Anna Maria Testi1, Mazin Faisal Al-Jadiry2, Maria Luisa Moleti1, Stefania Uccini3, Amir Fadhil Al-Darraij4, Raghad Majid Al-Saeed4, Hasanein Habeeb Ghali2 Ahmed Hatem Sabhan4, Samaher Abdulrazzaq Fadhil4, Safaa Abdulelah Al-Badri5, Adil Rabeea Alsaadawi6, Ameer Dh Hameedi7, Manhal Hashim Shanshal4, Yasir Saadoon Al-Agele4, Fatimah Abdul Ridha Al-Saffar4, Nihal Khalid Yaseen4, Alfonso Piciocchi8, Giovanni Marsili8 and Salma Abbas Al-Hadad2.
1 Department of Translational and Precision Medicine, Sapienza University, Rome, Italy
2 College of Medicine-University of Baghdad, Children Welfare Teaching Hospital-Medical City, Pediatrics, Baghdad, Iraq
3 Clinical and Molecular Medicine, Sapienza University of Rome, Rome, Italy.
4 Children Welfare Teaching Hospital-Medical City, Oncology Unit, Baghdad, Iraq.
5
College of Medicine-Wasit University, Children's Welfare Teaching
Hospital-Paediatric Oncology Unit, Medical City, Pediatrics, Baghdad,
Iraq.
6 Central Teaching Laboratory, Medical City, Pathology, Baghdad, Iraq.
7 College of Medicine, University of Baghdad, Pathology, Baghdad, Iraq.
8 GIMEMA Foundation, Rome, Italy, Statistical, Rome, Italy.
Correspondence to:
Anna Maria Testi, Hematology Institute; Department of Translational and
Precision Medicine, Sapienza University of Rome. Via Benevento 6, 00161
Rome, Italy. Phone: +39-3394723402 Fax: +39-06-44241984 Email:
testi@bce.uniroma1.it
Published: July 01, 2024
Received: February 08, 2024
Accepted: June 16, 2024
Mediterr J Hematol Infect Dis 2024, 16(1): e2024053 DOI
10.4084/MJHID.2024.053
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background. Childhood
Hodgkin lymphoma (HL) is an eminently curable disease. Good outcomes
can be achieved even in resource-limited settings, and the focus is
increasingly on limiting long-term toxicity. Contemporary treatment
incorporates a risk-stratified, response-adapted approach using
multiagent chemotherapy with/without low-dose radiotherapy. Many
developing countries continue to use ABVD-based regimens due to limited
acute toxicity, cost, and ease of delivery.
Objective. We herein report the outcomes of childhood HL diagnosed and treated in an Iraqi single centre over 16 years.
Methods. Children
≤14 years old with biopsy-proven HL were enrolled. Most patients
received ABVD chemotherapy or COPP/ABV when Dacarbazine was
unavailable. Radiotherapy was not available.
Results. Three
hundred-three children were consecutively newly diagnosed with HL; 284
were considered eligible for the retrospective analysis (treatment
refusals 9; deaths before therapy 5; initially diagnosed of non-Hodgkin
lymphoma 5). ABVD scheme was administered to 184 children (65%),
COPP/ABV to 83 (29%), and other schemes to the remaining 17 patients.
Complete response (CR) was achieved in 277 (98%); 4 (1.4%) showed
disease progression, and 1 had stable disease. Four patients in CR
abandoned therapy and were in CR at the time of analysis, 2 died from
infection. Relapse occurred in 42 patients (15%). The 15-year OS and
EFS are 89.7% and 70.3%, respectively.
Conclusion. In
this single Centre, over 16 years, almost 90% of children suffering
from HL survive, despite the numerous limitations in diagnostic
procedures, shortage of chemotherapy, no radiotherapy facilities,
absence of effective second-line treatments, and finally, therapy
abandonment for social and financial reasons.
|
Introduction
Hodgkin lymphoma (HL) accounts for 5%-6% of all childhood cancers.[1] This disease is highly responsive to treatment and represents one of the success stories of paediatric oncology.[2]
Current treatment protocols for childhood HL have undergone
considerable modifications in order to reduce both acute toxicity and
long-term therapy-related complications without compromising the
excellent clinical results.[3] In the Western World, the 5-year survival of paediatric HL exceeds 95%.[4]
However, survival in low-income countries is lower. Many factors,
including delayed diagnosis, withdrawal from therapy, and insufficient
intensive and supportive care, have resulted in decreased survival
rates for HL children living in these countries.[5-7]
In Iraq, numerous limitations in diagnostic procedures, shortage of
chemotherapy agents, no radiotherapy (RT) facilities, absence of
effective second-line therapies, and finally, therapy abandonment for
social and financial reasons have made it difficult to treat children
with HL. Since 2003, in the context of a Telemedicine Project between
Sapienza, University of Rome, Italy, and The College of Medicine in
Baghdad, Iraq, it has been possible to review the children’s
histological materials, to set up a prospective clinical registry and,
subsequently, to adopt guidelines for paediatric patients with HL in
Iraq.
The purpose of this study was to retrospectively analyse the
outcome of HL children treated with ABVD-based therapy, diagnosed and
managed at the Children’s Welfare Teaching Hospital (CWTH) in Baghdad
over 16 years.
Patients and Methods
Diagnostic evaluation. This
study includes children up to 14 years of age with a diagnosis of HL
treated at the CWTH in Baghdad between January 2004 and December 2019.
Histological diagnosis was based on a biopsy of a lymph node or of an
involved organ. From January 2007, the patients' pathology specimens
were reviewed in the Pathology Department of Sapienza University of
Rome. Immunohistochemistry was available in 61% of patients. Upon
admission, medical history, including the presence of B symptoms,
physical examination, blood chemistry, chest X-ray, superficial node
and abdominal sonography, and, when available, neck and chest computed
tomography (CT), bone marrow biopsy (BM) for stage III, IV, or presence
of B symptoms and cardiac function were obtained. Bulky disease was
defined as the presence of a lymph node mass of at least 10 cm in
diameter or a mediastinal mass with a diameter exceeding one-third of
the maximum mediastinal width on an upright posteroanterior chest
radiograph. Staging of the disease followed the Ann Arbor/Cotswold’s
classification.[8] For this analysis, children with
stages IA, IB, and IIA were classified as early diseases, and those
with stages IIB, III, and IV as advanced diseases.
