Andrea Nunzi1, Luigi Della Valle1, Elisa Linnea Lindfors Rossi1, Giorgia Ranucci1, Flavia Mallegni1, Federico Moretti1, Elisa Meddi1, Luca Guarnera1, Ilaria Tiravanti1, Kristian Taka1, Elisa Buzzatti1, Fabiana Esposito1, Roberto Secchi1, Francesca Di Giuliano2, Flavia Chirico3, Raffaele Palmieri1, Luca Maurillo4, Francesco Buccisano1, Carmelo Gurnari1,5, Giovangiacinto Paterno4, Adriano Venditti1 and Maria Ilaria Del Principe1.
1 Ematologia, Dipartimento di Biomedicina e Prevenzione, Università di Roma Tor Vergata, Viale Oxford 81, 00133, Roma, Italia.
2
Unità di Neuroradiologia, Dipartimento di Biomedicina e Prevenzione,
Università di Roma Tor Vergata, Viale Oxford 81, 00133, Roma, Italia.
3
Unità di Diagnostica per Immagini, Dipartimento di Biomedicina e
Prevenzione, Università di Roma Tor Vergata, Viale Oxford 81, 00133,
Roma, Italia.
4 Ematologia, Fondazione Policlinico Tor Vergata, Viale Oxford 81, 00133, Roma, Italia.
5 Department of Translational Hematology and Oncology Research, Taussig Cancer Institute, Cleveland Clinic, Cleveland, OH, USA.
Correspondence to:
Prof. Adriano Venditti, MD, Professor. Department of Biomedicine and
Prevention, Tor Vergata University, Viale Oxford 81, 00133, Rome,
Italy. Phone number: +39 0620903236. Email:
adriano.venditti@uniroma2.it; ORCID: 0000-0002-0245-055
Published: July 01, 2024
Received: February 22, 2024
Accepted: June 16, 2024
Mediterr J Hematol Infect Dis 2024, 16(1): e2024054 DOI
10.4084/MJHID.2024.054
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background and objectives.
Identification of latent tuberculosis infection (LTBI) is a critical
step of tuberculosis surveillance, especially in low-incidence
countries. However, it is limited to situations with a higher
probability of developing active disease, e.g., patients with
hematological malignancies. According to guidelines, in TB non-endemic
countries, no clear screening program is established at diagnosis for
patients with acute leukemia (AL).
The primary endpoint of this
study was to establish the prevalence of LTBI in patients with a
diagnosis of AL using QuantiFERON (QFT)-TB. Secondarily, radiological
and clinical features driving the increased risk of LTBI were evaluated.
Methods. QFT-TB
screening was performed before induction or consolidation in all
patients with AL (myeloid and lymphoid) treated at our Institution
between October 2019 and August 2023.
Results. We
accrued 62 patients, of whom 7 (11,3%) tested positive, without any
symptoms or signs of active TB, and 2 (3,2%) resulted as indeterminate.
All positive patients started prophylaxis with isoniazid 300 mg daily,
while patients whose test was indeterminate did not receive any
prophylaxis. Active TB was excluded by imaging, as well as microscopic,
cultural, and molecular examination on bronchoalveolar lavage if signs
of any infection were detected. During the 46 months of observation, no
patients developed TB reactivation.
Conclusions. Despite
the low sample size, 1/10 of our patients had prior TB exposure,
hinting that LTBI could be more common than expected in Italy. This
finding suggests implementing TB screening in the pre-treatment
setting, particularly at a time when more active treatments are
becoming available also for patients ineligible for intensive
chemotherapy.
|
Introduction
According
to data from the 2023 report published by the World Health Organization
and the European Centre for Disease Prevention and Control, Italy is a
low tuberculosis (TB) incidence country, with < 20 new cases/100.000
inhabitants.[1] Latent tuberculosis infection (LTBI) is defined as the presence of Mycobacterium tuberculosis in individuals without any symptoms or signs of active disease.[2]
While
most individuals with LTBI rarely develop active disease, up to 15% of
such cases with concurrent high-risk factors, such as Human
Immunodeficiency Virus (HIV) infection, malnutrition, active cancer,
solid organ transplant, or hematopoietic stem cell transplantation and
immunodepression, may progress to active TB.[3-6]
Moreover, a variety of environmental situations, workplace, and
personal habits concur with increasing the risk of developing active
TB.[7]
Patients with hematologic malignancies and LTBI have a 40 times higher risk of progression to fully-blown disease,[4,8]
with studies showing the prevalence of up to 30% of LTBI in patients
with hematological malignancies, even in those countries that are not
TB-endemic.[9]
According to current guidelines,
in non-endemic TB countries, no clear TB screening program is
established at diagnosis for patients with acute leukemia (AL), while
it is imperative prior to hematopoietic stem cell transplantation.
