Fatma Burçin Kurtipek1, Ayça Koca Yozgat1, Saliha Kanık Yüksek2, Dilek Kaçar1, Turan Bayhan1, Dilek Gürlek Gökçebay1, Aslınur Özkaya Parlakay2 and Neşe Yaralı1.
1 Ankara Bilkent City Hospital, Division of Paediatric Hematology and Oncology, Ankara, Turkey.
2 Ankara Bilkent City Hospital, Division of Paediatric Infectious Diseases, Ankara, Turkey.
Correspondence to:
Fatma Burçin Kurtipek, Ankara Bilkent City Hospital, Division of
Paediatric Hematology and Oncology. E-mail:
burcindogan86@gmail.com
Published: September 01, 2024
Received: June 24, 2024
Accepted: August 17, 2024
Mediterr J Hematol Infect Dis 2024, 16(1): e2024072 DOI
10.4084/MJHID.2024.072
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Introduction:
Port catheters facilitate the administration of chemotherapy,
antibiotics, blood products, fluid, and parenteral nutrition to
pediatric patients with hematological malignancies. However, as its use
has become widespread, local and systemic, catheter-related infections
have emerged as important causes of morbidity and mortality. In our
study, we aimed to evaluate the success of antibiotic lock therapy in
port catheter-related infections of pediatric patients followed up with
acute leukemia.
Methods: Port
catheter cultures taken from a total of 182 pediatric patients with
acute lymphoblastic/myeloblastic leukemia who were followed up at
Ankara City Hospital Pediatric Hematology Clinic between August 2019
and August 2023 were evaluated retrospectively.
Results:
Bacterial growth was identified in 739 port catheter culture specimens
of 182 patients. Closure or removal of the port was required in 91, and
removal of the port catheters in 49 patients due to port
catheter-related infections. Antibiotic lock therapy was started in 56
patients with bacterial growth in the port catheter. With antibiotic
lock therapy, port catheter-related infections of 42 patients were
eradicated, and their catheters began to be used again. As a result,
the port catheter-related infections of 42 of 56 (75%) patients whose
ports were closed and also received systemic antibiotic therapy were
eradicated, and no infection recurrence was observed.
Conclusion: Adding antibiotic lock therapy to systemic antibiotics in pediatric patients may be beneficial in terms of catheter salvage.
|
Introduction
Intravascular
catheter-related bloodstream infections (CRBSI) are significant
complications for leukemia patients with increased risk of morbidity
and mortality, longer hospital stays, and higher costs.[1,2] Coagulase-negative Staphylococci (CoNS), Staphylococcus aureus, Enterococcus spp., and Klebsiella spp. are the most common pathogens responsible for CRBSI in children.[3] Microorganisms may be transmitted through the infected closure sites during catheter insertion or via hematological routes.[4]
The risk of CRBSI is high in children receiving intensive chemotherapy
and those carrying external or double-lumen catheters.[5]
Although catheter removal is preferred to prevent the development of
CRBSI, catheter salvage may be attempted in patients whose alternative
venous access is challenging to construct and who continue to need a
catheter for infusions.
Antibiotic lock therapy (ALT) is a
treatment method in which high concentrations of antibiotics are
delivered through the lumen of the catheter.[2]
Microorganisms can migrate into the intraluminal space, forming a
pathogenic biofilm resulting in the onset of CRBSI. In ALT, the
catheter lumen is filled with a high concentration of antibiotics to
maximize bactericidal activity and penetrate the pathogenic biofilm.
The antibiotic concentration required to destroy causative
microorganisms in the catheter lumen should be 100-1000 times the
minimum inhibitor concentration.[6]
Re-catheterization complications and costs can be prevented with the
use of ALT. However, the catheter should be removed if the systemic
intravenous antibiotics and ALT are unable to destroy the
microorganisms.[1,7]
Data
concerning the efficacy and safety of ALT in children are limited. In
this study, we aim to determine its effectiveness in children with
leukemia and identify factors affecting treatment outcomes.
Methods
This
retrospective study assessed 182 children with acute leukemia treated
in the Ankara Bilkent City Hospital Pediatric Hematology Clinic between
August 2019 and August 2023. The patient demographic and clinical
characteristics, isolated microorganisms, and antibiotics used in lock
therapies were examined. Duration, success or failure of ALT,
catheter-related reinfections, catheter removal, and mortality data
were collected from patients' electronic medical files.
In cases
of febrile neutropenia (FN) (body temperature >37.5 ºC persisting
for at least 2 hours or >38 ºC once), a blood sample was drawn from
the peripheral vein and port catheter lumen for culture and empirical
antimicrobial treatment was started. Systemic empiric antibiotic
therapy was administered according to the Infectious Diseases Society
of America (IDSA) guidelines that recommend the use of antipseudomonal
broad-spectrum beta-lactamase antibiotics like piperacillin-tazobactam
or cefepime.[8] After the causative microorganism was
identified, the treatment might be modified if necessary. Glycopeptides
were added to the treatment protocol in patients with severe mucositis,
pneumonia, soft tissue infections, and methicillin-resistant S. aureus colonization.