Treatment.
Most children received ABVD courses or COPP/ABV when Dacarbazine was
unavailable. The number of cycles ranged from 4 to 8, based on the
initial stage and treatment response. Response was evaluated using the
same diagnostic techniques employed at diagnosis, during, and at the
end of chemotherapy. Complete response (CR) was defined as a >80%
regression of the clinical and radiological lesions. Partial response
(PR) was defined as the reduction in all disease sites by at least 70%
compared to the initial involvement. Stable disease (SD) was defined as
less than a 70% reduction in total tumour size. Disease progression
(DP, ≤ 3 months) or relapse (> 3 months from therapy completion) was
defined as an increase of at least one measurable lesion or the
appearance of new lesions. In January 2014, Treatment Guidelines were
designed and adapted to the local resources and modulated according to
patients’ risk and response. The “interim response” evaluation after
the first 2 cycles was introduced. The good interim response was
defined when a 2-dimensional reduction in size greater than 50% was
achieved. Patients were divided into 3 risk groups: standard, stage IA
or IIA with < 3 nodal sites and no bulky disease; intermediate:
stage IA, IIA or IIIA with ≥ 3 nodal sites or bulky disease; high:
stage IIB, IIIB or IV. The ABVD scheme was chosen as effective and safe
for all risk groups. The number of cycles was 4 for standard-risk, 6
for intermediate-risk, and 8 for high-risk patients (Table 1).
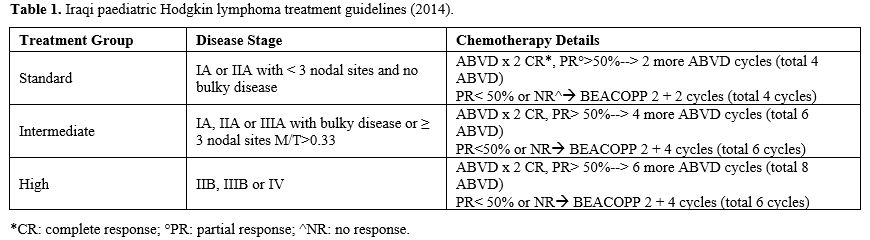 |
- Table
1. Iraqi paediatric Hodgkin lymphoma treatment guidelines (2014).
|
RT
was not available for the whole period, and patients with interim
response < 50% received treatment intensification with standard
BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide,
vincristine, procarbazine, prednisone) (Table 1).
ABVD chemotherapy was administered in daycare on an outpatient basis; BEACOPP cycles required the patient’s hospitalization.
The study received approval from the official local institutional review board.
Follow up. After
completion of therapy, the follow-up plan included clinical evaluation
every 3 months in the first year, every 4 months in the second year,
every six months from the third to the fifth year, and yearly after
that. At each visit, in addition to obtaining any relevant history and
a physical examination pertinent to HL, no imaging studies or blood
investigations to detect relapse were routinely recommended for
asymptomatic patients with normal physical examinations. Pulmonary
and/or cardiac assessments were only carried out for symptomatic
patients. In patients with a suspicion of relapse, imaging studies and
a new biopsy were performed. For patients who did not turn up for the
follow-up, clinical status was confirmed by phone.
Statistical analysis.
Patient’s characteristics were summarised by frequencies and percentage
values for categorical variables, while continuous variables were
described with median values and their relative ranges. Overall
survival (OS) was defined as the time from diagnosis to death or the
date of the last follow-up. Event-free survival (EFS) was defined as
the time from diagnosis to the date of failure (no response, treatment
abandonment, relapse, or death) or the date of the last follow-up. The
probabilities of OS and EFS were estimated following the Kaplan–Meier
product limit method, while the results of univariate comparisons were
performed according to the Log-Rank test. All tests were two-sided with
a significance level of 0.05, and confidence intervals were calculated
at a 95% level. All analyses were performed using the R version 4.2.2.
Results
At
the CWTH of Baghdad, from January 2004 to December 2019, 303 children
with a median age of 7.8 years (range 3–14) were newly diagnosed with
HL. Patients’ demographic profiles and diagnostic characteristics are
reported in Table 2. A male predominance was observed (215 vs 88), 47% of patients lived outside Baghdad and nutrition status was <3rd
percentile in 45 children (15%). The median duration of HL symptoms
before diagnosis was 5 months (range 1-60). Twenty-six children (9%)
presented co-morbidities. The most severe included: cerebral atrophy
(1), absence of the left kidney, nephrectomy or renal impairment (5),
ataxia telangiectasia (1), Castleman disease (1), Wilson disease (1),
congenital heart disease (1), immune thrombocytopenia (2), immune
deficiency (1), bone marrow fibrosis (1), anaemia (4). One hundred and
fifty-three children (51%) presented B symptoms and 105 (35%) had bulky
disease. Forty-seven children had stage I (IA 38; IB 9), 106 stage II
(66 IIA, 40 IIB), 122 stage III (IIIA 43, IIIB 79) and 28 stage IV (IVA
3, IVB 25). The most frequent histological subtype was mixed
cellularity (MC: 181 patients, 60%) followed by nodular sclerosis (NS:
86 patients, 28%). An immunohistochemical analysis was performed in 186
samples; EBV, as assessed by in situ hybridization for
EBER-(Epstein-Barr encoded)-RNA, was positive in 82/100 (82%) cases
studied at the Pathology Department in Rome.
