.
Matteo Molica1, Luca Maurillo2, Marco Rossi1, Massimo Breccia3, Carla Mazzone4, Paolo de Fabritiis2,4 and Salvatore Perrone5.
1 Department of Hematology-Oncology, Azienda Universitaria Ospedaliera Renato Dulbecco, 88100 Catanzaro, Italy.
2 Department of Biomedicina e Prevenzione, Tor Vergata University, 00133 Rome, Italy.
3
Hematology, Department of Translational and Precision Medicine, Az.
Policlinico Umberto I-Sapienza University, 00185 Rome, Italy.
4 Hematology, St. Eugenio Hospital, ASL Roma2, 00144 Rome, Italy.
5 Department of Hematology, Polo Universitario Pontino, S.M. Goretti Hospital, 04100 Latina, Italy.
Correspondence to:
Salvatore Perrone, MD. Department of Hematology, Polo Universitario
Pontino, S.M. Goretti Hospital, 04100 Latina, Italy. E-mail:
sperrone@hotmail.it
Published: July 01, 2024
Received: March 03, 2024
Accepted: June 14, 2024
Mediterr J Hematol Infect Dis 2024, 16(1): e2024055 DOI
10.4084/MJHID.2024.055
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
To the editor
In
fit patients with newly diagnosed acute myeloid leukemia (ND-AML),
immediate treatment is recommended due to the poor prognosis of
untreated acute leukemia; however, this paradigm has been challenged by
a German study.[1] The authors stratified patients
into 4 groups for a time from diagnosis to treatment start (TDT),
showing no difference in waiting before starting intensive
chemotherapy. The actual clinical practice, therefore, considers it
acceptable to delay ND-AML patients completing the laboratory work-up
recommended by the European Leukemia Net (ELN2022)[2]
and solve toxicities present at the time of diagnosis. Turnaround times
for targeted gene panels performed with next-generation sequence (NGS)
techniques are generally from 5 to 14 days.[3] so
several days can elapse before a definite clinical entity can be
assigned, and some mutations have an impact on the choice of the first
line therapy.[4] On the contrary, AML patients unfit
for intensive chemotherapy are candidates for agnostic therapy
encompassing hypomethylating drugs (HMAs) ± venetoclax (VEN)[5] or glasdegib + low-doses cytarabine,[6]
thus, laboratory work-up is not mandatory before choosing the frontline
treatment, but molecular determinants of outcome are of prognostic
significance.[7] Because of the scant data published
on TDT in the context of HMAs in ND-AML, we evaluated the prognostic
value of TDT in 220 patients with AML older than 75 years, already
reported in a multicentric retrospective study in a real-life setting
of HMAs usage.[8] Patients were treated with only HMAs
due to comorbidities. The main reason for treatment delay was the
presence of an infectious event at baseline.
In this study,
data were collected from patients treated with azacitidine (AZA) (164)
and decitabine (DEC) (56) between September 2010 and September 2023. We
recorded baseline patient-related and disease characteristics,
including age, ECOG, Charlson comorbidity index (CCI) scores, kidney
function (eGFR and CKD-EPI equation), molecular profiling, ELN 2017,
cytogenetic risk, dates of regimen initiation, and survival (Table 1).
Patients were divided into 4 cohorts: those that started therapy in
<15 days (n= 85 patients), 15-30 days (n = 64 patients), 31-45 days
(n= 33 patients), 46 days and beyond (n= 38 patients) from their
initial diagnosis of AML (Table 2).
These 4 cohorts received homogeneous treatment with HMAs. We analyzed
survival using the Kaplan-Meier curves, with significance determined by
the log-rank test. The event for calculating the overall survival (OS)
was the date of death, and for event-free survival (EFS), was the time
of progressive disease, relapse, or death. Patients were otherwise
censored at the date of the last follow-up.
