogram built for forecasting CALs. |
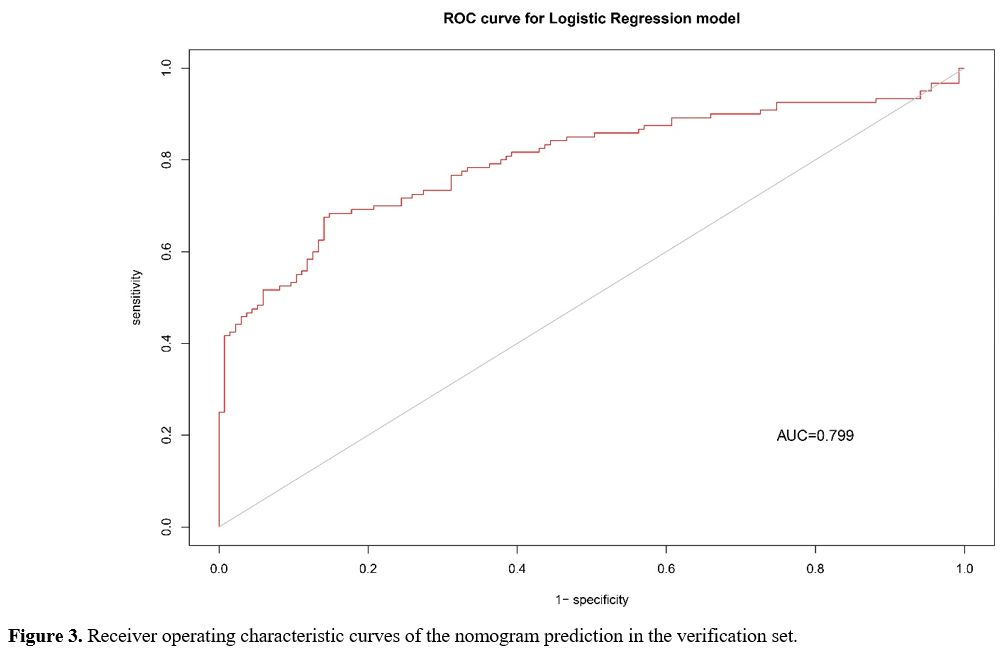 |
Figure 3. Receiver operating characteristic curves of the nomogram prediction in the verification set. |