Information about the efficacy and safety of TB preventive treatment in
patients with newly diagnosed AL is very limited, and there is a very
high heterogeneity in TB screening approaches across Centers of Care
for hematological patients at diagnosis. Search for LTBI at diagnosis
of AL could provide an opportunity to prompt recognition and subsequent
treatment or prophylaxis, thereby lowering morbidity and mortality
associated with TB.[10]
Considering our Italian
peninsula, only a few monocentric studies analyzed LTBI prevalence and
efficacy of prophylaxis in similar hematological populations,
underlining the unmet need to establish further evaluations to confront
data from different Centers and geographic areas. According to Bettelli
et al., 7,7% of patients with AL or aplastic anemia tested positive for
QuantiFERON (QFT)-TB test, concluding that LTBI is not uncommon as
expected in low-incidence countries.[11] For this
reason, it is of great utility to expand current evidence with
experiences and data from as many centers as possible to establish a
more solid picture of epidemiology in this category of patients and
attempt to develop common strategies of intervention. Herein, we aim to
study the prevalence of LTBI in our patients with AL.
Material and Methods
Study design and endpoints.
This is a retrospective, observational, monocentric study focused on
consecutive patients admitted to the Department of Hematology at
Policlinico Tor Vergata with a diagnosis of AL (myeloid, lymphoblastic,
promyelocytic) who underwent a QFT-TB test from October 2019 to August
2023, with a follow-up period of 1 to 46 months.
The primary endpoint was to establish the LTBI prevalence in patients with a diagnosis of AL using QFT-TB as screening.
Secondary endpoints included the evaluation of any possible correlations between radiological findings and the QFT-TB results.
Setting. Policlinico Tor Vergata, Rome, Italy.
Study population.
We enrolled consecutive patients aged ≥ 18 years, diagnosed with AL
according to the 2016 revision to the World Health Organization (WHO)
classification[12] and risk-stratified according to the European Leukemia Net (ELN) 2017[13] for patients diagnosed before the updated versions, and then according to the WHO 2022 classification[14] and the ELN 2022,[15]
when available. We selected patients undergoing induction or
consolidation treatment, both fit and unfit for intensive treatment.
Patients who were candidates for best supportive care were also
enrolled in the study. Indeed, the vast majority (n=58/62, 94%) of our
patients were tested before induction therapy and only 4 (6%) before
consolidation. However, we then focused (as detailed by patient
characteristics) on those naïve to treatment.
We collected
data on cases that underwent the QFT-TB test (using the QuantiFERON-TB
Gold Plus method) from October 2019 to August 2023. The test assessed
patients' TB status: positive, indeterminate, or negative (see below).
QuantiFERON-TB test. QFT-TB Gold Plus from peripheral blood was used to detect M. tuberculosis
infection. Briefly, the test evaluated the presence of Interferon
Gamma, a cytokine produced after T-cell stimulation by two highly
specific M. tuberculosis antigens (ESAT-6 and CFP-10). Moreover, this test was able to differentiate CD4+ and CD8+ -specific cellular responses.
Results
could be positive, negative, or indeterminate. A patient was considered
positive for M. tuberculosis infection if the IFN-γ response to TB
antigens was deemed above the test cutoff. A positive result may result
in either latent or active tuberculosis based on radiological imaging
and/or microbiological studies as per established guidelines.[1]
The
QFT-TB test in our institute is used in clinical practice for patients
with hematological malignancies before the start of treatment.
Statistical analysis.
Patients’ characteristics have been described by frequency tables for
qualitative variables and location indicators for quantitative
variables. For the univariate analysis, Chi-square or Fisher's exact
test for qualitative variables and Mann-Whitney test for quantitative
variables have been used. Confidence intervals have been calculated at
95%, and differences with p < 0.05 have been considered
statistically significant. All analyses were performed using IBM SPSS
Statistics 25 software.
Data collection.
Data were retrieved and tabulated by revision of patients’ medical
records. Variables of major interest included the following: gender,
age at diagnosis of AL and at QFT-TB test, type of diagnosis and
characteristics (molecular biology and cytogenetic alterations)
according to WHO 2016 and 2022 classification, risk according to ELN
2017 and 2022 classification, specific antineoplastic treatments,
QFT-TB test results, information on TB preventive treatment, adverse
events and possible pharmacological interactions were evaluated
according to Common Terminology Criteria for Adverse Events (CTCAE
v5.0)). Additional epidemiologic data were collected, such as place of
birth and living at the moment of diagnosis, work occupation, smoking
habits, comorbidities, blood cell count at diagnosis and QFT-TB test,
microbiology studies (direct microscopy, blood, and bronchoalveolar
lavage cultures, galactomannan assay in serum or bronchoalveolar
lavage), and imaging. Data from regular follow-up were collected: date
of allogeneic stem cell transplantation (alloHSCT) and date of death.
