.
Maria
Pina Dore1,2, Giovanni Mario Pes1,3,
Sandro Mereu1, Jessica Piroddu1,
Lorenzo Cavagna4 and Gian Luca Erre1.
1 Dipartimento
di Medicina, Chirurgia e Farmacia, University of Sassari, Sassari
07100, Italy.
2 Baylor College of Medicine, 77030 Houston,
Texas, USA.
3 Sardinia Blue Zone Longevity Observatory,
Ogliastra, Italy.
4 Division of Rheumatology, IRCCS Fondazione
Policlinico San Matteo, Pavia, Italy
Correspondence to:
Prof. Maria Pina Dore. Clinica Medica, Viale San Pietro - 8, PO Box
07100 Sassari, ITALY. Phone: +39 347 4539532. E-mail:
mpdore@uniss.it
Published: July 01, 2024
Received: May 03, 2024
Accepted: June 14, 2024
Mediterr J Hematol Infect Dis 2024, 16(1): e2024056 DOI
10.4084/MJHID.2024.056
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
To the editor
A decade ago,
Gheita et al. reported a high frequency of glucose-6-phosphate
dehydrogenase (G6PD, EC 1.1.1.49) deficiency in patients with
rheumatoid arthritis (R.A.) and concurrent metabolic syndrome.[1] More recently, in a retrospective
cohort study using a large, nationwide database of individuals with
known G6PD status, Israel et al. confirmed a significant association
between the occurrence of R.A. and G6PD enzyme deficiency.[2] In Sardinia, Italy, hereditary G6PD deficiency reaches a frequency as high as 8% and 12% in
the male and female population, respectively,[3]
which is among the highest in the world. It has already been proven
that 95% of cases are due to the G6PD Med mutation (Ser188Phe),[3] which entails a class II severe
deficiency, according to the World Health Organization, because it is
associated with a more than 90% reduction of catalytic activity.[4] Moreover, the prevalence of R.A. has
been estimated at 552 × 105
in the general Sardinian population,[5]
i.e., considerably higher than that recorded in the Italian peninsula
(330 × 105).[6]
Based on this claim and the particularly favourable setting, the
association between R.A. and G6PD deficiency was tested in the
population of northern Sardinia.
Methods
This was a
retrospective case-control study recruiting outpatients referred for
upper endoscopy to the Gastroenterology section of a teaching hospital
(University of Sassari, Italy). The section is the main referral centre
for rheumatological patients with dyspeptic complaints. Data from
January 2014 and January 2022 were retrieved from an electronic
database. Collected data included sex, age, smoking habits,
anthropometric parameters, and the presence of a defined diagnosis of
R.A.
Rheumatoid
arthritis. R.A. was diagnosed by the rheumatologist
according to national and international guidelines/expert consensuses
progressively developed and used in clinical practice.[7]
Rheumatoid factor (R.F.) levels and anti-citrullinated protein antibody
(ACPA) titres were also available in a subset (n=291) of patients with
R.A. Serum R.F. titers were measured in the hospital reference lab
using a commercial ELISA kit (Abcam®, Cambridge, MA, U.S.A.),[8] and ACPA titres (Euro Diagnostica
Immunoscan CCPlus®, Arnhem, The Netherlands) according to the
manufacturer’s instructions.
Glucose-6-phosphate
dehydrogenase deficiency. In all study participants, G6PD
status had been assessed using a previously described laboratory test
based on the measurement of the G6PD/6PGD ratio in red blood cells of
peripheral venous blood samples.[9]
Enzyme deficiency was classified as severe (<10% residual G6PD
activity) or intermediate (between 10% and 80% residual activity).
Molecular testing was not available.
Statistical
analysis. The body mass index (B.M.I.), calculated by
using the formula weight (kg) / height (m)², allowed to stratify
participants into normal, overweighted (B.M.I. between 25 and 29.9
kg/m²), and obese (B.M.I.≥ 30 kg/m²). According to smoking habits,
patients and controls were divided into never smokers or former
smokers/current smokers. Both severe and intermediate G6PD deficiency
were merged into the same category to strengthen the analysis.
