In our study, we retrieved retrospectively a population of 229 unselected ALL patients consecutively diagnosed in our Unit from 2001 to 2022. We selected the cohort of 86 (37.6%) patients aged over 55 years (median age: 70, range: 55-88) at the time of diagnosis, focusing on Ph negative ALL (Figure 1). We evaluated their characteristics and outcomes according to age and treatment received at dose adjusted by age, including, whenever possible, high-dose consolidation with stem cell transplantation (SCT).
Among the 86 patients aged more than 55 years, 29 (33%) were BCR::ABL1 positive, 16 (55%) were older than 65 years, and 57 were Ph-negative, 37 (65%) were older than 65 years.
According to physician choice, intensive treatment with chemotherapy was given to 40 patients (70%) according to paediatric-inspired protocols (NILG Protocols[4,5] or similar therapeutic program) with dose adjustment by age (in patients older than 65 years, reduction of idarubicin, cyclophosphamide, methotrexate, vincristine, and steroid doses and omission of L-asparaginase were applied) (Appendix 1). Written informed consent to treatment was obtained from patients in accordance with the Declaration of Helsinki. All patients under 65 years were intensively treated, while only 54% (20/37) of patients aged more than 65 years (p: 0.0002). Seventeen (30%) patients, aged more than 65 years, received only corticosteroids and best supportive care (BSC) (Figure 1).
The characteristics of the 57 Ph-negative patients are summarized in Table 1.
The median age of the 40 intensively treated patients was significantly lower than that of patients receiving BSC (65.5 vs. 78 years, p:<0.0001), and ECOG performance status was ≥2 in 30% of the intensively treated patients compared to 59% of the BSC subgroup patients (p: 0.07). At diagnosis, fewer patients treated intensively (10%) had a CCI >2 compared to those receiving BSC (29%), although not significant (p: 0.1). The two subgroups did not differ in phenotype, karyotype, white blood cell count and clinical risk profile at diagnosis.
In the intensively treated subgroup, the complete remission (CR) after induction therapy was 77.5% (31/40), without significant differences according to age (85% in 55-65 years vs 75% in >65 years; p: 0.7). Three patients died during induction (7.5%) (fungal infection n=1, multiorgan failure n=2) and six patients (15%) were refractory. Immunophenotype, age, and karyotype did not impact CR achievement. Evaluation of measurable residual disease (MRD) with RQ-PCR technology was performed,[6,7] obtaining one or more patient-specific probe(s) with a sensitivity of at least 10e-3 in 26 patients (45.6%). After about 10 weeks from diagnosis, MRD was negative in 17 patients (65%) and positive but not quantifiable in two. Overall, severe adverse events (grade >2) occurred in 4.5% and did not impact on subsequent chemotherapy.
Overall, 20 patients (50% of the intensively treated group), considered at high risk of relapse (HR) (Appendix 1), underwent SCT, both autologous or allogeneic, as part of their treatment program; 8 (40%) of them were over 65 years. Considering patients older than 65 years, only 22% (8/37) underwent SCT, compared to 60% (12/20) of younger patients (p: 0.0078). Ten received an allogeneic SCT and eight (80%) in the first CR. The donor was haploidentical in four patients (40%), sibling in two, and matched unrelated in four. Ten patients not eligible for allo-SCT received autologous SCT. The median age at the time of transplant was 70 years (range: 63-76) in autologous and 60.5 years (range: 55-70) in allogeneic SCT (p: 0.0051).
Twenty patients (50%) classified as standard risk (SR) at diagnosis or as HR but achieving MDR negativity, received 2-year maintenance chemotherapy.
The relapse rate was 62.5% (20/32), without differences according to age (53% under 65 vs 73% over 65 years, p: 0.29). The median time to relapse was 8.7 months (range: 2.7-43), with relapse-free survival at 1 and 3 years of 57.3% (95% CI: 38.7-72) and 36.7% (95% CI: 20-53.2), respectively, without differences according to age (p: 0.4). Three patients (15%) had an isolated central nervous system relapse, despite the intrathecal prophylaxis, and were treated with radiotherapy and with intensified intrathecal chemotherapy. Only five patients (5/20; 25%) received Blinatumomab and/or Inotuzumab Ozogamicin, and four subsequently underwent allo-SCT, with MRD negativity in three cases.