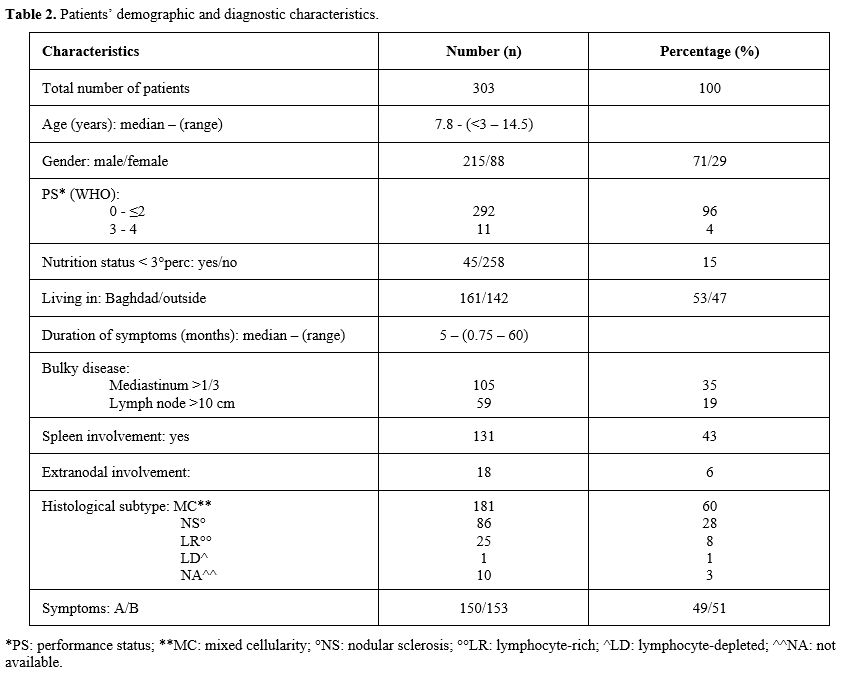 |
- Table 2. Patients’ demographic and diagnostic characteristics.
|
Two
hundred and eighty-four children were considered eligible for the study
evaluation. Nine were excluded because of treatment refusal, 5 (1 with
a previous renal impairment; 1 with Wilson disease) died before
treatment and 5 were initially diagnosed as non-Hodgkin lymphoma (NHL)
and received a different treatment. Of the 284 eligible patients, 84
(16%) aged less than 5 years and 239 (84%) were older; 47 (17%) were
classified as stage I (37 IA, 10 IB), 96 (34%) stage II (59 IIA, 37
IIB), 114 (40%) stage III (43 IIIA, 71 IIIB), 27 (10%) stage IV (3 IVA,
24 IVB). According to our analysis criteria, 107 children (38%) were
considered with early and 177 (62%) with advanced disease. ABVD scheme
was administered to 184 children (65%), COPP/ABV to 83 (29%), and the
remaining 17 patients received different HL chemotherapy schemes. One
hundred and forty-three children (78%) treated with ABVD received 6-8
cycles (median 6), and 41 (22%) received 2-5 cycles (median 4).
Response evaluation at the end of therapy documented a CR in 174
children (95%); 3 of them abandoned the treatment after the first 3-4
cycles and are still alive and in CR. One child died from DP, and
another 9 patients with early PR underwent treatment intensification
and achieved a CR. Forty-six of the 83 children treated with COPP/ABV
schema (55%) received 6-8 cycles (median 6), and 37 (45%) received 2-5
cycles (median 4). Eighty-two children achieved a CR; one patient died
of DP. Twelve of the 17 patients (71%) who received different HL
treatments achieved a CR; 2 died of DP; 1 child with a good response
abandoned therapy and is still alive, and 2 patients showed an NR. A
total of 268/284 treated patients (94%) achieved a CR with the
first-line therapy; another 9 further patients with a poor response to
first-line therapy intensified treatment and achieved a CR. The total
response rate for all the evaluable patients was 98% (277/284).
A
relapse was recorded in 42/277 patients (15%) (10 stage I, II; 32 stage
III, IV) at a median time from diagnosis of 20 months (range 9-84).
Twenty-nine had received an ABVD scheme, 9 COPP/ABV, and 4 different
front-line treatments. All relapsed children received salvage schemes,
and 28 (67%) achieved a second CR; 13 died during treatment, and 1 is
still alive with persistent disease.
At a median follow-up of 6.42
years (4.11-10.12), the 15-year OS and EFS for the entire cohort of
children are 89.7% and 70.3%, respectively (Figure 1).
There is no difference in outcome in children under or over 5 years
(15-year OS and EFS: 82.9% and 65.8% vs 91.1% and 71.2%, respectively;
p=0.43 and 0.46), histological subtype MC and NS (10-year OS and EFS:
89.8% and 71.7% vs 90.3% and 66.9%, respectively; p=0.85 and p=0.19,)
and living in Baghdad or outside (15-year OS and EFS: 91.4% and 75% vs
87.5% and 64.3%; p=0.095; p=0.076). Based on the type of treatment
(ABVD vs COPP/ABV), we did not observe a difference in the OS (10-year
OS 93.3% vs. 87.8%; p=0.2), even if the EFS at 10 years resulted in
inferior in the group of children that had received ABVD scheme (66.5%)
compared to those treated with COPP/ABV (82.9%). A significantly better
outcome has been observed in stage I-II patients compared to stage
III-IV children (15-year OS and EFS: 96.9% and 82.6% vs 82.8% and
57.7%, respectively; p<0.0001), and in patients classified as early
compared to advanced stages; the 15-year OS is 97.4% vs 85.7%
(p=0.0007) and EFS 89% vs 59.4% (p<0.0001), respectively (Figure 2).
Multivariate analysis confirmed the statistically better OS and EFS for
children classified as early compared with advanced stage (HR 16.0,
[95%CI 2.13-12.1]; p=0.007 and 4.32, [95%CI 2.20-8.49]; p<0.001) (Supplemental Table 1 and Table 2).
The
5-year OS of the 82 EBV-positive children was 90.1% compared to 74.7%
for the EBV-negative cases (p=0.046). The 5-year EFS was also
statistically better in EBV-positive vs EBV-negative patients (75.4% vs
36.8%; p=0.00017) (Figure 3).