Differences between means were evaluated by the Student’s t-test for
continuous variables and by the Pearson χ² test for categorical
variables. The association between G6PD deficiency and R.A. was
determined using univariable and multivariable logistic regression
models by calculating unadjusted and adjusted odds ratios (O.R.s) and
their 95% confidence intervals (CI). The analysis was conducted
separately in males and females. All statistical analyses were
performed using SPSS statistical software (version 22.0, Chicago, IL,
U.S.A.). P-values lower than 0.05 were considered statistically
significant.
Ethical
Considerations. Ethical review and approval were
waived for this study due to observational retrospective design by the
Italian law (GU No. 76 31/Mar/2008). The procedures followed in the
study were in accordance with the ethical standards of the Declaration
of Helsinki.
Results
A total of
5,279 study participants (mean age 53.0 ± 17.1 years; 66.5% female)
were included in the analysis. In 594 participants, a diagnosis of R.A.
(11.3%) was posed by the rheumatologist. Table 1 shows the
features of the study participants separately in males and females.
There were 661 patients (12%) with G6PD deficiency, reflecting the
regional frequency,[3] with an F: M
ratio of 1.86, and 4618 patients without. No significant differences
were observed in B.M.I. and smoking habits. The prevalence of R.A. was
14.4% in males and 16.6% in females, respectively, among carriers of
G6PD deficiency, whereas it was 8.5% and 11.7% among males and females
with normal enzyme activity (p < 0.0001).
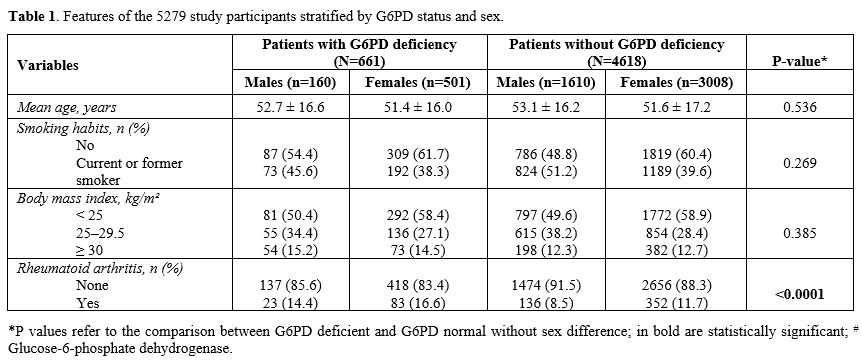 |
- Table
1. Features of the 5279 study participants stratified by G6PD status and sex.
|
The results of
univariable and multivariable logistic regression analysis, using the
presence or absence of R.A. as the outcome, according to the exposure
to G6PD deficiency, are reported in Table
2. The odds for an R.A. diagnosis in subjects with G6PD
deficiency were statistically significant (OR 2.21, 95%CI 1.54‒3.18)
after adjusting for the covariates included in the study (Table 2) and higher
in males [OR 1.82, 95%CI 1.13–2.93] compared with females [OR 1.49,
95%CI 1.15–1.94].
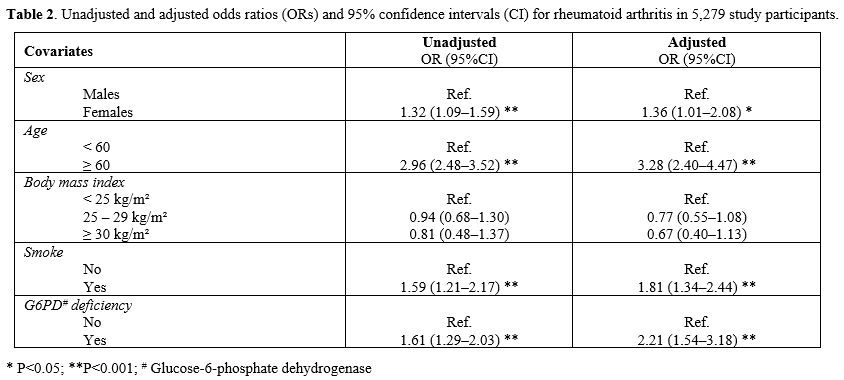 |
- Table
2. Unadjusted and adjusted odds ratios (ORs) and 95% confidence
intervals (CI) for rheumatoid arthritis in 5,279 study participants.