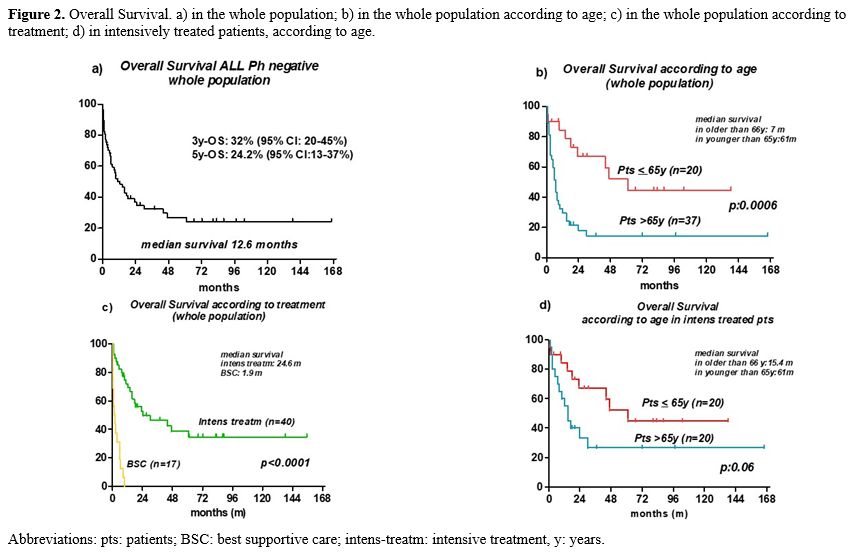
With a median follow-up of 66.5 months, 3- and 5-year survival for the entire cohort, including patients receiving only BSC, was 32% (95% CI: 20-45%) and 24.2% (95% CI: 13-37%), respectively, with significant age-related differences for patients aged 55-65 years and over 65 years (median survival 61 vs 6 months; p: 0.0005) (Figure 2a and 2b).
No significant differences in survival were observed between patients diagnosed during the first decade (2001-2011) and the second (2012-2022) (median survival 6 vs 14 months; p: 0.3).
The median survival in the BSC group was 1.9 months (range, 0.2-9.7), significantly lower than in the 40 patients treated intensively (24.6 months, p<0.0001) (Figure 2c).
In the intensively treated group, the 3- and 5-year survival was 46.3% (95% CI: 29.6-61.4%) and 34.7% (95% CI: 18.8-51%), respectively; 17 patients were alive (43%), 11 in first CR and 6 beyond first CR. Most patients died of progressive disease (65%); eight (35%) died while on CR (COVID-19 =1, secondary myelodysplastic syndrome =1, allo-transplant related mortality =3, death in aplasia =3), independently from age. According to age, 5-year survival was better in younger patients (<65 years) compared to their older counterparts, with a borderline statistically significant difference [47% (95% CI: 19.7-67%) vs 26.7% (95% CI: 9-48%); p: 0.06] (Figure 2d). Adverse karyotype, ECOG, CCI (CCI 0-1 vs. CCI >/=2), risk at diagnosis, and phenotype had no impact on survival. In contrast, MRD positivity had a negative impact on outcome (median survival: 14.6 months vs. undefined in MRD negative, p: 0.046).
In patients undergoing allo-SCT, the 3-year survival was 54.8% (95% CI: 29-91%), and the median survival was not reached. Three patients relapsed (33%) after transplant, and one of them died. Survival at 3 years of patients receiving autologous SCT was 46.7% (95% CI: 9.5-73.7%), with a median of 30.2 months. The relapse rate was 50%; transplant-related mortality (TRM) was 0%.
At multivariable analyses for survival, only BSC remained significantly associated with reduced survival (p: 0.001). When achieving CR was added to the model, it proved to be independently associated with better survival.
In Ph negative ALL patients, chemotherapy with pediatric-inspired regimens still represents the standard treatment backbone, even if the rotating and long-term use of chemotherapeutic agents, even at high doses, and of corticosteroids can be particularly detrimental in older adults.
Therefore, we addressed our analysis of the feasibility and treatment outcomes in 57 Ph-negative ALL patients. We used a relatively low age cutoff (55 years) to identify older patients, lower than in Acute Myeloid Leukemia but similarly adopted in most clinical trials[8-10] and used in recent ELN recommendations.[2]
We treated most patients intensively, although with reduced doses of chemotherapy, particularly in patients older than 65 years, according to protocol guidelines (see Appendix 1), to allow them to tolerate therapy better. Only 29.8% received palliation with corticosteroids and/or BSC. Clinical judgment and chronological age itself represented the main criteria for excluding a patient from potentially curative treatment. Their proportion and their features are like those reported in other studies.[11,12] Their median survival was very poor, and in the multivariate analysis, BSC was the only variable that independently predicted an adverse outcome.