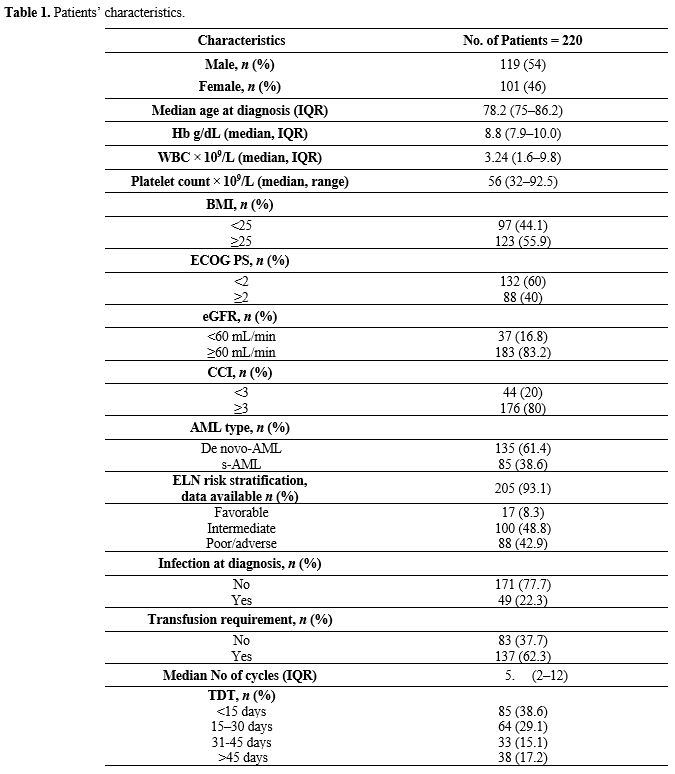 |
Table
1. Patients’ characteristics.
|
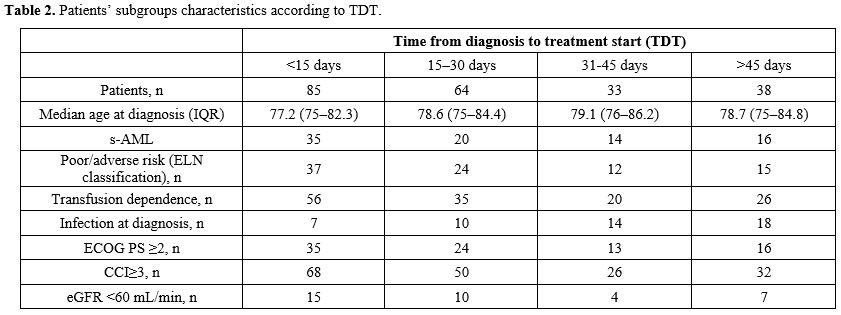 |
Table 2. Patients’ subgroups characteristics according to TDT.
|
The median TDT of patients treated with AZA or DEC was 21 or 15 days, respectively; overall, it was 19 days. The median OS (Figure 1)
was 7.5 months (95%CI 4.5-10.5 mts), 11 months (95%CI 4.5-17.4), 7.4
months (95%CI 4.7-10.1), and 7.6 months (95%CI 2.3-13) for the
subgroups of TDT <15, 15-30, 31-45 and >46 days, respectively
(p=0.224). The median EFS was 5.8 months (95%CI 2.4-9.1), 9.8 months
(95%CI 7.9-11.7), 5.0 months (95%CI 0.9-9.1), and 5.7 months (95%CI
0.5-12.2), for the subgroups of TDT <15, 15-30, 31-45 and >45
days, respectively (p=0.187). No statistically significant difference
was noted when considering patients receiving AZA or DEC In terms of OS
(p=0.08) and EFS (p=0.083) considering the four different groups.
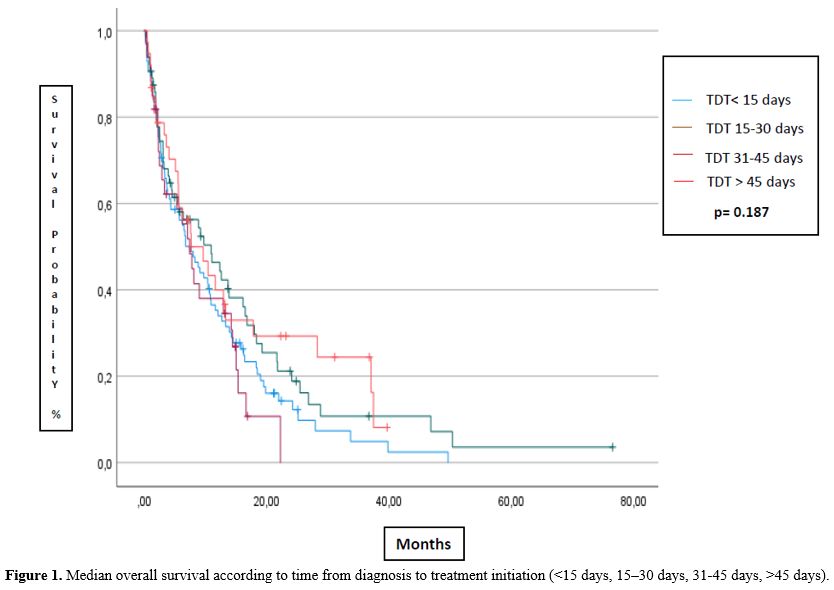 |
- Figure 1. Median
overall survival according to time from diagnosis to treatment
initiation (<15 days, 15–30 days, 31-45 days, >45 days).
|
In
conclusion, TDT did not show prognostic significance in our patients
with AML treated with HMAs. However, a trend for better outcomes can be
seen in the subgroup that started treatment 15-30 days after initial
diagnosis. These results confirm the lack of difference found in the
German study[1] but, at the same time, do not contrast
with the results from Bouligny et al., who considered the impact of TDT
on patients treated with VEN + HMAs.[9] Indeed, in
this study, OS was 5.8 months for patients included in the 0-7 day
cohort of VEN+HMA initiation, significantly worse than 8.9 months for
the 8-14 day cohort and the 12.7 months for 15 days and beyond (p =
0.023). Our study also found that the earliest TDT and the two delayed
groups had worse results than the intermediate 15-30 days TDT group.
