.
Elaiza Almeida Antônio de Kós1,2, Viviane Lamim Lovatel1, Rita de Cássia Barbosa Tavares3, Gerson Moura Ferreira4, Bernadete Gomes5, Ana Paula Silva Bueno6, Elaine Sobral da Costa6 and Teresa de Souza Fernandez1,2.
1 Cytogenetic Laboratory, Cell and Gene Therapy Program, Instituto Nacional de Câncer (INCA), Rio de Janeiro, RJ, Brazil.
2 Post-Graduate Program in Medical Sciences, Universidade do Estado do Rio de Janeiro (UERJ), Rio de Janeiro, RJ, Brazil.
3 Bone Marrow Transplantation Center, Instituto Nacional de Câncer (INCA), Rio de Janeiro, RJ, Brazil.
4 Stem Cell Laboratory, Instituto Nacional de Câncer, Rio de Janeiro 20230-130, Brazil.
5 Immunology Laboratory, Cell and Gene Therapy Program, Instituto Nacional de Câncer (INCA), Rio de Janeiro, RJ, Brazil.
6
Instituto de Puericultura e Pediatria Martagão Gesteira (IPPMG),
Universidade Federal do Rio de Janeiro (UFRJ), Rio de Janeiro, RJ,
Brazil.
Correspondence to:
Teresa de Souza Fernandez. Instituto Nacional de Câncer (INCA), Centro
de Transplante de Medula Óssea, Laboratório de Citogenética, Praça Cruz
Vermelha nº 23, 6º andar. Centro, Rio de Janeiro, RJ, Brazil. CEP:
20230-130. Tel +55 21 3207-1701. e-mail:
teresafernandez@inca.gov.br ORCID:
https://orcid.org/0000-0003-1299-4666
Published: May 01, 2024
Received: March 14, 2024
Accepted: April 07, 2024
Mediterr J Hematol Infect Dis 2024, 16(1): e2024040 DOI
10.4084/MJHID.2024.040
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
To the editor
Allogeneic
hematopoietic stem cell transplantation (allo-HSCT) represents the only
potentially curative treatment for myelodysplastic neoplasm (MDS).[1,2,3] However, post-transplant disease relapse emerges as the major cause of treatment failure in MDS patients.[3,4]
Although a standard approach for MDS patients with post-transplant
relapse is not established, some salvage therapies have been reported
with variable effectiveness.[3,4] Here, we report a
clonal cytogenetic evolution (CCE) in a child with MDS who relapsed
after allo-HSCT, showing a complex karyotype and a variant in the ETV6
at diagnosis.
A three-year-old boy with severe thrombocytopenia,
mild macrocytic anemia, leukopenia, and 12% myeloid peripheral blasts
was admitted at Instituto de Pediatria e Puericultura Martagão
Gesteira, Universidade Federal do Rio de Janeiro, Brazil, in January
2016. Bone marrow (BM) evaluation evidenced marked erythroid dysplasia,
megakaryocytic dysplasia, and 14% of myeloid blasts. Cytogenetic
analysis of BM cells by G-banding revealed the complex karyotype: 49,
XY, del(3)(q21),
del(6)(q21),+der(6)del(6)(q21),+8,+der(12)del(12)(p11)[21] (Figure 1A). FISH analysis confirmed the +8 (Figure 1B). The patient was diagnosed with MDS with increased blasts (MDS-IB).[5]
Next-generation sequencing (NGS) analysis using the Ion Torrent
Personal Genome Machine (PGM) platform (Life Technologies) was
performed for the genes: GATA2, RUNX1, CEBPA, ANKRD26, ETV6, SAMD9,
SAMD9L, PTPN11, NRAS, SETBP1, DDX41, TP53, FLT3, SRP72 and JAK3. An
ETV6 likely pathogenic variant was identified, with the molecular
consequence of the loss of the termination codon (stop-loss variant) (Table 1).