The first important management issue in older patients is the selection of frail patients who do not deserve intensive treatment, even at reduced doses. While in Acute Myeloid Leukemia and in hematological diseases, a comprehensive geriatric assessment, including the evaluation of daily life activities, cognitive and psychological functions, and other geriatric parameters, has proven useful for selecting the most appropriate treatment intensity in older patients,[13,14] in ALL, there are no validated criteria to define their fitness.
In our experience, more than 70% of patients have been treated intensively, obtaining a median survival of 24.6 months and a 3-year survival of 46.3%, without differences according to karyotype, ECOG PS or CCI. With the predefined dose adjustments of treatment protocols, chronological age did not represent a limitation to the use of an intensive treatment in selected fit patients, particularly in very old patients. Chemotherapy was given up to 79 years, and autologous transplants up to 76 years of age. Overall, 54% of patients aged more than 65 years were treated intensively, and 22% received a transplant procedure, respectively, obtaining an unsatisfactory but acceptable median survival of 15 months with a plateau, particularly compared to patients receiving only BSC. These results are in line with data reported by the PETHEMA group that showed a superiority of intensive treatment in event-free survival and overall survival. The intensity of treatment was the only variable with independent significance for event-free survival in multivariate analysis.[8]
The intensive chemotherapy used in this study proved feasible even if the treatment‐related mortality was quite high (20%) and mortality in CR was 12.5%, like those reported by other studies.[8,9] Notably, the program was also well tolerated by 14 selected patients aged over 70 years included in the study, whose mortality in CR was 7.1% and whose survival was not significantly different compared to younger patients.
The use of SCT as consolidation treatment in an older population is also debatable. In our series, 17% of patients (median age: 60.5) received an allotransplant, and despite a TRM of 33%, they achieved a satisfactory 3 year-survival of 54.8%. An additional 17% of patients (median age: 70) received an autologous SCT with no TRM. The proportion of older ALL patients submitted to allo-SCT in other studies was lower than in the present study, 8% in the GMALL trial[10] and 9% in a real-life Canadian trial.[15] A recent study comparing patients >55 years, treated with reduced intensity-allo-SCT vs auto-SCT, showed no significant difference between the two options [5 year-survival: p: 0.23]. Non-relapse mortality was higher with allogeneic SCT (25% versus 10%: p: 0.001).[16]
The limitations of the present study include its retrospective nature, the relatively low number of patients, and the long duration of the study, which spanned periods when the new drugs were not yet available and supportive therapy progressively improved. In addition, patients treated intensively were selected by medical judgment without using objective criteria. Nevertheless, considering the rarity of this disease in older adults and the paucity of prospective studies in this patient's population, the study supports the concept that it is important to consider old ALL patients for curative treatment, which can be successful in a significant proportion of cases, without excluding them “a priori” based on age.
Overall, the present study's results showed that the majority of older ALL patients can receive curative treatment with dose-adjusted chemotherapy, including transplantation. It was desirable to identify objective criteria for patient selection and incorporate novel, more efficient, and less toxic immunologic agents into the treatment algorithm.[17-20]
References
- Guru
Murthy GS, Venkitachalam R, Mehta P. Trends in survival outcomes of
B-lineage acute lymphoblastic leukemia in elderly patients: analysis of
Surveillance, Epidemiology, and End Results database. Leuk Lymphoma.
2015; 56: 2296-2300 https://doi.org/10.3109/10428194.2014.991921
- Gökbuget
N, Boissel N, Chiaretti S, Dombret H, Doubek M, Fielding A, Foà R,
Giebel S, Hoelzer D, Hunault M, Marks DI, Martinelli G, Ottmann O,
Rijneveld A, Rousselot P, Ribera J, Bassan R. Management of ALL in
adults: 2024 ELN recommendations from a European expert panel. Blood.