This result can be explained by the time used to treat disease-related
complications and mitigate existent comorbidities that can reduce
toxicities related to HMA treatment.
On the other hand, waiting
too long to start HMAs worsens the prognosis of AML patients, therefore
suggesting a possible temporal window to optimize results. Our study
has several limitations, given its retrospective nature and the fact
that time-passing bias can be present, considering that HMAs are
actually rarely given without venetoclax. Therefore, more studies have
to be developed to find the best temporal window in TDT when starting
HMA + VEN, which could help to improve the results of patients with AML.
References
- Röllig, C.; Kramer, M.; Schliemann, C.; Mikesch,
J.-H.; Steffen, B.; Krämer, A.; Noppeney, R.; Schäfer-Eckart, K.;
Krause, S.W.; Hänel, M.; et al. Does Time from Diagnosis to Treatment
Affect the Prognosis of Patients with Newly Diagnosed Acute Myeloid
Leukemia? Blood 2020, 136, 823-830, doi:10.1182/blood.2019004583. https://doi.org/10.1182/blood.2019004583 PMid:32496541
- Döhner,
H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret,
H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al.
Diagnosis and Management of AML in Adults: 2022 Recommendations from an
International Expert Panel on Behalf of the ELN. Blood 2022, 140,
1345-1377, doi:10.1182/blood.2022016867. https://doi.org/10.1182/blood.2022016867 PMid:35797463
- Duncavage,
E.J.; Bagg, A.; Hasserjian, R.P.; DiNardo, C.D.; Godley, L.A.;
Iacobucci, I.; Jaiswal, S.; Malcovati, L.; Vannucchi, A.M.; Patel,
K.P.; et al. Genomic Profiling for Clinical Decision Making in Myeloid
Neoplasms and Acute Leukemia. Blood 2022, 140, 2228-2247,
doi:10.1182/blood.2022015853. https://doi.org/10.1182/blood.2022015853 PMid:36130297 PMCid:PMC10488320
- Molica,
M.; Perrone, S. Molecular Targets for the Treatment of AML in the
Forthcoming 5th World Health Organization Classification of
Haematolymphoid Tumours. Expert Review of Hematology 2022, 15, 973-986,
doi:10.1080/17474086.2022.2140137. https://doi.org/10.1080/17474086.2022.2140137 PMid:36271671
- DiNardo,
C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei,
A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P.; et al.
Azacitidine and Venetoclax in Previously Untreated Acute Myeloid
Leukemia. N Engl J Med 2020, 383, 617-629, doi:10.1056/NEJMoa2012971. https://doi.org/10.1056/NEJMoa2012971 PMid:32786187
- Cortes,
J.E.; Heidel, F.H.; Hellmann, A.; Fiedler, W.; Smith, B.D.; Robak, T.;
Montesinos, P.; Pollyea, D.A.; DesJardins, P.; Ottmann, O.; et al.
Randomized Comparison of Low Dose Cytarabine with or without Glasdegib
in Patients with Newly Diagnosed Acute Myeloid Leukemia or High-Risk
Myelodysplastic Syndrome. Leukemia 2019, 33, 379-389,
doi:10.1038/s41375-018-0312-9. https://doi.org/10.1038/s41375-018-0312-9 PMid:30555165 PMCid:PMC6365492
- DiNardo,
C.D.; Tiong, I.S.; Quaglieri, A.; MacRaild, S.; Loghavi, S.; Brown,
F.C.; Thijssen, R.; Pomilio, G.; Ivey, A.; Salmon, J.M.; et al.
Molecular Patterns of Response and Treatment Failure after Frontline
Venetoclax Combinations in Older Patients with AML. Blood 2020, 135,
791-803, doi:10.1182/blood.2019003988. https://doi.org/10.1182/blood.2019003988 PMid:31932844 PMCid:PMC7068032
- Molica,
M.; Mazzone, C.; Niscola, P.; Carmosino, I.; Di Veroli, A.; De
Gregoris, C.; Bonanni, F.; Perrone, S.; Cenfra, N.; Fianchi, L.; et al.
Identification of Predictive Factors for Overall Survival and Response
during Hypomethylating Treatment in Very Elderly (≥75 Years) Acute
Myeloid Leukemia Patients: A Multicenter Real-Life Experience. Cancers
2022, 14, 4897, doi:10.3390/cancers14194897. https://doi.org/10.3390/cancers14194897 PMid:36230820 PMCid:PMC9564161
- Bouligny,
I.; Murray, G.; Ho, T.; Gor, J.; Zacholski, K.; Wages, N.; Grant, S.;
Maher, K. The Impact of Delayed Venetoclax Initiation on Overall
Survival in Acute Myeloid Leukemia. Blood 2023, 142, 4233-4233,
doi:10.1182/blood-2023-188249. https://doi.org/10.1182/blood-2023-188249