|
The subset of
291 patients with R.A. and availability of immunological markers was
stratified according to R.F. and ACPA positivity (Table 3).
Interestingly, the frequency of G6PD deficiency was significantly
increased in R.A. patients positive for both markers compared with
controls (17.6% vs 12.1%, p = 0.029). In comparison, the frequency of
G6PD deficiency was non-significantly increased (14.8% vs 12.1%, p =
0.537) in patients negative for RA-specific markers. However, the small
number of patients with G6PD deficiency in this subset did not allow us
to draw definitive conclusions.
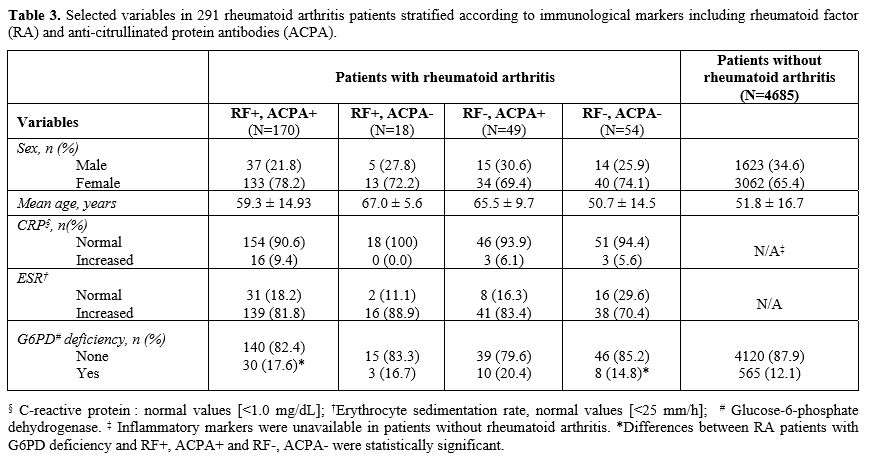 |
- Table
3. Selected variables in 291 rheumatoid arthritis patients stratified
according to immunological markers including rheumatoid factor (RA) and
anti-citrullinated protein antibodies (ACPA).
|
Discussion
The present
study involved a large cohort of patients. Our findings suggest that
similar to Israel's results,[2]
in the Northern Sardinian population, G6PD deficiency significantly
increased the odds of R.A. This association was found in both sexes,
especially in male patients. It is reasonable considering that among
females, a higher frequency of heterozygosity was reported, resulting
in greater residual enzyme activity. Interestingly, in a subanalysis,
the increased frequency of G6PD deficiency was detected in R.A.
patients with at least one positive immunologic marker.