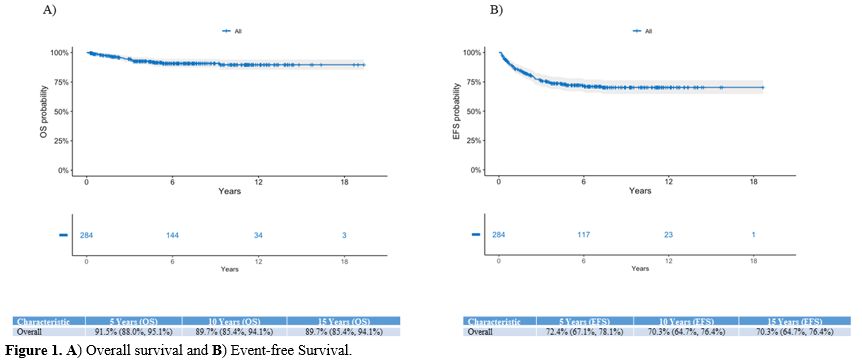 |
Figure
1. A) Overall survival and B) Event-free Survival. |
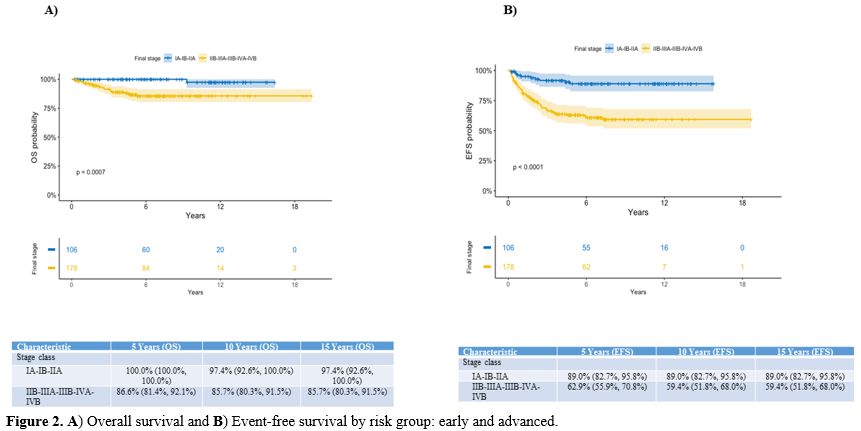 |
Figure
2. A) Overall survival and B) Event-free survival by risk group: early and advanced.
|
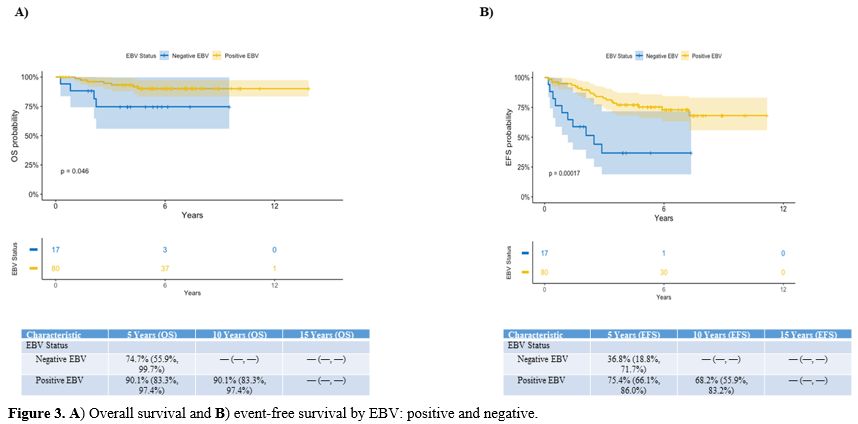 |
Figure 3. A) Overall survival and B) Event-free survival by EBV: positive and negative.
|
Results after Guideline Implementation. Since
January 2014, 130 newly diagnosed HL children entered the new protocol
guidelines. One hundred and twenty-four were evaluated, and 6 children
were moved to another treatment centre. Sixteen patients had stage I
(11 IA, 5 IB), 41 stage II (23 IIA, 18 IIB), 52 stage III (17 IIIA, 35
IIIB), and 15 stage IV (2 IVA, 13 IVB) disease. One patient with stage
IIA abandoned therapy, and 123 continued the treatment as planned.
These children were evaluated for disease response after the second
ABVD course; 109 (89%) showed a good response, while 14 (11%) had a
poor response. Intensification of treatment (7 BEACOPP, 4
IGEV-ifosfamide, gemcitabine, vinorelbine, prednisone) was administered
to 11 patients; 9 achieved a CR, and 2 showed a DP and died. Of the
remaining 3 poor responders, 1 abandoned treatment, 1 died before
starting intensification therapy, and 1 continued the ABVD cycles but
relapsed and is still alive after salvage treatment. The good
responders continued ABVD treatment. Seventeen (17/118; 14%) patients
relapsed at a median of 20 months (range 9-78) from diagnosis; 11 are
still alive and 6 have died of DP. A total of 113/123 (92%) patients
treated with the new HL guidelines were still alive at the last
follow-up (Table 3).
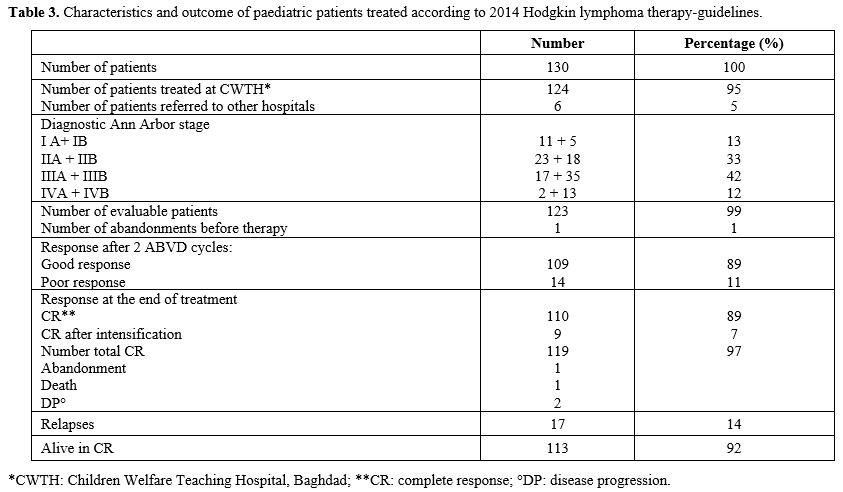 |
- Table 3. Characteristics and outcome of paediatric patients treated according to 2014 Hodgkin lymphoma therapy-guidelines.
|
An
improved outcome was observed in children treated with the 2014
guidelines compared to the others, but the difference is so far not
statistically significant (5-year OS and EFS 93.1% and 75.7% vs 90.4%
and 70.3%, respectively; p=0.67, p=0.37).