2024;143(19):1903-30 https://doi.org/10.1182/blood.2023023568
- Aldoss I, Forman SJ, Pullarkat V. Acute Lymphoblastic Leukemia in the Older Adult. J Oncol Pract. 2019; 15: 67-75. https://doi.org/10.1200/JOP.18.00271
- Bassan
R, Spinelli O, Oldani E, Intermesoli T, Tosi M, Peruta B, Rossi G,
Borlenghi E, Pogliani EM, Terruzzi E, Fabris P, Cassibba V,
Lambertenghi-Deliliers G, Cortelezzi A, Bosi A, Gianfaldoni G, Ciceri
F, Bernardi M, Gallamini A, Mattei D, Di Bona E, Romani C, Scattolin
AM, Barbui T, Rambaldi A. Improved risk classification for
risk-specific therapy based on the molecular study of minimal residual
disease (MRD) in adult acute lymphoblastic leukemia (ALL). Blood 2009;
113(18): 4153-62 https://doi.org/10.1182/blood-2008-11-185132
- Bassan
R, Pavoni C, Intermesoli T, Spinelli O, Tosi M, Audisio E, Marmont F,
Cattaneo C, Borlenghi E, Cortelazzo S, Cavattoni I, Fumagalli M, Mattei
D, Romani C, Cortelezzi A, Fracchiolla N, Ciceri F, Bernardi M,
Scattolin AM, Depaoli L, Masciulli A, Oldani E, Rambaldi A. Updated
risk-oriented strategy for acute lymphoblastic leukemia in adult
patients 18-65 years: NILG ALL 10/07. Blood Cancer Journal 2020; 10:
119 https://doi.org/10.1038/s41408-020-00383-2
- Van
Dongen JJ, Langerak AW, Brüggemann M, Evans PA, Hummel M, Lavender FL,
Delabesse E, Davi F, Schuuring E, García-Sanz R, van Krieken JH, Droese
J, González D, Bastard C, White HE, Spaargaren M, González M, Parreira
A, Smith JL, Morgan GJ, Kneba M, Macintyre EA. Design and
standardization of PCR primers and protocols for detection of clonal
immunoglobulin and T-cell receptor gene recombinations in suspect
lymphoproliferations: report of the BIOMED-2 Concerted Action
BMH4-CT98-3936. Leukemia 2003; 17: 2257-2317 https://doi.org/10.1038/sj.leu.2403202
- Van
der Velden VH, Cazzaniga G, Schrauder A, Hancock J, Bder P,
Panzer-Grumayer ER, Flohr T, Sutton R, Cave H, Madsen HO, Cayela JM,
Trka J, Eckert C, Foroni L, Stadt UZ, Beldjord K, Raff T, van der
Schoot CE, van Dongen JJM. European Study Group on MRD detection in ALL
(ESG-MRD-ALL). Analysis of minimal residual disease by Ig/TCR gene
rearrangements: guidelines for interpretation of real-time quantitative
PCR data. Leukemia 2007; 21: 304-611 https://doi.org/10.1038/sj.leu.2404586
- Ribera
JM, García O, Gil C, Mercadal S, García-Cadenas I, Montesinos P, Barba
P, Vives S, González-Campos J, Tormo M, Esteve J, López A, Moreno MJ,
Ribera J, Alonso N, Bermúdez A, Amigo ML, Genescà E, García D,
Vall-Llovera F, Bergua JM, Guàrdia R, Monteserín MC, Bernal T, Calbacho
M, Martínez MP, Feliu E; PETHEMA Group. Comparison of intensive,
pediatric-inspired therapy with non-intensive therapy in older adults
aged 55-65 years with Philadelphia chromosome-negative acute
lymphoblastic leukemia. Leuk. Res. 2018; 68: 79-84 https://doi.org/10.1016/j.leukres.2018.03.010
- Wenge
D, Wethmar K, Klar CA, Kolve H, Sauer T, Angenendt L, Evers G, Call S,
Kerkhoff A, Khandanpour C, Kessler T, Mesters R, Schliemann C, Mikesch
JH, Reicherts C, Brüggemann M, Berdel WE, Lenz G, Stelljes M.