Antioxidant
mechanisms protecting the body from the harmful action of reactive
oxygen species (R.O.S.) are hinged on several enzymes found in most
cells, one of the most important being G6PD. More specifically, in the
first reaction of the pentose phosphate pathway, G6PD supplies reducing
equivalents (NADPH) necessary to maintain high intracellular levels of
reduced glutathione (G.S.H.), a thiol-containing tripeptide active
against R.O.S., especially the superoxide anion.[10]
Subjects with G6PD deficiency are generally asymptomatic, but they may
experience more or less serious haemolytic crises following the intake
of specific drugs, especially NSAIDs, or the consumption of certain
foods, such as fava beans.[4]
Beyond haemolytic crises and neonatal jaundice, more recent literature
has reported how G6PD deficiency, affecting any cell of the organism,
can be implicated in other disorders, including the cardiovascular
system,[11] as well as various
autoimmune diseases[12] and, more
specifically, R.A. Pathogenic pathways underlying R.A. onset and
progression are largely unknown, with different mechanisms, including
genetic predisposition, environmental risk factors, microbial exposure,
and increased oxidative stress, being proposed until now.[13,14] The R.A. is characterized by
impaired antioxidant defense, although the precise mechanisms involved
are relatively poorly understood. Experimental[15]
and human studies[16] seem to
corroborate the notion that a defect in antioxidant mechanisms,
whatever the cause, concurs with inflammation in determining joint
tissue injury as well as systemic damage.
Several
studies and meta-analyses have shown that impaired antioxidant defense
is one of the pathogenic hallmarks of R.A. disease.[17]
Suggested hypotheses to explain how failure to counteract oxidative
stress can contribute to maintaining the autoimmune mechanism in R.A.
are structured along two lines of reasoning: (i) a decrease in the
intracellular glutathione, whose sulfhydryl (‒SH) moiety is
responsible for R.O.S. neutralization, and (ii) the establishment of a
chronic inflammatory dysregulation, globally affecting cell
signalling including that of the immune system. Evidence from different
studies highlighted the large amount of R.O.S. produced by monocytes
from R.A. patients. In particular, oxygen and nitrogen-reactive species
can directly degrade some components of the synovial tissues―more
specifically, hyaluronic acid and other proteoglycans―contributing to
joint damage. Furthermore, activated T-cells themselves are highly
sensitive to R.O.S. damage, and this might contribute to the
establishment of an altered immune response in R.A. via a
self-sustained pathogenic loop. In such circumstances, it is reasonable
to assume that any alteration of the antioxidant system due to
inherited defects can exacerbate the intracellular redox status,
thereby interfering with the mechanisms involved in immune tolerance
and increasing the chance of developing R.A. Consequently, tentative
speculation to explain our findings could be that G6PD deficiency, via
intracellular NADPH depletion, may hamper the conversion of GSSG to
G.S.H. necessary for R.O.S. disposal. Some intracellular components,
critical for immune tolerance, may be permanently modified and trigger
the sustained immune activation conducive to R.A.
G6PD
deficiency might also enhance R.A. risk via additional mechanisms, such
as dysregulation of the inflammatory response. Several in vitro studies
have demonstrated that G6PD-deficient cells release several regulatory
cytokines in excess, such as the transforming growth factor beta
(TGF-β), which plays a major role in inflammation and oxidative stress,
as confirmed by the lowering R.O.S. effect of TGF-β inhibitors.[18] Clinical evidence suggests that
TGF-β regulates the function of fibroblasts and might have a pathogenic
role in R.A..[19] Bira et al.
reported an increased TGF-β in the synovial tissue and fluids of R.A.
patients.[20] Through its action
on pivotal mechanisms of innate (natural killer cells) and adaptive
(Tregs cells) immunity, TGF-β upregulation may contribute to the
autoimmune response mounted in R.A..[20]
The
present study has some limitations due to its retrospective design and
the lack of molecular typing of G6PD deficiency. However, it is
reasonable to assume that the majority of patients carried the
Mediterranean variant.[3] The
frequency of G6PD deficiency in patients without R.A. was comparable to
that reported for the Sardinian population in the same area,[12] making a bias unlike. Although the
database used included patients with clinical complaints requiring
endoscopy, we are confident there were no reasons to think that the
comorbidity distribution was dissimilar between subjects with and
without G6PD deficiency.
Conclusions
Our
study confirmed previous results of an association between G6PD
deficiency and R.A., especially in patients with R.A. positive for R.F.
and/or ACPA. At present, it is not justified to recommend systematic
screening for G6PD in patients with R.A., and further evidence from a
larger case series is needed. Nonetheless, the identification of a new
risk factor, such as G6PD deficiency, opens a new avenue in research to
understand R.A. pathogenesis better.