Computed
Tomography (CT) scan was performed using a 256-slice scanner (GE
Medical System, Revolution CT) with the following parameters: slice
acquisition 2.5 mm, reconstructed to 1.25 with soft tissue windows for
mediastinal revision and lung windows for parenchymal revision, pitch
0.5, rotation time 0.7 s, tube voltage 120 kVp, with adaptive mA. Two
experienced radiologists reviewed the chest CT scans to assess whether
the positivity of the QFT-TB test had a radiological counterpart
consistent with a diagnosis of typical or atypical tuberculosis. All
patients, regardless of the results of the QFT-TB test, underwent chest
CT before starting treatment as part of the initial staging of the
disease or due to respiratory symptoms.
Ethics approval and consent to participate.
The review and collection of clinical and molecular data were performed
in accordance with the protocols and written consent approved by our
institution's Institutional Review Board (number of Ethical Committee
approval: 105.23 CET2 PTV) and the guidelines set forth by the
Declaration of Helsinki.
Results
From
October 2019 to August 2023, a total of 62 consecutive patients (36
male/26 female) were included in the study, of whom 47 (76%) had Acute
Myeloid Leukemia (AML), 12 (19%) with Acute Lymphoblastic Leukemia
(ALL) and 3 (5%) with Acute Promyelocytic Leukemia (APL). The median
age at AL diagnosis was 64 years (range 18-83). Charlson Comorbidity
Index (CCI) was used to evaluate comorbidity burden, and the median CCI
was 4 (range 2-7).[15] Of all patients, 34 (55%) underwent intensive treatment, and 3 proceeded to alloHSCT.
QFT-TB
test was performed before induction therapy in 58 (94%) patients and
before consolidation in 4 (6%) patients. In our cohort, 7 cases (11,3%)
tested positive: 6 (9,6%) before induction chemotherapy and 1 (1,6%)
before first consolidation, without any symptoms or signs of active TB.
Four patients tested indeterminate: 2 of them (both with a diagnosis of
AML) tested negative in a subsequent evaluation, while the remaining 2
(one diagnosed with ALL and the other one with AML) were confirmed as
indeterminate.
Several variables were analyzed in an attempt to
establish a correlation with QFT-TB test results, but no significant
differences were observed, as shown in Table 1.
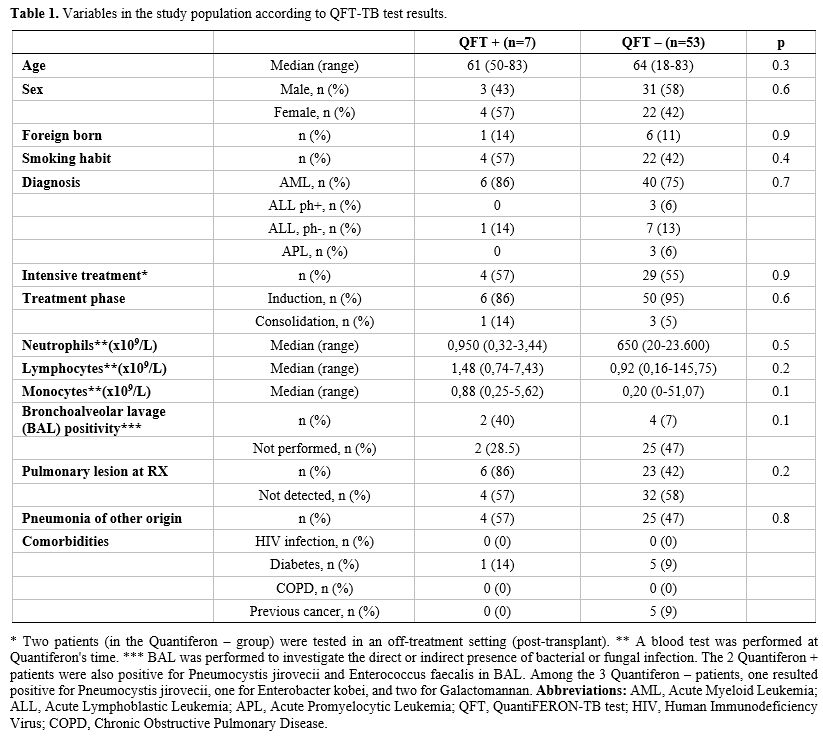 |
- Table
1. Variables in the study population according to QFT-TB test results.
|
We
then focused on potential epidemiological indicators of the 7 patients
who tested positive. Three patients (4.8%) had occupational-related
risk, but all tested negative for the QFT-TB test, while 22 (35,5%)
patients had a smoking habit: Four (57%) of the 7 QFT-positive patients
were in this subgroup.
6 (9,6%) of 62 patients were foreign born:
2 patients from Peru, 2 from Romania, 1 from Albania, and 1 from
Uganda. Of these, only Uganda and Peru are listed by the World Health
Organization as having high TB burden profiles. None of the 62 patients
were living in foreign countries. Of the 6 foreign-born patients, just
one (1 of the 2 from Romania) tested positive for the QFT-TB test. None
of the 62 patients had ever been tested before for tuberculosis, and
only 1 (1,6%) of 62 patients referred previous exposure to a relative
with active tuberculosis and tested positive for the QFT-TB test. All
62 patients were tested for HIV serology, all resulting negative.