Characteristics and Outcome of Elderly Patients (>55 Years) with
Acute Lymphoblastic Leukemia. Cancers. 2022; 14(3): 565 https://doi.org/10.3390/cancers14030565
- Gökbuget
N, Viardot A, Steffen B, Hahn J, Spriewald B, Martin S, Raffel S,
Teichmann LL, Trummer A, Alsdorf W, Morgner A, Schwartz S, Stelljes M,
Vucinic V, Alakel N, Stoltefuß A, Baldus CD, Brüggemann M, Serve H,
Hoelzer D. Outcome of 841 Older Patients (>55 yrs) with Newly
Diagnosed Ph/BCR-ABL Negative ALL Prospectively Treated According to
Pediatric-Based, Age-Adapted GMALL Protocols. Blood. 2022; 140:121-123 https://doi.org/10.1182/blood-2022-158934
- Kozlowski
P, Lennmyr E, Ahlberg L, Bernell P, Hulegardh E, Karbach H, Karlsson K,
Tomaszewska-Toporska B, Astrom M, Hallbook H. Age but not Philadelphia
positivity impairs outcome in older/elderly patients with acute
lymphoblastic leukemia in Sweden. Eur. J. Haematol. 2017; 99: 141-149 https://doi.org/10.1111/ejh.12896
- Miller
KC, Al-Kali A, Shah M, Hogan WJ, Elliott MA, Begna KH, Gangat N,
Patnaik MM, Viswanatha DS, He R, Greipp PT, Sproat LZ, Foran JM, Litzow
MR, Alkhateeb HB. Elderly acute lymphoblastic leukemia: A Mayo Clinic
study of 124 patients. Leuk. Lymphoma. 2019; 60: 990-999 https://doi.org/10.1080/10428194.2018.1509318
- Tucci
A, Ferrari S, Bottelli C, Borlenghi E, Drera M, Rossi G. A
comprehensive geriatric assessment is more effective than clinical
judgment to identify elderly diffuse large cell lymphoma patients who
benefit from aggressive therapy. Cancer 2009; 115: 4547-53 https://doi.org/10.1002/cncr.24490
- Ferrara
F, Barosi G, Venditti A, Angelucci E, Gobbi M, Pane F, Tosi P, Zinzani
P, Tura S. Consensus-based definition of unfitness to intensive and
non-intensive chemotherapy in acute myeloid leukemia: a project of SIE,
SIES and GITMO group on a new tool for therapy decision making.
Leukemia. 2013; 27(5): 997-9 https://doi.org/10.1038/leu.2012.303
- Perusini
MA, Jad Sibai J, Atenafu EG, Bankar AMinden MD, Gupta V, Maze D,
Davidson M, Chan S, Schimmer AD, Carpentier GR, Lucero JA, Andrews C,
Kim DD, Yee K, Schuh AC, Sibai HReal-World Outcomes and Adverse Events
of Elderly Patients with Ph-Negative Acute Lymphoblastic Leukemia
Treated with a Pediatric-Inspired Protocol. Blood 2022; 140 (Supplement
1): 6039-6041. https://doi.org/10.1182/blood-2022-169406
- Giebel
S, Labopin M, Houhou M, Caillot D, Finke J, Blaise D, Fegueux N, Ethell
M, Cornelissen JJ, Forcade E, Yakoub-Agha I, Lussana F, Maertens J,
Bourhis JH, Jindra P, Gorin NC, Nagler A, Mohty M. Autologous versus
allogeneic hematopoietic cell transplantation for older patients with
acute lymphoblastic leukemia. An analysis from the Acute Leukemia
Working Party of the European Society for Blood and Marrow
Transplantation. BMT. 2023; 58(4): 393-400 https://doi.org/10.1038/s41409-022-01904-2
- Jabbour
E, Short NJ, Senapati J, Jain N, Huang X, Daver N, DiNardo CD,
Pemmaraju N, Wierda W, Garcia-Manero G, Montalban Bravo G, Sasaki K,
Kadia TM, Khoury J, Wang S, Haddad FG, Jacob J, Garris R, Ravandi F,
Kantarjian HM. Mini-hyper-CVD plus inotuzumab ozogamicin, with or
without blinatumomab, in the subgroup of older patients with newly
diagnosed Philadelphia chromosome-negative B-cell acute lymphocytic