Acute and late toxicities.
Acute toxicity observed during first-line treatment included fatal
pneumonia in 1 child after the second chemotherapy course. Acute
varicella complicated by a fatal pneumonia occurred in a child 6 months
after treatment completion.
Symptomatic bleomycin pulmonary
toxicity and cardiomyopathy were not observed. A second malignant
neoplasm in the form of osteosarcomas and acute lymphoblastic leukaemia
occurred in 2 children, after 8 and 10 years, respectively, from
chemotherapy completion. Both patients had received treatment with 6
COPP/ABV courses.
Discussion
Paediatric HL is a highly curable malignant tumour with overall survival rates exceeding 95%.[2,3]
These excellent outcomes, however, come at the cost of an increased
risk of long-term toxicities due to chemotherapy and/or radiation.[9-11]
The current risk- and response-adapted treatment for children with HL,
which aims at maximizing survival while minimizing toxicity, should be
the standard of care for the disease in this patient population.
In
adults with HL, the ABVD scheme has been considered the de facto
standard of care for several decades due to its effectiveness and
excellent toxicity profile.[12,13] ABVD continues to
be widely used in resource-limited settings owing to low costs, ease of
delivery, and limited acute toxicity, allowing for safe delivery, even
at centres with suboptimal supportive care. In a survey of paediatric
oncology providers in India, ABVD was the first-line chemotherapy in
73% of paediatric oncologic centers.[5] Satisfactory results have been reported in these children treated with ABVD[5,6,14]
but at the cost of 6 or more cycles of chemotherapy with RT delivered
to a varying proportion of patients. ABVD was the treatment of choice
for paediatric patients with HL in 2 Egyptian centres. Good results
were reported in 59 children treated over 8 years; with a median
follow-up of 39 months, the 5-year OS and EFS were 96.6% and 84.7%,
respectively. Patients with advanced stages received 8 ABVD courses.[7]
In
this present retrospective analysis, we have reported the real-life
experience in Iraq in treating children with HL over 16 years with an
ABVD-based therapy. RT was not feasible in the country with a long
waiting list. The pretreatment disease characteristics such as low
median age at presentation (7.8 years), male preponderance (71%),
proportion of patients with B symptoms (51%), and high-stage disease
(63%) are similar to those reported in previous studies from India and
Latin-America.[5-7,15-17] The
treatment outcome in terms of 15-year OS and EFS was 89.7% and 70.3%,
respectively, comparing favourably with the published literature from
India and limited resource centers.[5-7]
However,
most children (141/184, 77%) received 6-8 chemotherapeutic ABVD cycles,
accounting for doxorubicin and bleomycin exposures of ≥ 400 and ≥ 160
mg/m2, respectively. The feasibility
of reducing the chemo-radiotherapeutic burden for early and
intermediate-risk patients has been demonstrated by the German Society
of Paediatric Oncology, the Children Oncology Group (COG), and the
European EuroNet-PHL-C1 (EudraCT number: 2006-000995-33).[18-21]
The Stanford, Dana Farber, and St. Jude Consortium and German trials
have demonstrated that for patients who achieved a CR after 2 cycles
with [F-18]2-fluoro-2-deoxyglucose (FDG)-positron emission tomography
(PET)-based response assessment, treatment can be reduced to 4 cycles
without adjuvant RT. In a recent study (POG-HL-15-01) conducted in low-
and middle-income countries, the impact of using CT and PET after 2
ABVD courses on treatment decisions and outcomes was compared. In a
cohort of 382 HL children, the use of PET as the modality for early
response evaluation clearly indicated a satisfactory response compared
to CT.[22] In Baghdad, during the study period, no
patients could be staged and reassessed with an FDG-PET due to the
impossibility of carrying out the procedure. In Iraqi children who
entered the 2014 new guidelines, the early response evaluation was not
based on PET, and this could have produced inconsistent results.
Furthermore, greater uncertainty relating to less accurate staging and
response assessments, as well as the unavailability of RT, has led to
an increase in the number of chemotherapy cycles, even in more limited
stages of disease.
In Western countries, in order to decrease late
chemotherapy-related side effects and restrict exposure to alkylating
agents and anthracyclines, new treatment schemas have been employed in
paediatric HL. In the EuroNet-PHL-C1,[21] OEPA
(prednisone, vincristine, doxorubicin, etoposide) for the first 2
courses, followed by COPDAC (prednisone, vincristine, doxorubicin,
etoposide, cyclophosphamide), was administered to children with newly
diagnosed HL. However, it is well recognized that OEPA chemotherapy is
associated with significantly more acute toxicity, especially in the
first cycles. In a study from India,[23] 69 febrile
episodes during neutropenia were reported in 54 patients with a
treatment-related mortality of 5.3% (7/132) and a treatment abandonment
of 10%.[23] Parambil et al. also reported a no-relapse mortality of 4.3% in advanced-stage HL with the same strategy.[24] The reported incidence of febrile neutropenia and toxic mortality following ABVD is much lower.[5]
In the present experience, only 1 child died during treatment due to a
pulmonary infection; no other acute toxicities were observed.
Abandonment during first-line therapy was also limited (<2% of
patients) despite limitations in the country's social and financial
resources. However, 2 patients presented a second neoplasm after 8 and
10 years, respectively, even without RT. Hence, in resource-limited
settings, there is a need to discern how best to balance the risk of
early treatment-related toxicity versus late sequelae.