Relevant comorbidities are listed in Table 1. None of the 62 patients was undergoing immunomodulatory or steroid therapy at the time of the QFT-TB test.
All
7 positive patients started prophylaxis with isoniazid 300 mg daily
with pyridoxine supplementation without any side effects; 4 (57%) of
them underwent intensive chemotherapy. No significant pharmacological
interactions were recorded, and none of the patients required
discontinuation of therapy due to side effects possibly registered with
isoniazid (hepatotoxicity, peripheral neuropathy, neurological
symptoms). In our QFT-indeterminate subgroup of patients, no
prophylaxis was administered without any sign or symptom of subsequent
active disease. One of the two QFT-indeterminate patients had more than
100.000/mmc lymphoid blasts at diagnosis of ALL, while the other
patient, diagnosed with AML, had more than 10.000/mmc white blood cells
at the QFT-TB test.
If lung infiltrates were detected, patients
developing febrile neutropenia underwent a chest CT scan and
bronchoalveolar lavage (BAL). Imaging, as well as microscopic,
cultural, and molecular examination on BAL, excluded active TB when
imaging showed suspicious findings of any possible infection. M. tuberculosis was not found on any microbiological or molecular examination performed in QFT-positive patients.
In accordance with the literature, radiologic signs such as cavitation, consolidation (Figure 1), unilateral pleural effusion, pericardial effusion, miliary nodules, centrilobular and tree-in-bud nodules (Figure 2),
ground-glass opacity, bronchiectasis, and other more specific signs
such as galaxy and marginal sign were sought. The imaging revision by
two radiologists demonstrated an interobserver agreement of 100% in the
interpretation of CT patterns: 5 of the seven patients with a positive
QFT test and 5 of the total number of patients with a negative QFT test
had radiological findings indicative of pulmonary infection (71% vs.
9%, respectively; p < 0.001) in the context of febrile neutropenia
during induction. All patients with pulmonary infection, regardless of
their previous QFT result, underwent BAL, and active TB was excluded
through cultural and molecular examination. Given the peculiarity of
patients, most of them being neutropenic, radiologic evaluation was
challenging, and positive findings were generally interpreted as
non-specific (e.g., consolidation, pleural effusion). In the 5
QFT-positive patients, the chest CT scan showed areas of consolidation
with air bronchogram. Two of 5 showed a tree-in-bud pattern, 2 carried
pleural effusions, and one had ground glass opacities type
densitometric changes. Eventually, tree-in-bud patterns and
ground-glass opacities were considered typical radiological signs of TB.
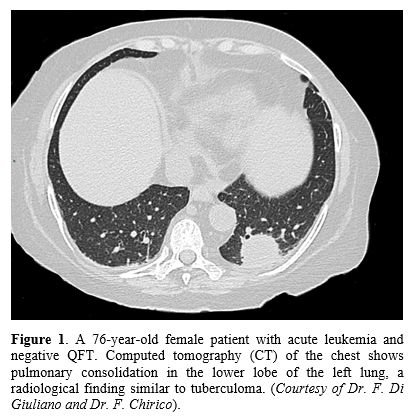 |
Figure
1. A
76-year-old female patient with acute leukemia and negative QFT.
Computed tomography (CT) of the chest shows pulmonary consolidation in
the lower lobe of the left lung, a radiological finding similar to
tuberculoma. (Courtesy of Dr. F. Di Giuliano and Dr. F. Chirico). |
 |
Figure
2. A
56-year-old male patient with acute leukemia and QFT positivity. CT
shows a tree-in-bud radiological pattern in the left lung, a typical
finding in post-primary TB. (Courtesy of Dr. F. Di Giuliano and Dr. F. Chirico).
|
Over
the 46-month observation period (median time 6 months, range 1 - 46
months), none of the QFT-positive patients developed TB reactivation.
Moreover, 3 (5%) of the 62 patients underwent alloHSCT; 2 with AML
received stem cells from 10/10 HLA-identical sibling donors, 1 with ALL
underwent alloHSCT from a haploidentical sibling donor after having
received Chimeric Antigens Receptor Cells-T (CAR-T). All 3 of these
patients were QFT-negative at diagnosis; in addition, the Tuberculin
Skin Test performed during the pre-alloHSCT screening was negative in
all 3 of them.
