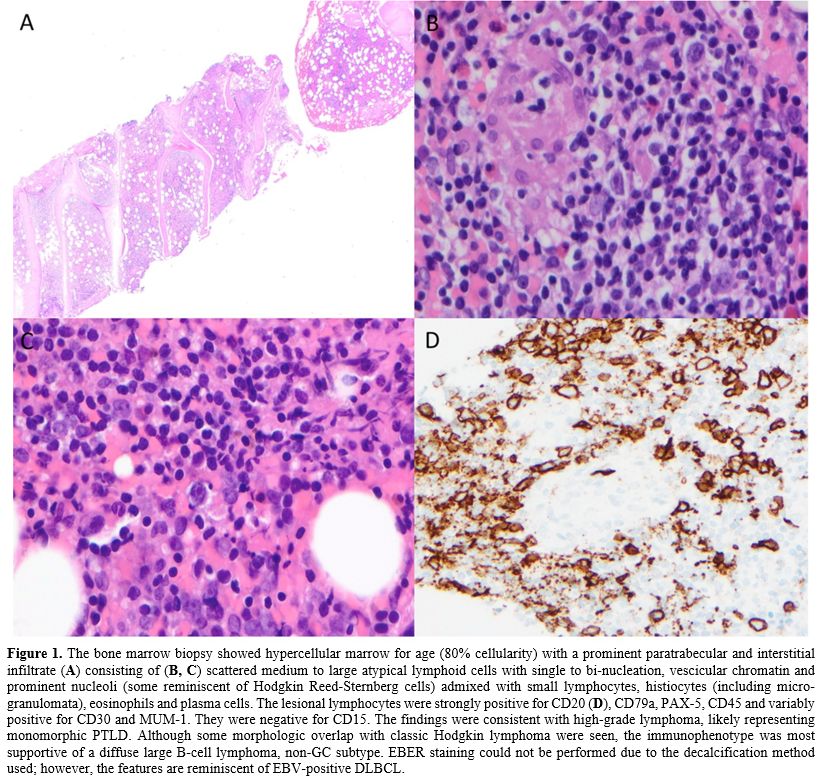
Post-transplant
lymphoproliferative disorders (PTLDs) are a catastrophic complication
of both solid organ (SOT) and haematopoietic stem cell transplant
(HCT). Iatrogenic immune suppression following HCT can result in
EBV-driven unchecked proliferation of neoplastic lymphoid or
plasmacytic cells.[1] PTLDs are traditionally
classified as early (within one-year post-transplant) and late (usually
around five years post-transplant). Very late PTLDs have been described
as presenting after ten years post-transplant.[2] These are much more common post-SOT and are extremely rare post-HCT.[3] We report a case of PTLD developing 17 years post-transplant for relapsed acute myeloid leukaemia (AML).
In
March 2004, a 49-year-old man was diagnosed with AML M4 with the
inversion of chromosome 16. He started induction chemotherapy with
daunorubicine, cytarabine and thioguanine, achieving complete remission
(CR). He then underwent 3 consolidation courses of chemotherapy with
high-dose Cytosine. In January 2006, he developed relapsed disease and
started salvage chemotherapy with FLAG-Ida [fludarabine, cytarabine,
G-CSF (granulocyte-colony stimulating factor), idarubicin], to which he
responded well and went into complete remission; he subsequently
received a second cycle of FLAG-Ida as consolidation. He underwent a
matched volunteer unrelated haematopoietic stem cell transplant (10/10
HLA match) with Fludarabine 150 mg/msq, Busulphan 6.4 mg/Kg and
Alemtuzumab (Campath) 60 mg (FBC) on 29th
June 2006. Graft-versus-host disease (GVHD) prophylaxis consists of a
Cyclosporine single agent. On D+76, while cyclosporine was being
tapered, he developed acute grade 2 skin GVHD, which progressed to
chronic GVHD requiring treatment with Psoralen plus UltraViolet A
radiation (PUVA) and prolonged systemic immune suppression.