In developing countries, HL patients are likely to be younger and more likely to have EBV-driven disease.[25-27]
Whether EBV-driven disease is more responsive to treatment is actively
debated, and treatment strategies for these young patients need to be
more focused.[28] In our cohort of patients up to 14
years of age, EBV could only be assessed in one-third of cases; 82 out
of 100 patients tested resulted in EBV-positive. The outcome of these
children, regardless of the stage and type of treatment received, was
significantly better than that of those who resulted negative (5-year
OS and EFS 90.1% and 75.4% vs 74.7% and 36.8%, respectively). The high
frequency of childhood EBV-associated HL is also described in other
developing countries,[28] and some studies report a
favourable effect of EBV-positive HL on survival, as we have observed
in our cohort of Iraqi children. This finding supports further
investigation of EBV as a prognostic marker for children with HL living
in these countries.
There are, however, some limitations to this
study. Many patients could be under staged (extra nodal involvement
only 6% of cases) because the diagnostic tools, such as PET and CT,
were not always available in the centre, and the initial staging was
assessed only with chest X-ray, superficial node, and abdominal
sonography. Moreover, before January 2014, this was not a clinical
trial and chemotherapy regimens were chosen based on the availability
of drugs. The number of chemotherapy courses was often based on each
child's clinical outcome. Finally, the number of late side effects may
be underestimated because the status of the patients who did not turn
up for follow-up was confirmed by phone.
Conclusions
In
this large paediatric HL series managed in Baghdad with long-term
follow-up, good results in terms of OS and EFS have been achieved
despite the numerous limitations in diagnostic procedures, shortage of
chemotherapy, and no RT facilities. This analysis has provided a
platform for planning future prospective studies. Furthermore, PET is
now available in the centre, and in the near future, it will be used
for diagnostic staging and assessing the early response to therapy. RT
is currently organized in the country also for paediatric patients.
There is clearly a need to adapt the intensity of treatment to the
initial disease’s stage and the early response to treatment in order to
improve the long-term results further and avoid acute and late
therapy-related side effects.
References
- Flerlage JE, Hiniker SM, Armenian S, Benya EC,
Bobbey AJ, Chang V, Cooper S, Coulter DW, Cuglievan B, Hoppe BS,
Isenalumbe L, Kelly K, Kersun L, Lamble AJ, Larrier NA, Magee J, Oduro
K, Pacheco M, Price AP, Roberts KB, Smith CM, Sohani AR, Trovillion EM,
Walling E, Xavier AC, Burns JL, Campbell M. Pediatric Hodgkin Lymphoma,
Version 3.2021. J Natl Compr Canc Netw. 2021;19(6):733-754. doi:
10.6004/jnccn.2021.0027. https://doi.org/10.6004/jnccn.2021.0027 PMid:34214968
- Childhood
Hodgkin Lymphoma Treatment (PDQ®): Health Professional Version. PDQ
Pediatric Treatment Editorial Board. PDQ Cancer Information Summaries
[Internet]. Bethesda (MD): National Cancer Institute (US);2002. 2023
Sep 14.
- New guidelines for pediatric Hodgkin lymphoma. The Lancet Haematol. 2020;7(12):e851. doi: 10.1016/S2352-3026(20)30371-9. https://doi.org/10.1016/S2352-3026(20)30371-9 PMid:33242438
- Lo
AC, Dieckmann K, Pelz T, Gallop-Evans E, Engenhart-Cabillic R,
Vordermark D, Kelly KM, Schwartz CL, Constine LS, Roberts K, Hodgson D.
Pediatric classical Hodgkin lymphoma. Pediatr Blood Cancer. 2021. 68
(Suppl 2):e28562. doi: 10.1002/pbc.28562. https://doi.org/10.1002/pbc.28562 PMid:33818890
- Jain
S, Kapoor G, Bajpai R. ABVD-based Therapy for Hogkin Lymphoma in
Children and Adolescents: lessons Learnt in a Tertiary Care Oncology
Center in a Developing Country. Pediatr Blood Cancer.
2016;63:1024-1030. doi: 10.1002/pbc.25935. https://doi.org/10.1002/pbc.25935 PMid:26855007
- Ghafoor
T. Prognostic factors in pediatric Hodgkin lymphoma: experience from a
developing country. Leuk Lymphoma. 2020;61(2):344-350. doi:
10.1080/10428194.2019.1665666. https://doi.org/10.1080/10428194.2019.1665666 PMid:31535950
- Sherief
LM, Elsafy UR, Abdelkhalek ER, Kamal NM, Elbehedy R, Hassan TH,
Sherbiny HS, Beshir MR, Saleh SH. Hodgkin lymphoma in childhood:
clinicopathological features and therapy outcome at 2 centers from a
developing country. Medicine. 2015; 94(15):e679. doi:
10.1097/MD.0000000000000670. https://doi.org/10.1097/MD.0000000000000670 PMid:25881843 PMCid:PMC4602501
- Lister
TA, Crowther D, Sutcliffe SB, Glatstein E, Canellos GP, Young RC,
Rosenberg SA, Coltman CA, Tubiana M. Report of a commitiee convened to
discuss the evaluation and staging of patients with Hodgkin's disease:
Cptswolds meeting. J Clin Oncol. 1989;7(11):1630-1636. doi:
10.1200/JCO.1989.7.11.1630. https://doi.org/10.1200/JCO.1989.7.11.1630 PMid:2809679
- Van
Leeuwen, Ng AK. Long-term risk of second malignancy and cardiovascular
disease after Hodgkin lymphoma treatment. Hematology, Am Soc Hematol
Educ Program 2016. Dec 2, 2016;(1):323-330. doi:
10.1182/asheducation-2016.1.323. https://doi.org/10.1182/asheducation-2016.1.323 PMid:27913498 PMCid:PMC6142518
- Andersson
A, Enblad G, Erlanson M, Johansson A-S, Molin D, Tavelin B, Näslund U,
Melin B. High risk of cardiovascular side effects after treatment of
Hodgkin's lymphoma - is there a need for intervention in long-term
survivors ? Ups J Med Sci. 2021;15:126. doi:
10.48101/ujms.v126.6117.eCpllection 2021. https://doi.org/10.48101/ujms.v126.6117 PMid:33889307 PMCid:PMC8043572
- Mittal
A, Bhethanabhotla S, Ganguly S, Vishnubhatla S, Khadgawat R, Patel C,
Mohan A, Biswas A, Bakhshi S. Late effects in pediatric Hodgkin
lymphoma survivors after uniform treatment with ABVD with or without
radiotherapy. Pediatr Blood Cancer. 2021;68(11):e29293. doi:
10.1002/pbc.29293. https://doi.org/10.1002/pbc.29293 PMid:34431211
- Brockelmann
PJ, Eichenauer DA, Jakob T, Follmann M, Engert A, Skoetz N. Hodgkin
lymphoma in Adults. Dtsch Arztebl Int. 2018;115(31-32):535-540. doi:
10.3238/arztebl.2018.0535. https://doi.org/10.3238/arztebl.2018.0535 PMid:30149835 PMCid:PMC6131364
- Santoro
A, Mazza R, Spina M, Califano C, Specchia G, Carella M, Consoli U,
Palombi F, Musso M, Pulsoni A, Kovalchuk S, Bonfichi M, Ricci F, Fabbri
A, Liberati AM, Rodari M, Giordano L, Chimienti E, Balzarotti M,
Sorasio R, Gallamini A, Ghiggi C, Ciammella P, Ricardi U, Chauvie S,
Carlo-Stella C, Merli F. Dose-dense ABVD as first-line therapy in early
stage unfavorable Hodgkin lymphoma: results of a prospective,
multicenter double-step phase II study by Fondazione Italiana Linfomi.