Discussion
It is estimated that M. tuberculosis
infection affects approximately one-third of the world's population. TB
is especially concerning for individuals with compromised immune
systems, such as patients with hematological malignancies. The
increased risk in such a setting is due to both the underlying
hematological disease and the specific antineoplastic therapies
administered.[17] Since anti-leukemia treatment could
affect the outcome of the test, with possible indeterminate or false
negative results, it seems of major utility to evaluate QFT-TB results
in the pre-treatment setting. Moreover, LTBI patients with radiological
pulmonary lesions suspected to be related to M. tuberculosis
infection should be carefully observed during the treatment period,
given the well-recognized risk of TB reactivation, even in a
disseminated form.[18] In our experience, 71% of patients positive for QFT also carried radiological abnormalities consistent with TB.
A relevant issue pertains to the site of M. tuberculosis dwelling during latent infection. It is assumed that latent bacilli primarily reside within fibrotic pulmonary granulomas.[19] However, some studies demonstrated the presence of M. tuberculosis
DNA not only in the macrophages of old granulomas but also in
non-professional phagocytic cells found within histologically normal
lung tissue specimens.[20,21] This discovery suggests
that latent mycobacteria may also exist within non-specialized
phagocytic cells residing in other health tissues. This finding bears a
critical significance as approximately 15% of TB reactivation cases
manifest in extrapulmonary sites.[22] Taking this
into consideration, performing QFT-TB test in the pre-treatment setting
and subsequent TB preventive therapy appears extremely important also
for those patients without pulmonary lesions.
Patient-specific
factors, such as diabetes, exposure to indoor air pollution, alcohol
consumption, immunosuppressive drugs, and smoking, need to be
considered possible contributors to the transition from LTBI to the
development of active TB. These factors are in addition to widely
acknowledged risk factors, e.g., HIV infection, malnutrition, and young
age. Moreover, socioeconomic and behavioral habits have been shown to
heighten susceptibility to TB infection.[23]
The
diagnosis of LTBI is commonly achieved through two widely used methods:
the Tuberculin Skin Test (TST) and Interferon Gamma Release Assays
(IGRAs), including the QFT-TB test. In individuals with intact immune
systems, the QFT-TB test displays sensitivity similar to the TST but
offers improved specificity when it comes to LTBI diagnosis. QFT-TB
offers several advantages: it provides numerical results, reduces the
potential for bias in interpretation, and does not yield false-positive
results due to prior Bacillus Calmette–Guérin (BCG) vaccination.
Additionally, QFT-TB offers convenience, eliminating the need for a
follow-up visit to interpret the results,[24] resulting in more effective and less expensive than TST.[25]
Nonetheless, a notable drawback of the QFT-TB test is its inability to
provide a clear interpretation of the response to TB-specific antigens
when an indeterminate result occurs. Abnormalities in the number and
differentials of white blood cells have been found to be possible
predictors of indeterminate results.[26]
As
regards preventive treatment, few studies have been carried out to
evaluate toxicity, adherence to treatment, and possible pharmacological
interactions.[27] Notably, the latest guidelines on the management of M. tuberculosis
infections in patients with hematological malignancies point out the
possibility of administering TB preventive therapy in selected cases
regardless of TST or IGRA status, considered at high risk of TB
infection (e.g., patients with close and long-lasting contact with
active pulmonary or laryngeal tuberculosis). On the other hand, they
suggest evaluating the prognosis of the hematological malignancy and
patients' characteristics, especially age and therefore decide whether
to omit LTBI treatment in individuals with poor prognosis.[28]
As
regards which preventive therapy to use, international guidelines point
out the non-inferiority of regimens containing rifamycin in terms of
efficacy compared with isoniazid; nevertheless, shorter rifamycin-based
treatment shows an improvement in adherence and completion rates.[29,30]
However, drug-to-drug interactions remain still a concern, not only
considering the anti-leukemia specific drugs (e.g., daunorubicin) but
also for the relative prophylaxis (e.g., posaconazole) due to the
documented CYP450 induction by rifampin.[31]
Therefore, in our institution, we consider it more practical to
administer isoniazid as a preventive treatment, given the
non-inferiority in terms of efficacy, even though we are now evaluating
the possible administration of rifamycin-based treatment in accordance
with our infectious disease specialists.
Early investigation and
management can help prevent the progression of TB in this cohort of
high-risk patients. This approach is even more relevant with the new
emerging cellular therapies, e.g., bispecific antibodies and CAR-T
cells, which impact the T-cell compartment even more, the major
responsible for controlling LTBI when present.
Even though this
study has limitations, such as its retrospective monocentric approach
and the low sample size, it may contribute to highlighting that even in
countries considered to have low TB burdens, such as Italy, LTBI could
be not so uncommon as expected, also given the evolving characteristics
and epidemiology of patients.
Considering the increasing
globalization, immigration - also from TB-endemic countries - and the
fact that patients with hematological malignancies are now receiving
more active treatments (including those who may not be eligible for
intensive chemotherapy or stem cell transplantation), enforcing TB
screening in the pre-treatment setting becomes of critical importance.
This approach not only could protect individual patients but may also
help prevent the spread of TB in healthcare settings.