leukaemia: long-term results of an open-label phase 2 trial. Lancet
Hematology 2023; 10: 433-444 https://doi.org/10.1016/S2352-3026(23)00073-X
- Chevallier
P, Leguay T, Kim R, Delord M. Fractionated Inotuzumab Ozogamicin
combined with Low-Intensity chemotherapy in older patients with newly
diagnosed CD22+ Philadelphia chromosome (Ph)-Negative B-Cell Precursor
(BCP) Acute Lymphoblastic Leukemia (ALL): Results of the EWALL-INO
Study. Blood. 2022; 140: 6114- 6116. 102. https://doi.org/10.1182/blood-2022-166035
- Gökbuget
N, Schwartz S, Faul C, Topp M, Subklewe M, Renzelmann A, Stoltefuss A,
Hertenstein B Wilke A, Raffel S, Jäkel N, Vucinic V,Niemann DM, Reiser
L, Serve H, Brüggemann M, and Viardot A, Dose reduced chemotherapy in
sequence with Blinatumomab for newly diagnosed older patients with
Ph/BCR::ABL negative B-precursor Adult Lymphoblastic Leukemia (ALL):
preliminary results of the GMALL Bold Trial. Blood 2023; 142
(Supplement 1): 964 https://doi.org/10.1182/blood-2023-180472
- Salas MQ, Atenafu EG, Pasic I, Al-Shaibani E, Bascom O, Wilson L, Chen C, Law AD, Lam W, Novitzky-Basso I, Kim DDH, Gerbitz A, Viswabandya A, Michelis FV, Lipton JH, Mattsson J, Alibhai S, Kumar R. Impact of hematopoietic cell transplant frailty scale on transplant outcome in adults. BMT 2023; 58(3): 317-324 https://doi.org/10.1038/s41409-022-01892-3
Appendix 1
A) Treatment programBefore 2013, the patients were treated according to NILG protocols (NCT 00358072 and NCT 00795756, ClinicalTrial.gov). The scheme of therapy in older patients was similar and it was summarized below.
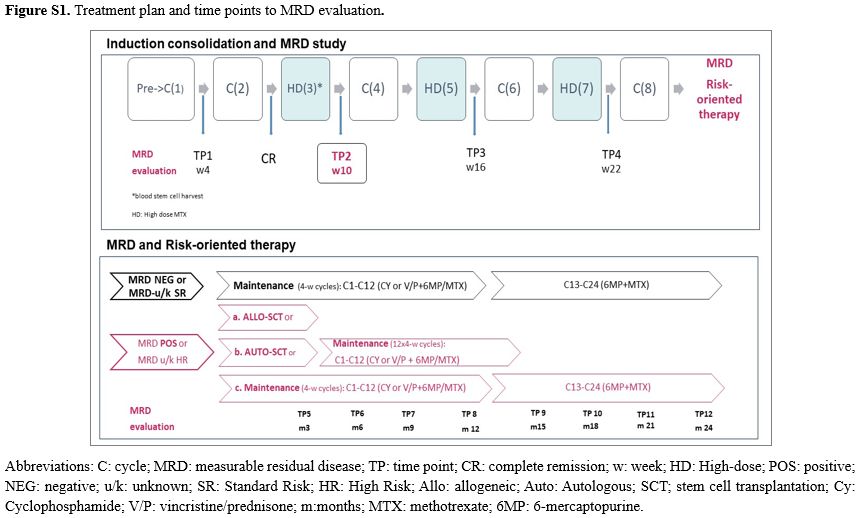
The protocol plan consisted of chemotherapy blocks administered over 25 weeks in association with central nervous system (CNS) prophylaxis Collection of autologous blood stem cells was performed after cycle 3 (Figure S1).
Risk stratification and measurable residual disease evaluation. At diagnosis, patients were stratified in standard risk (SR) and high risk (HR) according to molecular and clinical patient’s features. The HR group included patients with the Ph translocation [ie, t(9;22)], or t(4;11), or with corresponding gene rearrangements (BCR::ABL1, KMT2A::AFF1), or adverse cytogenetics (monosomy 7, trisomy 8, 6q deletion, 7p deletion, t(8;14), low-hypodiploidy with 30 to 39 chromosomes, near-triploidy with 60 to 78 chromosomes, complex karyotype with ≥5 unrelated clonal abnormalities) or patients with white blood cell count (WBC) higher than 30 x 109/L in B-ALL or 100 x 109/L in T-ALL, respectively, patients achieving late complete remission (CR) after cycle 2 of chemotherapy, and those with an adverse EGIL immunophenotype (pro-B or pre-/mature-T). Patients with MRD ≥ 10-4 at week 10 were reclassified as HR independently from their initial risk group. The SR group included all patients without any of the HR features
For risk-oriented therapy, the consolidation program was considered concluded for MRDneg cases. These patients began 2-year continuous maintenance therapy, reinforced by pairs of drugs alternated monthly.
In contrast, all MRDpos patients with an available HLA-identical related or unrelated volunteer donor would undergo allogeneic SCT.