Ann Hematol. 2021;100(10):2547-2556. doi: 10.1007/s00277-021-04604-x. https://doi.org/10.1007/s00277-021-04604-x PMid:34327561
- Kapoor
G, Advani SH, Dinshaw KA, Muckaden MA, Soman CS, Saikia TK, Gopal R,
nair CN, Kurkure PA, Pai SK. Treatment results of Hodgkin's disease in
Indian children. Pediatr Hematol Oncol. 1995;12(6):559-568. doi:
10.3109/08880019509030770. https://doi.org/10.3109/08880019509030770 PMid:8589001
- Hessissen
L, Khtar R, Madani A, El Kababri M, Kili A, Harif M, Khattab M,
Saharoui S, Benjaafar N, Ahid S, Howard SC, Benchekroun S. Improving
the prognosis of pediatric Hodgkin lymphoma in developing countries: a
Maroccan Society of Pediatric Hematology and Oncology study. Pediatr
Blood Cancer. 2013;60(9):1464-1469. doi: 10.1002/pbc.24534. https://doi.org/10.1002/pbc.24534 PMid:23606223
- Castellanos
EM, Barrantes JC, Baez LF, Gamboa Y, Peña A, Alabi S, Bonilla M, Wang
H, Metgzer ML, de Alarcón PA. A chemotherapy only therapeutic approach
to pediatric Hodgkin lymphoma: AHOPCA LH 1999. Pediatr Blood Cancer.
2014;61(6):999-1002. doi: 10.1002/pbc.24905. https://doi.org/10.1002/pbc.24905 PMid:24347509
- Luna-Fineman
S, Castellanos M, Metzger ML, Baez LF, Hernandez AP, Bonilla M,
Fuentes-Alabi S, Nieves R, Blanco J, Rossi E, Devidas M, Chen Y,
Arreola M, de Alarcon PA. Treatment of high-risk Hodgkin lymphoma with
a modified Stanford V regimen in the AHOPCA: Substituting chemotherapy
agents and hampered outcomes. Pediatr Blood Cancer. 2023;71(2):e30792.
doi: 10.1002/pbc.21003. https://doi.org/10.1002/pbc.21003 PMid:16883601
- Dorfell
W, Ruhl U, Luders H, Claviez A, Albrecht M, Bökkerink J, Holte H,
Karlen J, Mann G, Marciniak H, Niggli F, Schmiegelow K, Schwarze E-W,
Potter R, Wickmann L, Schellong G. Treatment of children and
adolescents with Hodgkin lymphoma without radiotherapy for patients in
complete remission after chemotherapy: final results of the
multinational trial GPOH-HD95. J Clin Oncol. 2013;31(12):1562-1568.
doi: 10.1200/JCO.2012.45.3266. https://doi.org/10.1200/JCO.2012.45.3266 PMid:23509321
- Friedman
DL, Chen L, Wolden S, Buxton A, McCarten K, FitzGerald TJ, Kessel S, De
Alarcon PA, Chen AR, Kobrinsky N, Ehrlich P, Hutchison RE, Constine LS,
Schwartz CL. Dose-intensive response-based chemotherapy and radiation
therapy for children and adolescents with newly diagnosed
intermediate-risk Hodgkin lymphoma: a report from the Children's
Oncology Group Study AHOD0031. J Clin Oncol. 2014;32(32):3651-3658.
doi: 10.1200/JCO.2013.52.5410. https://doi.org/10.1200/JCO.2013.52.5410 PMid:25311218 PMCid:PMC4220044
- Donaldson
SS, Link MP, Weinstein HJ, Rai SN, Brain S, Billett AL, Hurwitz CA,
Krasin M, Kun LE, Marcus KC, Tarbell NJ, Young JA, Hudson MM. Final
results of a prospective clinical trial with VAMP and low-dose
involved-field radiation for children with low-risk Hodgkin's disease.
J Clin Oncol. 2007;25(3):332-337. doi: 10.1200/JCO.2006.08.4772 https://doi.org/10.1200/JCO.2006.08.4772 PMid:17235049
- Mauz-Korholz
C, Landman-Parker J, Balwierz W, Ammann RA, Anderson RA, Attarbaschi A,
Bartelt JM, Beishuizen A, Boudjemaa S, Cepelova M, Claviez A, Daw S,
Dieckmann K, Fernandez-Teijeiro A, Fossa A, Gattenlohner S, Georgi T,
Hjalgrim LL, Hraskova A, Karlen J, Kluge R, Kurch L, Leblanc T, Mann G,
Montravers F, Pears J, Pelz T, Rajic V, Ramsay AD, Stoevesandt D,
Uyttebroeck A, Vordermark D, Korholz D, Hasenclever D, Wallace WH.