Conclusions
Our
study adds to the current evidence regarding the prevalence of LTBI in
hematological patients, emphasizing the importance of implementing
pre-treatment screening approaches for LTBI in this setting, as well as
the need to establish consensus guidelines. By doing so, clinicians can
develop and share consistent and effective strategies to address the
unique challenges presented by this high-risk population. Sharing data
and experiences could help identify trends, common risk factors, and
the most effective strategies for TB screening and prevention in this
heterogeneous population.
Acknowledgments
We
would like to thank all the patients who participated in this study
Doctor Stefano Di Carlo and Professor Sergio Bernardini, who provided
the laboratory data.
References
- World Health Organization. (2023). Tuberculosis
surveillance and monitoring in Europe 2023: 2021 data. Accessed October
30, 2023. Available at: https://www.ecdc.europa.eu/sites/default/files/documents/tuberculosis-surveillance-monitoring-2023.pdf
- Getahun,
H., Matteelli, A., Abubakar, I., Aziz, M. A., Baddeley, A., Barreira,
D., Den Boon, S., Borroto Gutierrez, S. M., Bruchfeld, J., Burhan, E.,
Cavalcante, S., Cedillos, R., Chaisson, R., Chee, C. B.-E., Chesire,
L., Corbett, E., Dara, M., Denholm, J., de Vries, G., Raviglione, M.
(2015). Management of latent Mycobacterium tuberculosis infection: WHO
guidelines for low tuberculosis burden countries. European Respiratory
Journal, 46(6), 1563-1576. https://doi.org/10.1183/13993003.01245-2015 PMid:26405286 PMCid:PMC4664608
- Erkens
CG, Kamphorst M, Abubakar I, Bothamley GH, Chemtob D, Haas W, Migliori
GB, Rieder HL, Zellweger JP, Lange C. Tuberculosis contact
investigation in low prevalence countries: a European consensus. Eur
Respir J. 2010;36(4):925-49. https://doi.org/10.1183/09031936.00201609 PMid:20889463
- Compagno
M, Navarra A, Campogiani L, Coppola L, Rossi B, Iannetta M, Malagnino
V, Parisi SG, Mariotti B, Cerretti R, Arcese W, Goletti D, Andreoni M,
Sarmati L. Latent Tuberculosis Infection in Haematopoietic Stem Cell
Transplant Recipients: A Retrospective Italian Cohort Study in Tor
Vergata University Hospital, Rome. Int J Environ Res Public Health.
2022;19(17):10693. https://doi.org/10.3390/ijerph191710693 PMid:36078409 PMCid:PMC9518118
- Cheng
MP, Kusztos AE, Bold TD, Ho VT, Glotzbecker BE, Hsieh C, Baker MA,
Baden LR, Hammond SP, Marty FM. Risk of Latent Tuberculosis
Reactivation After Hematopoietic cell Transplantation. Clin Infect Dis.
2019;69(5):869-72. https://doi.org/10.1093/cid/ciz048 PMid:30689792 PMCid:PMC6938207
- Litvoc
M.N., Leal F.E., Ferreira D.B., Ferreira Lopes M.I.B., Capuani L.,
Rocha V.G., Costa S.F. High tuberculosis density incidence rate in
matched unrelated allogeneic stem cell transplantation recipients in
the state of São Paulo, Brazil. Mediterr J Hematol Infect Dis 2023,
15(1): e2023037, https://doi.org/10.4084/MJHID.2023.037 PMid:37435037 PMCid:PMC10332347
- Corradi
M, Durando P, Lamberti M, Lodi V, Matteelli A, Nicosia V, Pagliaro G,
Placidi D, Verso MG, Sotgiu G. (2018). Gestione e prevenzione della
tubercolosi in ambito occupazionale in paesi a bassa incidenza.
Giornale italiano di Medicina del Lavoro ed Ergonomia. 2018;40,
27-27.
- Bumbacea
D, Arend SM, Eyuboglu F, Fishman JA, Goletti D, Ison MG, Jones CE,
Kampmann B, Kotton CN, Lange C, Ljungman P, Milburn H, Morris MI,
Muller E, Muñoz P, Nellore A, Rieder HL, Sester U, Theodoropoulos N,
Wagner D, Sester M. The risk of tuberculosis in transplant candidates
and recipients: a TBNET consensus statement. Eur Respir J. 2012 Apr
10;40(4):990-1013. https://doi.org/10.1183/09031936.00000712 PMid:22496318
- Osorio-López,
E. A., Vilar-Compte, D., García-Tirado, J., & Martin-Onraet, A.
(2021). Prevalence of latent tuberculosis in patients with
hematological neoplasms in a cancer referral hospital in Mexico City.
BMC Infectious Diseases, 21(1). https://doi.org/10.1186/s12879-021-06236-y PMid:34059022 PMCid:PMC8168316
- Taha
R, Kothari S, Foroutan F, Gitman M, Gupta V, Nguyen T, Rotstein C.