He evolved with worsening cytopenias, transfusion requirements, and
progression to MDS/AML. He was treated with thioguanine but he did not
show response to this treatment. More intensive chemotherapy was
performed for induction of
minimal residual disease (MRD); then, he was referred to Bone Marrow
Transplantation Center, Instituto Nacional de Cancer, and underwent
allo-HSCT from his nine years old female HLA-matched sibling donor,
with minor ABO incompatibility. The myeloablative conditioning regimen
consisted of busulfan/cyclophosphamide (BU/CY) and graft-versus-host
disease (GVHD) prophylaxis of methotrexate (MTX) and cyclosporine
(CSA). The engraftment occurred on D+21. BM evaluation at D+45
post-transplant showed negative MRD by flow cytometry, donor karyotype
46, XX[35], and mixed donor chimerism by PCR short tandem repeats (STR)
analysis (96.9% in mononuclear cells and 100% in granulocytic
population). However, at D+75, peripheral blood and BM analysis
revealed pancytopenia, myeloid dysplasia, and a decline in donor
chimerism to 87.5% in the mononuclear population. Attempts to carry out
preemptive donor lymphocyte infusions (DLIs) did not materialize
because the donor had recurrent respiratory infections at that time.
The patient received one cycle of azacitidine (AZA) with improvement of
hepatomegaly, bone pain, and hematological counts, but soon after, he
evolved with severe thrombocytopenia and respiratory infection. At D16
of AZA, the patient showed 2% of blasts compatible with megaloblasts
and 12% of dysplastic megakaryocytic lineage by flow cytometry. His
clinical condition worsened around D+137, with aggravation of
pancytopenia due to a progressive decrease in donor chimerism (47.8% in
the mononuclear population). At that point, flow cytometry showed 18%
of dysplasia in the megakaryocytic sector. Overt disease relapse
occurred at D+180 post-HSCT. The cytogenetic analysis showed the CCE:
50, XY, del(3)(q21),+der(3)del(3)(q21), del(6)(q21),+der(6)
del(6)(q21),+8,+der(12)del(12)(p11)[2]/46, XX[19] (Figure 1C). The patient had 14.2% of positive cells for +8 by FISH (Figure 1D).
The immunophenotyping showed 5% of blasts, and STR detected mixed donor
chimerism in both lineages (59.9% mononuclear and 77.3% in granulocytic
populations). Salvage chemotherapy with fludarabine plus cytarabine and
idarubicin was started. Despite attempts to control the disease, it
progressed; the patient developed severe persistent pancytopenia (with
transfusion dependency) and massive pulmonary aspergillosis that led to
his death after 8 months post-HSCT.
 |
Figure 1. Conventional and molecular cytogenetics of BM cells in pediatric MDS at diagnosis and post-HSCT. (A)
G-banding showing the complex karyotype:
49,XY,del(3)(q21),del(6)(q21),+der(6)del(6)(q21),+8,+der(12)del(12)(p11);
(B) FISH analysis using the c-MYC probe (LSI MYC spectrum orange, 8q24, Vysis) showing trisomy 8 (three red signals); (C)
G-banding analysis post-HSCT showing a cytogenetic clonal evolution:
50,XY,del(3)(q21),+der(3)del(3)(q21),del(6)(q21),+der(6)del(6)(q21),+8,+der(12)del(12)(p11);
(D) FISH analysis using the
c-MYC probe (LSI MYC spectrum orange, 8q24, Vysis) showing cells with
trisomy 8 and normal cells (two red signals).