Author
contributions statement
G.M.P. and
M.P.D. were responsible for conceptualization, study design, literature
search, analysis, interpretation, writing the original draft,
reviewing, and editing. G.M.P. contributed with formal analysis. S.M.,
J.P., and L.C. collected data and performed data curation and writing.
G.L.E. contributed to the analysis, data interpretation, writing,
review, and editing. All authors had full access to all the data in the
study and were the final ones responsible for deciding to submit for
publication.
References
- Gheita TA, Kenawy SA, El
Sisi RW, Gheita HA, Khalil H. Subclinical reduced G6PD activity in
rheumatoid arthritis and Sjogren's Syndrome patients: relation to
clinical characteristics, disease activity and metabolic syndrome. Mod
Rheumatol. Jul 2014;24(4):612-7. doi:10.3109/14397595.2013.851639 https://doi.org/10.3109/14397595.2013.851639
PMid:24252052
- Israel
A, Schäffer AA, Berkovitch M, et al. Glucose-6-phosphate dehydrogenase
deficiency and long-term risk of immune-related disorders. Original
Research. Frontiers in Immunology. 2023-September-11
2023;14doi:10.3389/fimmu.2023.1232560 https://doi.org/10.3389/fimmu.2023.1232560
PMid:37753082 PMCid:PMC10518697
- Fiorelli
G, Meloni T, Palomba V, Manoussakis C, Villa S, Cappellini MD. Gene
frequency of glucose-6-phosphate dehydrogenase (G6PD) polymorphic
variants in Sardinia. Gene Geogr. Dec 1990;4(3):139-42.
- Luzzatto
L, Ally M, Notaro R. Glucose-6-phosphate dehydrogenase deficiency.
Blood. Sep 10 2020;136(11):1225-1240. doi:10.1182/blood.2019000944 https://doi.org/10.1182/blood.2019000944
PMid:32702756
- Sardu
C, Cocco E, Mereu A, et al. Population based study of 12 autoimmune
diseases in Sardinia, Italy: prevalence and comorbidity. PLoS One.
2012;7(3):e32487. doi:10.1371/journal.pone.0032487 https://doi.org/10.1371/journal.pone.0032487
PMid:22396771 PMCid:PMC3292563
- Rossini
M, Rossi E, Bernardi D, et al. Prevalence and incidence of rheumatoid
arthritis in Italy. Rheumatol Int. May 2014;34(5):659-64.
doi:10.1007/s00296-014-2974-6 https://doi.org/10.1007/s00296-014-2974-6
PMid:24610538
- Aletaha
D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification
criteria: an American College of Rheumatology/European League Against
Rheumatism collaborative initiative. Arthritis Rheum. Sep
2010;62(9):2569-81. doi:10.1002/art.27584 https://doi.org/10.1002/art.27584
PMid:20872595
- Bampton
JL, Cawston TE, Kyle MV, Hazleman BL. Measurement of rheumatoid factors
by an enzyme-linked immunosorbent assay (ELISA) and comparison with
other methods. Ann Rheum Dis. Jan 1985;44(1):13-9.
doi:10.1136/ard.44.1.13 https://doi.org/10.1136/ard.44.1.13
PMid:3970590 PMCid:PMC1001560
- Mosca
A, Paleari R, Rosti E, et al. Simultaneous automated determination of
glucose 6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase
activities in whole blood. Eur J Clin Chem Clin Biochem. May
1996;34(5):431-8. doi:10.1515/cclm.1996.34.5.431 https://doi.org/10.1515/cclm.1996.34.5.431
PMid:8790979
- Weiss
SJ. The role of superoxide in the destruction of erythrocyte targets by
human neutrophils. J Biol Chem. Oct 25 1980;255(20):9912-7. https://doi.org/10.1016/S0021-9258(18)43479-5
PMid:6253458
- Dore
MP, Portoghese M, Pes GM. The Elderly with Glucose-6-Phosphate
Dehydrogenase Deficiency are More Susceptible to Cardiovascular
Disease. J Atheroscler Thromb. Jun 1 2021;28(6):604-610.