For MRDpos patients who were unable to undergo an allogeneic SCT, an intensification treatment supported by autologous blood stem cells was proposed, followed by the maintenance program
When the MRD risk class was unknown (MRDu/k), maintenance was the therapy in clinical SR subsets and allograft in HR patients, respectively. If an allogeneic SCT was not possible, the autologous SCT plus maintenance option was indicated.
The cytotoxic humanized monoclonal antibody rituximab was given to patients with CD20+ ALL after 2020.
Induction/Consolidation Therapy
Including subset-specific elements for patients, aged >55 years (y) and aged>65y. CR evaluation bone marrow is checked on days 28 and/or 56. Consolidation cycles are administered at 21-28 day intervals.
Induction/early consolidation therapy for patients aged more than 55 y
Pre-induction: prednisone 20 mg/m2/bd per os (PO) on days -5 to -1, cyclophosphamide 200 mg/m2/d intravenously (IV) on days -3 to –1
Cycle 1: idarubicin 9 mg/m2/d IV on days 1 and 2, vincristine 1.4 mg/m2/d (max. 2 mg) on days 1, 8, 15 and 22, L-asparaginase (E.Coli) 3.000 U/m2 IV on days 8, 10, 12, 15, 17 and 19, dexamethasone 5 mg/m2/bd IV on days 1-5, 15-19, G-CSF from day 5 (induction).
Cycle 2: idarubicin 9 mg/m2/d IV on day 1, cyclophosphamide 1000 mg/m2 IV on day 1, dexamethasone 5 mg/m2/bd IV/PO on days 1-5, cytarabine 75 mg/m2/d IV/subcutaneous (SC) on days 2-5, 6-mercaptopurine 60 mg/m2/d PO on days 1-10, G-CSF from day 8 to resolution of absolute neutropenia <1 x109/L.
Cycles 3,7: methotrexate 1.5 g/m2/d IV on day 1 (24-h infusion, folinic acid rescue), cytarabine 2 g/m2/bd IV on days 3 and 4, G-CSF from day 8 (collection/cryopreservation of autologous blood stem cells at cycle 3).
Cycles 4,6: idarubicin 9 mg/m2/d IV on day 1, cyclophosphamide 1000 mg/m2 IV on day 1, vincristine 1.4 mg/m2/d (max. 2 mg) IV on days 1 and 8, dexamethasone 5 mg/m2/bd IV/PO on days 1-5, cytarabine 75 mg/m2/d IV/SC on days 2-5, 6-mercaptopurine 60 mg/m2/d PO on days 1-10, G-CSF from day 8 to resolution of absolute neutropenia <1 x109/L.
Cycle 5: methotrexate 1.5 g/m2/d IV on day 1 (24-h infusion, folinic acid rescue), L-asparaginase (E. Coli) 10.000 U/m2 IV on days 3 and 8.
Cycle 8: idarubicin 7.5 mg/m2/d IV on days 1 and 8, vincristine 1.4 mg/m2/d (max. 2 mg) IV on days 1 and 8, cyclophosphamide 200 mg/m2/d IV on days 1-3, dexamethasone 5 mg/m2/bd IV/PO on days 1-5, prednisone 20 mg/m2/bd PO on days 8-12, G-CSF from neutropenia <0.5 microl to its resolution.
· CNS prophylaxis: Intrathecal MTX 12.5 mg, Ara-C 50 mg, and PDN 40 mg or dexa 4 mg during induction/consolidation and maintenance for a total of 12 IT
Variations for age >65 y:
Pre-induction: cyclophosphamide 100 mg/m2/d IV on days -3 to –1
Cycle 1: idarubicin 10 mg/d IV on days 1 and 2, vincristine 1 mg/m2/d (max. 2 mg) on days 1, 8, 15 and 22, dexamethasone 5 mg/m2/d IV on days 1-5, 15-19, G-CSF from day 5 (induction).
Cycle 2: idarubicin 10 mg/d IV on day 1, cyclophosphamide 500 mg/m2 IV on day 1, dexamethasone 5 mg/m2/d IV/PO on days 1-5, cytarabine 60 mg/m2/d IV/SC on days 2-5, 6-mercaptopurine 40 mg/m2/d PO on days 6-10, G-CSF from day 8 to resolution of absolute neutropenia <1 x109/L
Cycles 3: methotrexate 0.5 g/m2, cytarabine 1 g/m2/bd IV on days 3 and 4, G-CSF from day 8 (collection/cryopreservation of autologous blood stem cells at cycle 3).