Implementation of a Routine Screening Program for Latent Tuberculosis
Infection among Patients with Acute Leukemia at a Canadian Cancer
Center. Curr Oncol. 2022;29(12):9325-34. https://doi.org/10.3390/curroncol29120731 PMid:36547145 PMCid:PMC9777027
- Bettelli
F, Giusti D, Morselli M, Colaci E, Nasillo V, Pioli V, Gilioli A, Iotti
S, Galassi L, Giubbolini R, Colasante C, Catellani H, Barozzi P,
Lagreca I, Vallerini D, Maffei R, Franceschini E, Mussini C, Banchelli
F, D'Amico R, Marasca R, Narni F, Potenza L, Comoli P, Luppi M,
Forghieri F. Epidemiology and clinical outcomes of latent tuberculosis
infection in adults affected with acute leukemia or aplastic anemia: a
retrospective single-center study. Ann Hematol. 2020;99(9):2201-3. https://doi.org/10.1007/s00277-020-04191-3 PMid:32699943
- Arber
DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM,
Bloomfield CD, Cazzola M, Vardiman JW. The 2016 revision to the World
Health Organization classification of myeloid neoplasms and acute
leukemia. Blood. 2016;127(20):2391-405. https://doi.org/10.1182/blood-2016-03-643544 PMid:27069254
- Döhner
H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, Dombret H,
Ebert BL, Fenaux P, Larson RA, Levine RL, Lo-Coco F, Naoe T,
Niederwieser D, Ossenkoppele GJ, Sanz M, Sierra J, Tallman MS, Tien HF,
Wei AH, Löwenberg B, Bloomfield CD. Diagnosis and management of AML in
adults: 2017 ELN recommendations from an international expert panel.
Blood. 2017 Jan 26;129(4):424-47. https://doi.org/10.1182/blood-2016-08-733196 PMid:27895058 PMCid:PMC5291965
- Khoury
JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, Bejar R, Berti
E, Busque L, Chan JK, Chen W, Chen X, Chng WJ, Choi JK, Colmenero I,
Coupland SE, Cross NC, De Jong D, Elghetany MT, Takahashi E, Emile JF,
Ferry J, Fogelstrand L, Fontenay M, Germing U, Gujral S, Haferlach T,
Harrison C, Hodge JC, Hu S, Jansen JH, Kanagal-Shamanna R, Kantarjian
HM, Kratz CP, Li XQ, Lim MS, Loeb K, Loghavi S, Marcogliese A,
Meshinchi S, Michaels P, Naresh KN, Natkunam Y, Nejati R, Ott G, Padron
E, Patel KP, Patkar N, Picarsic J, Hochhaus A. The 5th edition of the
World Health Organization Classification of Haematolymphoid Tumours:
Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia https://doi.org/10.1038/s41375-022-01613-1 PMid:35732831 PMCid:PMC9252913
- Döhner
H, Wei AH, Appelbaum FR, Craddock C, DiNardo CD, Dombret H, Ebert BL,
Fenaux P, Godley LA, Hasserjian RP, Larson RA, Levine RL, Miyazaki Y,
Niederwieser D, Ossenkoppele GJ, Röllig C, Sierra J, Stein EM, Tallman
MS, Tien HF, Wang J, Wierzbowska A, Löwenberg B. Diagnosis and
Management of AML in Adults: 2022 ELN Recommendations from an
International Expert Panel. Blood. 2022. https://doi.org/10.1182/blood.2022016867 PMid:35797463
- Charlson
ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying
prognostic comorbidity in longitudinal studies: Development and
validation. J Chronic Dis. Jan 1987;40(5):373-83.
https://doi.org/10.1016/0021-9681(87)90171-8 PMid:3558716
- Anibarro L, Pena A. Tuberculosis in patients with hematological malignancies. Mediterr J Hematol Infect Dis. 2014;6(1). https://doi.org/10.4084/mjhid.2014.026 PMid:24803999 PMCid:PMC4010605
- Al-Anazi,
K., Al-Jasser, A., & Evans, D. (2007). Infections caused by
mycobacterium tuberculosis in patients with hematological disorders and
in recipients of hematopoietic stem cell transplant, a twelve-year
retrospective study. Annals of Clinical Microbiology and
Antimicrobials, 6(1), 16. https://doi.org/10.1186/1476-0711-6-16 PMid:18021401 PMCid:PMC2200647
- Parrish,
N. M., Dick, J. D., & Bishai, W. R. (1998). Mechanisms of latency
in Mycobacterium tuberculosis. Trends in Microbiology, 6(3), 107-112. https://doi.org/10.1016/S0966-842X(98)01216-5 PMid:9582936
- Hernández-Pando,
R., Jeyanathan, M., Mengistu, G., Aguilar, D., Orozco, H., Harboe, M.,
Rook, G., & Bjune, G. (2000). Persistence of DNA from Mycobacterium
tuberculosis in superficially normal lung tissue during latent
infection. The Lancet, 356(9248), 2133-2138. https://doi.org/10.1016/S0140-6736(00)03493-0 PMid:11191539