doi:10.5551/jat.56531 https://doi.org/10.5551/jat.56531
PMid:32908034 PMCid:PMC8219535
- Dore
MP, Fanciulli G, Pes GM. Is Glucose-6-Phosphate Dehydrogenase
Deficiency a Risk Factor for Autoimmune Thyroid Disease? A
Retrospective Case-Control Study. Int J Environ Res Public Health. Feb
3 2023;20(3)doi:10.3390/ijerph20032709 https://doi.org/10.3390/ijerph20032709
PMid:36768075 PMCid:PMC9916078
- Erre
GL, Mameli G, Cossu D, et al. Increased Epstein-Barr Virus D.N.A. Load
and Antibodies Against EBNA1 and E.A. in Sardinian Patients with
Rheumatoid Arthritis. Viral Immunol. Sep 2015;28(7):385-90.
doi:10.1089/vim.2015.0035 https://doi.org/10.1089/vim.2015.0035
PMid:26083265
- Bo
M, Erre GL, Bach H, et al. PtpA and PknG Proteins Secreted by
Mycobacterium avium subsp. paratuberculosis are Recognized by Sera from
Patients with Rheumatoid Arthritis: A Case-Control Study. J Inflamm
Res. 2019;12:301-308. doi:10.2147/JIR.S220960 https://doi.org/10.2147/JIR.S220960
PMid:31819587 PMCid:PMC6899068
- Wruck
CJ, Fragoulis A, Gurzynski A, et al. Role of oxidative stress in
rheumatoid arthritis: insights from the Nrf2-knockout mice. Ann Rheum
Dis. May 2011;70(5):844-50. doi:10.1136/ard.2010.132720 https://doi.org/10.1136/ard.2010.132720
PMid:21173018
- Hassan
MQ, Hadi RA, Al-Rawi ZS, Padron VA, Stohs SJ. The glutathione defense
system in the pathogenesis of rheumatoid arthritis. J Appl Toxicol.
Jan-Feb 2001;21(1):69-73. doi:10.1002/jat.736 https://doi.org/10.1002/jat.736
PMid:11180282
- Quinonez-Flores
CM, Gonzalez-Chavez SA, Del Rio Najera D, Pacheco-Tena C. Oxidative
Stress Relevance in the Pathogenesis of the Rheumatoid Arthritis: A
Systematic Review. Biomed Res Int. 2016;2016:6097417.
doi:10.1155/2016/6097417 https://doi.org/10.1155/2016/6097417
PMid:27340664 PMCid:PMC4906181
- Sanna
F, Bonatesta RR, Frongia B, et al. Production of inflammatory molecules
in peripheral blood mononuclear cells from severely glucose-6-phosphate
dehydrogenase-deficient subjects. J Vasc Res. 2007;44(4):253-63.
doi:10.1159/000100903 https://doi.org/10.1159/000100903
PMid:17361089
- Parsanathan
R, Jain SK. Glucose-6-phosphate dehydrogenase (G6PD) deficiency is
linked with cardiovascular disease. Hypertens Res. Jun
2020;43(6):582-584. doi:10.1038/s41440-020-0402-8 https://doi.org/10.1038/s41440-020-0402-8
PMid:31974484
- Bira
Y, Tani K, Nishioka Y, et al. Transforming growth factor beta
stimulates rheumatoid synovial fibroblasts via the type II receptor.
Mod Rheumatol. 2005;15(2):108-13. doi:10.1007/s10165-004-0378-2 https://doi.org/10.1007/s10165-004-0378-2
PMid:17029045