Cycles 4,6: idarubicin 10 mg/d IV on day 1, cyclophosphamide 500 mg/m2 IV on day 1, vincristine 1 mg/m2/d (max. 2 mg) IV on days 1 and 8, dexamethasone 5 mg/m2/d IV/PO on days 1-5, cytarabine 60 mg/m2/d IV/SC on days 2-5, 6-mercaptopurine 40 mg/m2/d PO on days 6-10, G-CSF from day 8 to resolution of absolute neutropenia <1 x109/L.
Cycle 5: methotrexate 0.5 g/m2, L-asparaginase (E. Coli) 5.000 U/m2 IV on days 3
Cycle 7-8: omitted
· CNS prophylaxis: Intrathecal MTX 10 mg, Ara-C 40 mg, and PDN 40 mg or dexa 4 mg during induction/consolidation and maintenance for a total of 12 IT
MRD/Risk-Oriented Therapy
MRD-NEG/SR patients: Maintenance (24 4-week cycles)
Aged >55y
Cycles 1, 3, 5, 7, 9, 11: cyclophosphamide 50 mg/m2/d PO on days 1-4 (100mg or 50 mg total dose day 1 cycles 1-3), 6-mercaptopurine 75 mg/m2/d PO on days 8-28, methotrexate 15 mg/m2/d PO/intramuscular (IM) on days 8, 15 and 22.
Cycles 2, 4, 6, 8, 10, 12: vincristine 1 mg/m2 IV on day 1, prednisone 40 mg/m2/d PO on days 1-5, 6-mercaptopurine 75 mg/m2/d PO on days 8-28, methotrexate 15 mg/m2/d PO/IM on days 8, 15 and 22.
Cycles 13-24: 6-mercaptopurine 75 mg/m2/d PO on days 1-28, methotrexate 15 mg/m2/d PO/IM on days 1, 8, 15 and 22.
Aged >65y
Cycles 1, 3, 5, 7, 9, 11: cyclophosphamide 50 mg/d PO on days 1-4, 6-mercaptopurine 60 mg/m2/d PO on days 8-28, methotrexate 10 mg/m2/d PO/IM on days 8, 15 and 22.
Cycles 2, 4, 6, 8, 10, 12: vincristine 1 mg IV on day 1, prednisone 20 mg/m2/d PO on days 1-5, 6-mercaptopurine 50 mg/m2/d PO on days 8-28, methotrexate 10 mg/m2/d PO/IM on days 8, 15 and 22.
Cycles 13-24: 6-mercaptopurine 50 mg/m2/d PO on days 1-28, methotrexate 10 mg/m2/d PO/IM on days 1, 8, 15 and 22.
MRD-POS/HR patients: 1st option Allogeneic SCT
Allogeneic SCT: first choice option, from sibling/unrelated donor or cord blood. SCT procedure. SCT timing is by risk class and MRD study results (positive timepoint 2: early; others: at end of consolidation, with interim maintenance).
MRD-POS/HR: 2nd option Autologous SCT with Maintenance (12 4-week cycles)
Autologous SCT: second choice option if allogeneic SCT not possible (maintenance only if autologous SCT not feasible), with melphalan 100 mg/m2/d IV on days 1 and 2 (100mg/mq days 1 if aged>65y), plus autologous CD34+ blood cells (2-6x106/kg) on day 4, and G-CSF.
MRD-POS/HR patients excluded from SCT: 3rd option Maintenance (24 4-week cycles)
B) Generation of patient-specific probes for MRD study
The molecular evaluation of MRD was performed. DNA was extracted from mononuclear marrow cells using commercially available kits [QIAamp DNA Blood Kit (QIAGEN, Hilden, Germany)].
Leukemia-specific forward oligonucleotides were generated after genomic amplification and sequencing of the Leukemia-specific junctional regions of the rearranged IG heavy chain (H) or/and kappa light chain (K), and TCR gamma (G), delta (D), and beta (B) genes [27-29]. MRD quantification was performed by amplification of 500 ng sample DNA and the 10-fold dilution series of the the diagnostic DNA specimen in DNA obtained from mononuclear cells from a pool of five to 10 healthy donors. All samples were amplified in triplicate, and the MRD level was expressed as the logarithmic reduction of the leukemic burden detected at diagnosis, after correction for DNA quantity by amplification of a control gene.