- Arriaga,
A. K., Orozco, E. H., Aguilar, L. D., Rook, G. A. W., & Pando, R.
H. (2002). Immunological and pathological comparative analysis between
experimental latent tuberculous infection and progressive pulmonary
tuberculosis. Clinical & Experimental Immunology, 128(2), 229-237. https://doi.org/10.1046/j.1365-2249.2002.01832.x PMid:11985512 PMCid:PMC1906395
- Barrios-Payán,
J., Saqui-Salces, M., Jeyanathan, M., Alcántara-Vazquez, A.,
Castañon-Arreola, M., Rook, G., & Hernandez-Pando, R. (2012).
Extrapulmonary Locations of Mycobacterium tuberculosis DNA During
Latent Infection. The Journal of Infectious Diseases, 206(8),
1194-1205. https://doi.org/10.1093/infdis/jis381 PMid:22732919
- Narasimhan,
P., Wood, J., MacIntyre, C. R., & Mathai, D. (2013). Risk Factors
for Tuberculosis. Pulmonary Medicine, 2013, 1-11.
https://doi.org/10.1155/2013/828939 https://doi.org/10.1155/2013/828939 PMid:23476764 PMCid:PMC3583136
- Pai,
M., Denkinger, C. M., Kik, S. V., Rangaka, M. X., Zwerling, A., Oxlade,
O., Metcalfe, J. Z., Cattamanchi, A., Dowdy, D. W., Dheda, K., &
Banaei, N. (2014). Gamma Interferon Release Assays for Detection of
Mycobacterium tuberculosis Infection. Clinical Microbiology Reviews,
27(1), 3-20. https://doi.org/10.1128/CMR.00034-13 PMid:24396134 PMCid:PMC3910908
- Deuffic-Burban,
S., Atsou, K., Viget, N., Melliez, H., Bouvet, E., & Yazdanpanah,
Y. (2010). Cost-effectiveness of QuantiFERON®-TB test vs. tuberculin
skin test in the diagnosis of latent tuberculosis infection. The
International journal of tuberculosis and lung disease, 14(4), 471-481.
- Huang,
C.-C., Jerry Teng, C.-L., Wu, M.-F., Lee, C.-H., Chen, H.-C., &
Huang, W.-C. (2021). Features of indeterminate results of
QuantiFERON-TB Gold In-Tube test in patients with haematological
malignancies. Therapeutic Advances in Hematology, 12, 204062072110284. https://doi.org/10.1177/20406207211028437 PMid:34285787 PMCid:PMC8264733
- Sánchez-García,
E. M., Gamallo, R., Blanco-Moure, A., Viejo, M. A., Amador, L., &
Anibarro, L. (2013). Toxicity and adherence to treatment for latent
tuberculosis infection in patients with hematologic malignancies.
Infection, 41(5), 903-907. https://doi.org/10.1007/s15010-013-0489-9 PMid:23737388
- Bergeron
A, Mikulska M, De Greef J, Bondeelle L, Franquet T, Herrmann JL, Lange
C, Spriet I, Akova M, Donnelly JP, Maertens J, Maschmeyer G, Rovira M,
Goletti D, de la Camara R, Greinix H, Maertens J, De Greef J, Slavin M,
Spriet I, Hubacek P, Bergeron A, Cordonnier C, Kanerva J, Herbrecht R,
Herrmann JL, Lanternier F, Bondeelle L, Robin C, Einsele H, Lehrnbecher
T, Groll A, Maschmeyer G, Lange C, von Lilienfeld-Toal M, Pana D,
Roilides E, Kassa C, Averbuch D, Engelhard D, Cesaro S, Mikulska M,
Pagano L, Castagnola E, Compagno F, Goletti D, Mesini A, Donnelly PJ,
Styczynski J, Chemaly R. Mycobacterial infections in adults with
haematological malignancies and haematopoietic stem cell transplants:
guidelines from the 8th European Conference on Infections in Leukaemia.
Lancet Infect Dis. 2022 May. https://doi.org/10.1016/S1473-3099(22)00227-4 PMid:35636446
- Organizat
WH. WHO consolidated guidelines on tuberculosis. Module 1: Prevention.
Tuberculosis preventive treatment. Tuberc Lung Dis HIV Infect.
(2):86-92. https://doi.org/10.30978/TB2021-2-86
- Krishnan S, Chaisson RE. US Guidelines Fall Short on Short-Course TB Preventive Therapy. Clin Infect Dis 2023. https://doi.org/10.1093/cid/ciad659 PMid:37879092
- Hohmann
C, Kang EM, Jancel T. Rifampin and Posaconazole Coadministration Leads
to Decreased Serum Posaconazole Concentrations. Clin Infect Dis.
50(6):939-40. https://doi.org/10.1086/650740 PMid:20166829