Response-adapted omission of radiotherapy and comparison of
consolidation chemotherapy in children and adolescents with
intermediate-stage classical Hodgkin lymphoma (EURONet-PHL-C1): a
titration study with an open-label, embedded, multinational,
non-inferiority, randomized controlled trial. Lancet Oncol.
2022;23(1):125-137. doi: 10.1016/S1470-2045(21)00470-8. https://doi.org/10.1016/S1470-2045(21)00470-8 PMid:34895479
- Karla
M, Bakhshi S, Singh M, Seth R, Verma N, Jain S, Radhakrishnan V, Mandal
P, Mahajan A, Arora RS, Dinand V, Kapoor G, Sajid M, Kumar R, Taluja A,
Mallick S, Chandra J. Response assessment by positron emission
tomography-computed tomography as compared with contrast-enhanced
computed tomography in childhood Hodgkin lymphoma can reduce the need
for radiotherapy in low- and middle-income countries. J Pediatr Blood
Cancer. 2023;70(2):e30091. doi: 10.1002/pbc.30091. https://doi.org/10.1002/pbc.30091 PMid:36411263
- Palayullakandi
A, Trehan A, Jain R, Kumar R, Mittal BR, Kapoor R, Srinivasan R, Kakkar
N, Bansal D. Retrospective single-center experience with OEPA/COPDAC
and PET-CT based strategy for pediatric Hodgkin lymphoma in a LMIC
setting. Pediatr Hematol Oncol. 2022;39(7):587-599. doi:
10.1080/08880018.2022.2044418. https://doi.org/10.1080/08880018.2022.2044418 PMid:35271413
- Parambil
BC, Narula G, Prasad M, Shah S, Shet T, Shridhar E, Khanna N, Laskar S,
Gujral S, Sankaran H, Banavali S. Clinical profile and outcome of
classical Hodgkin lymphoma treated with a risk-adapted approach in a
tertiary cancer center in India. Pediatr Blood Cancer.
2020;67(2):e28058. doi: 10.1002/pbc.28058. https://doi.org/10.1002/pbc.28058 PMid:31724304
- Araujo
I, Bittencourt AL, Barbosa HS, Netto EM, Mendonca N, Foss H-D, Hummel
M, Stein H. The high frequency of EBV infection in pediatric Hodgkin
lymphoma is related to the classical type in Bahia, Brazil. Virchows
Arch. 2006; 449(3):315-319. doi: 10.1007/s00428-006-0244-z. https://doi.org/10.1007/s00428-006-0244-z PMid:16896892
- Al-Salam
S, John A, Daoud S, Chong SM, Castella A. Expression of Epstein-Barr
virus in Hodgkin lymphoma in a population of United Arab Emirates
nationals. Leuk Lymphoma. 2008;49(9):1769-1777. doi:
10.1080/10428190802270894. https://doi.org/10.1080/10428190802270894 PMid:18661399
- Mahaian
A, Bakhshi S, Seth R, Verma N, Mandal P, Singh M, Jain S, Radhakrishnan
V, Kanvinde S, Arora RS, Dinand V, Kalra M, Taluja A, Mallick S, Kumar
R, Chandra J. Hodgkin lymphoma in Children Under 5 years: Do They
Behave Differently? J Pediatr Hematol Oncol. 2022;44(4):186-190. doi:
10.1097/MPH.0000000000002423. https://doi.org/10.1097/MPH.0000000000002423 PMid:35293880
- Nohtani
M, Vrzalikova K, Ibrahim M, Powell JE, Fennell E, Morgan S, Grundy R,
McCarthy K, Dewberry S, Bouchal J, Bouchalova K, Kearns P, Murray PG.
Impact of Tumour Epstein-Barr Virus Status on Clinical Outcome in
Patients with Classical Hodgkin Lymphoma (cHL): A Review of the
Literature and Analysis of a Clinical Trial Cohort of Children with
cHL. Cancers (Basel). 2022;14(17):4297. doi: 10.3390/cancers14174297. https://doi.org/10.3390/cancers14174297 PMid:36077832 PMCid:PMC9454639
Supplementary materials
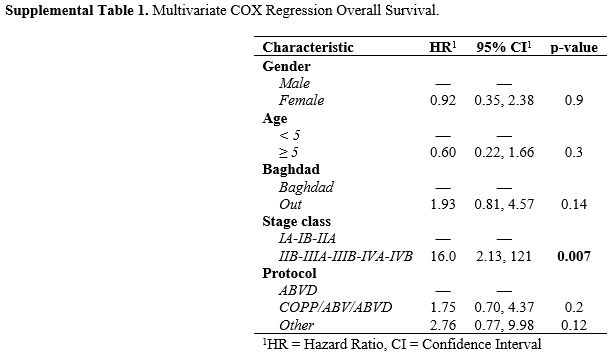 |
Supplemental Table 1. Multivariate COX Regression Overall Survival.. |
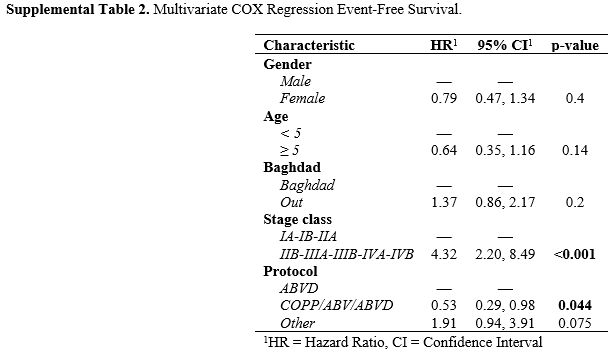 |
Supplemental Table 2. Multivariate COX Regression Event-Free Survival.
|