Cyclosporine was stopped one-year post-transplant. Repeated BM
examinations, until five years post-transplant confirmed molecular
remission (CBFb/MYH11 by Real-Time PCR). In 2016, routine evaluation
revealed neutropenia and reflex testing with a bone marrow showed
molecular Minimal Residual Disease (MRD) negative AML, but granulocytic
hypoplasia and the presence of a small non-clonal T-LGL expansion (4% T
cells with CD3+, CD57+, CD5+, CD16neg, CD56neg, CD2+, CD7+ and
alpha/beta +). He was initiated on prednisolone and G-CSF, to which
there was an improvement. However, on tapering of steroids, the
cytopenia recurred, necessitating the use of cyclosporin followed by
Sirolimus as a steroid-sparing agent. The blood counts normalised until
December 2022, when he again developed profound neutropenia. A repeat
bone marrow examination revealed para-trabecular and interstitial
infiltrate of B cells, likely monomorphic PTLD consistent with Diffuse
Large B Cell Lymphoma (DLBCL) of non-GC type [PET CT did not reveal any
nodal disease, and EBV viral load showed a peak of 25000 DNA
copies/ml]. Treatment with Rituximab 375mg/m2
weekly for four weeks was commenced, and that resulted in complete
resolution of cytopenia, and the end of treatment bone marrow confirmed
in February 2023 the absence of AML (MRD negative by flowcytometry and
RTPCR) and DLBCL. Due to the persistence of an enlarged LGL population
(7% T LGL cells), he was continued on prednisolone 5mg and Sirolimus 2
mg. In September 2023, he had a new episode of pancytopenia, and bone
marrow showed relapsed high-grade B-cell lymphoma (very low volume,
~1-2%) with AML molecular remission. PET CT revealed generalised
lymphadenopathy on both sides of the diaphragm. A Lymph node biopsy
revealed EBV-positive DLBCL, and he has been commenced on R-CHOP
chemotherapy.
A biphasic pattern of PTLD has been recognised,
with early disease within the first year of transplant and a second
peak 7 to 10 years after transplant.[3]
Presentations
after ten years of transplant have been defined as extremely late PTLD,
and this is the first report of an adult presenting with it in the
post-HCT setting, though there are few reports in the paediatric and
post-SOT setting.[2]
For HCT, PTLD risk factors
include EBV recipient-donor sero-mismatch, selective donor T-cell
depletion, haploidentical donor, unrelated or HLA-mismatched related
donor, umbilical cord blood transplant, use of reduced intensity
conditioning regimens, age of recipient older than 50, the use of
anti-thymocyte globulin (ATG) or anti-CD3 monoclonal antibody and
chronic GvHD requiring prolonged immunosuppression.[5] Much of the data for PTLD, and especially very late PTLD, comes from solid organ transplants.[6] PTLD is lowest after HCT and liver transplants compared to the other transplants[7]
since immune suppression is typically stopped post-HCT if there are no
complications like chronic GVHD. In the landmark large series
describing 18,014 patients who underwent allogeneic bone marrow
transplantation (BMT) at 235 centres worldwide,[7] the
authors concluded that altered immunity and T-cell regulatory
mechanisms like chronic GVHD were responsible for PTLD. Notably, EBV
and CMV reactivation did not correlate with late-onset PTLD.[8] The use of Campath and ATG has been implicated in the risk of developing PTLD.[3,9]
In our case report, there was the presence of chronic GVHD
necessitating the use of systemic immune suppression until 1-year
post-transplant, followed by nearly six years of treatment of immune
cytopenia with prednisolone, cyclosporin and Sirolimus prior to
developing the PTLD. Thus, there is a need to recognise atypical risk
factors for late-onset PTLDs and to keep the suspect of this entity in
the differential diagnosis process.
In the absence of EBV as a
driver, other postulated theories include the presence of lymphoid load
in the allograft and chronic antigen stimulation, which may result in a
dysregulated immune response leading to PTLD.[10] The
major factors influencing both B cell and T cell immune reconstitution
are GVHD and the use of immune suppressive agents. Generally, B cell
numbers recover to normal counts within 12 months after HSCT,[11]
although complete recovery may take up to 2 years. Patients receiving
antithymocyte globulin-fresenius (ATG-F) are known to have delayed
CD19+ B cell recovery compared to non-ATG patients,[12] and Campath is well known to offer a deeper lymphodepletion resulting in even later immune reconstitution.[13]
T lymphopenia and inadequate repertoire of CD4+ and CD8+ T cells,
lasting 1 year or more after transplant, foster recurrent infections
with latent viruses.[14]
The optimal treatment
of PTLD is still evolving. Quick withdrawal or reduction of immune
(RIS) suppression and induction therapy with weekly rituximab for
CD20-positive cases is the standard of care for PTLD.
The option of RIS alone may not be feasible because of the risk of GVHD[5]
and because the still immune incompetent host may not be able to mount
a cytotoxic response to halt the proliferative process.[6]
However, the response rates to rituximab monotherapy in HSCT PTLD have
been reported to be only 20% (ORR 60%–65%) with a 2-year median OS of
50%,[7] hence second line options practically include
chemotherapy (R-CHOP), Immunotherapy with Brentuximab if CD30+
and ideally EBV specific Cytotoxic Lymphocyte therapy (CTLs) where
available to offset infective complications and regimen related
toxicities of systemic chemotherapy. This patient responded to
first-line therapy despite the bone marrow involvement, which has been
described to have worse outcomes.
In conclusion, this case
highlights the complexity of very prolonged immune suppressive therapy
and shows that very late-onset PTLS post-HCT can occur in a setting of
persistent immunosuppression.