|
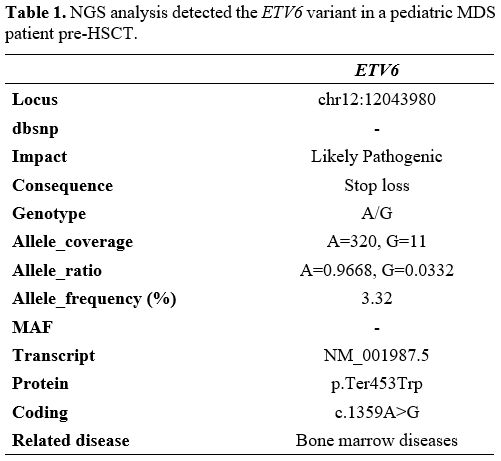 |
Table 1. NGS analysis detected the ETV6 variant in a pediatric MDS patient pre-HSCT.
|
The complex karyotype is a cytogenetic biomarker indicative of poor survival after HSCT in MDS patients.[4]
The MDS genetic diversity amongst coexisting subclones may result in a
more heterogeneous and complex disease, as some of the subclones may be
resistant to specific types of therapy.[6] In the
present report, the patient pre-HSCT had a complex karyotype.
Post-HSCT, the patient showed disease relapse and CCE, represented by
the acquisition of a der(3)del(3)(q21). The del(3)(q21) involves the
loss of important genes such as GATA2,[7] BCL6[8] and MECOM.[9]
The del(3q) was also present at the abnormal cytogenetic clone detected
at diagnosis with other chromosomal abnormalities involving important
genes such as MYB in del(6)(q21); c-MYC in +8; and ETV6 in
del(12)(p11). It is interesting to note the high genomic instability in
this patient, who also acquired the gain of these abnormal chromosomes,
resulting in chromosome derivatives. These extra copies can lead to
overexpression of important genes mapped to these chromosome regions as
FANCD2, RASSF1 in 3p; DEK, CDKN1A in 6p, and WNT1, HOXC13 in 12q.[8]
ETV6 is subject to heterozygous mutations in hematologic malignancies,
including MDS. ETV6 is a major intrinsic regulator of megakaryocytes.[10,11]
Besides that, ETV6 is one of the key regulators of sepsis, a major
cause of morbidity and mortality in the intensive care unit.[12]
In this case, the complex karyotype and the loss of heterozygosity in
ETV6 (chromosomal deletion and genetic variant) may be associated with
disease relapse and unfavorable clinical outcome post-transplant.
Ertz-Archambault and colleagues observed cytogenetic evolution in
myeloid neoplasms in adult patients who had disease recurrence after
HSCT. The authors observed that an unfavorable cytogenetic profile at
the initial diagnosis may represent an important pre-diagnosis factor
of a predisposition for clonal evolution. The acquisition of more
complex cytogenetic alterations is associated with lower survival.[6]
Our study suggests that the treatment of MDS patients with predictive
factors of poor prognosis, such as complex karyotypes and ETV6 variant,
remains a challenge. Prospective studies are necessary to characterize
the biology of MDS and identify molecular biomarkers associated with
disease relapse in order to develop precision medicine to improve the
survival of this group of patients.
Acknowledgements
This
study was supported by Fundação Carlos Chagas Filho de Amaro à Pesquisa
do Estado do Rio de Janeiro (FAPERJ) (FAPERJ/E-26/201.2018/2022) and
the Brazilian Ministry of Health (Instituto Nacional de Câncer/INCA,
Brazil).
Author Contributions
EAAK,
VLL, and TSF wrote the manuscript. TSF designed the study. EAAK and VLL
performed the cytogenetic and FISH analysis. RCBT, APB, and ESC
analyzed the clinical data. GMF, VLL, and TSF performed the NGS
analysis. BEG performed the flow cytometry analysis. TSF and RCBT
reviewed critically the manuscript for important intellectual content.
All authors have read and approved the manuscript.
Ethics Approval and Consent to Partecipate
This
study was approved by the Ethics and Research Committee of the National
Cancer Institute (reference number # 3401739) in accordance with the
Declaration of Helsinki. Informed consent was obtained from the
children’s parents.
References
- Locatelli F, Strahm B. How I treat myelodysplastic syndromes of childhood. Blood. 2018; 131(13):1406-1414. https://doi.org/10.1182/blood-2017-09-765214 PMid:29438960
- Hasle
H, Niemeyer CM. Advances in the prognostication and management of
advanced MDS in children. Br J Haematol. 2011; 154(2):185-95. https://doi.org/10.1111/j.1365-2141.2011.08724.x PMid:21554264
- Du
Y, Li C, Zhao Z, Liu Y, Zhang C, Yan J. Efficacy and safety of
venetoclax combined with hypomethylating agents for relapse of acute
myeloid leukemia and myelodysplastic syndrome post allogeneic
hematopoietic stem cell transplantation: a systematic review and
meta-analysis. BMC Cancer. 2023;23(1):764. https://doi.org/10.1186/s12885-023-11259-6 PMid:37592239 PMCid:PMC10433628
- De
Witte T, Bowen D, Robin M, Malcovati L, Niederwieser D, et al.
Allogeneic hematopoietic stem cell transplantation for MDS and CMML:
recommendations from an international expert panel. Blood. 2017;
129:1753-1762. https://doi.org/10.1182/blood-2016-06-724500 PMid:28096091 PMCid:PMC5524528
- Khoury
JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, et al. The 5th
edition of the World Healthy Organization Classification of
Haematolymphoid Tumors: Myeloid and Histiocytic/Dendritic Neoplasms.
Leukemia 2022; 36:1703-1719. https://doi.org/10.1038/s41375-022-01613-1 PMid:35732831 PMCid:PMC9252913
- Ertz-Archambault
N, Kosiorek H, Slack JL, Lonzo ML, Greipp PT, et al. Cytogenetic
Evolution in Myeloid Neoplasms at Relapse after Allogeneic
Hematopoietic Cell Transplantation: Association with Previous
Chemotherapy and Effect on Survival. Biol Blood Marrow Transplant.
2017; 23(5):782-789. https://doi.org/10.1016/j.bbmt.2017.02.003 PMid:28189903
- Greenmyer
JR, Thompson WS, Hoppman NL, Khan S, Patnaik MS, Schimmenti LA, Kohorst
MA. 3q21 deletion affects GATA2 and is associated with myelodysplastic
syndrome. Br J Haematol. 2022;196(4):1120-1123. https://doi.org/10.1111/bjh.17902 PMid:34651298
- Atlas
of Genetics and Cytogenetics in Oncology and Haematology in 2013. Huret
JL, Ahmad M, Arsaban M, et al. Nucleic Acids Res. 2013 Jan;41(Database
issue):D920-4. doi: 10.1093/nar/gks1082. https://doi.org/10.1093/nar/gks1082 PMid:23161685 PMCid:PMC3531131
- Voit
RA, Sankaran VG. MECOM Deficiency: from Bone Marrow Failure to Impaired
B-Cell Development. J Clin Immunol. 2023;43(6):1052-1066. https://doi.org/10.1007/s10875-023-01545-0 PMid:37407873
- Bejar
R, Stevenson K, Abdel-Wahab O, Galili N, Nilsson B, Garcia-Manero G,
Kantarjian H, Raza A, Levine RL, Neuberg D, Ebert BL. Clinical effect
of point mutations in myelodysplastic syndromes. N Engl J Med.
2011;364(26):2496-506. https://doi.org/10.1056/NEJMoa1013343 PMid:21714648 PMCid:PMC3159042
- Hock H, Shimamura A. ETV6 in hematopoiesis and leukemia predisposition. Semin Hematol. 2017;54(2):98-104. https://doi.org/10.1053/j.seminhematol.2017.04.005 PMid:28637624 PMCid:PMC5584538
- Zhang
Z, Chen L, Xu P, Xing L, Hong Y, Chen P. Gene correlation network
analysis to identify regulatory factors in sepsis. J Transl Med.
2020;18(1):381. https://doi.org/10.1186/s12967-020-02561-z PMid:33032623 PMCid:PMC7545567