Diagnosis
of CRBSI was based on the updated 2009 version of IDSA Clinical and
Practice guidelines for the diagnosis and management of intravascular
catheter-related infections.[8] Accordingly, in cases
where the same microorganism was detected in both the cultures of blood
samples drawn from the catheter tip and peripheral blood if the
microorganism was identified at least two hours earlier in the cultures
of the blood samples drawn from the catheter, then the presence of
CRBSI was considered. If nonpathogenic microorganisms of normal skin
flora (CoNS, S. viridans, Propionibacterium spp., Bacillus spp.)
were isolated from at least two blood cultures, it was accepted as
CRBSI. The clinical signs of infection were noted simultaneously at the
time when the blood culture samples were obtained.
Catheter removal was performed in catheter-related infections associated with S. aureus, Pseudomonas spp., fungi, and mycobacteria,
all tunnel infections, complicated catheter-related infections, and
severely septic patients. If the patient was clinically stable and the
catheter removal was not required, ALT combined with systemic
intravenous antibiotic therapy was used. In ALT, heparin (100 U/mL)
with saline and vancomycin (5 mg/mL), gentamicin (1 mg/mL), amikacin (2
mg/mL), colistin (0.1 mg/mL) or trimethoprim-sulfamethoxazole (10
mg/mL) were used according to identified microorganisms.[8,9]
The locking solution was introduced into the catheter lumen (usually 3
mL), and then the catheter was locked. This solution was renewed every
24 hours. Duration of ALT varied depending on the causative
microorganisms as follows: Enterococcus spp.: 7-14 days; CoNS: 10s-14 days; gram-negative bacilli: 10-14 days.[8] Control catheter and peripheral blood cultures were taken after 72 hours of ALT.
Treatment
failure was defined as persistent bacterial growth of the same
microorganism in blood cultures obtained within 72 hours of ALT. If the
same pathogen was isolated from the blood culture within three months,
it was accepted as a relapse.[9] The catheter was
removed if the fever persisted or a sterile blood culture could not be
obtained. All patients were followed for at least six months after
CRBSI was diagnosed.
This study was approved by the University of
Health Sciences Ankara City Hospital Ethics Committee (ethics approval
number: E2-23 -3816) and performed according to the World Medical
Association Declaration of Helsinki Ethical Principles for Medical
Research involving Human Subjects and its latest amendments.
Statistical analysis.
Statistical analyses were performed using SPSS for Windows 16.
Categorical variables were expressed as numbers and percentages, and
numerical variables were defined using mean ± standard deviation,
minimum–maximum, and median values. Survival curves were estimated
according to the Kaplan-Meier method. Adjusted hazard ratio and 95%
confidence interval were used to estimate survival. The level of
statistical significance was set at p <0.05.
Results
The
febrile neutropenia (FN) episodes of 182 children with acute leukemia
were examined. Bacterial growth was detected in port catheter cultures
in 91 FN episodes of 79 patients, including cases with acute myeloid
leukemia (AML) (n:14) and acute lymphoblastic leukemia (ALL) (n:65).
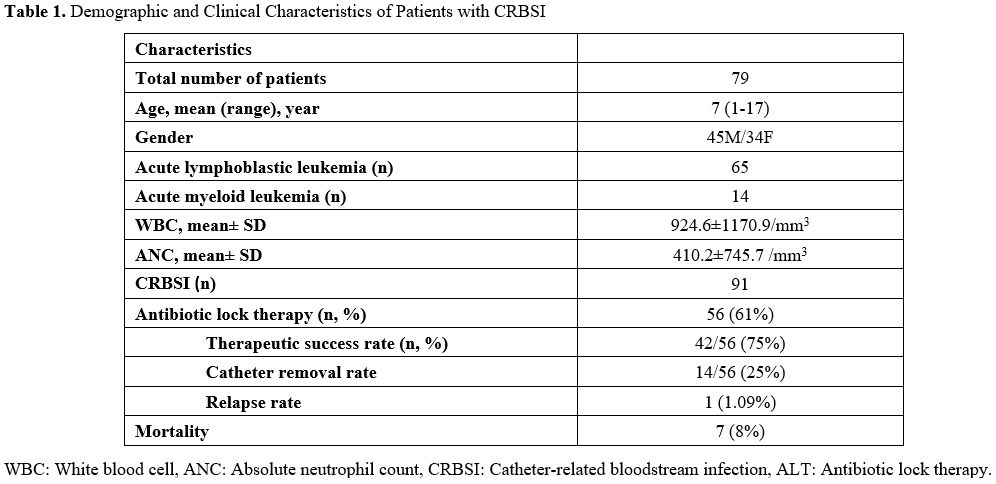
Demographic and clinical characteristics of patients are summarized in Table 1.
CRBSI was detected in 34 female and 45 male patients. The mean age of
the patients with CRBSI was 6.8 years (min-max: 1- 17 years).
 |
- Table
1. Demographic and Clinical Characteristics of Patients with CRBSI.
|
Patient
management was individualized depending on the causative microorganism,
the patient's clinical condition, and the catheter type. Ninety-two
catheters of 35 patients (44.3%) were removed as soon as possible
without using ALT due to the patient's unstable condition (hypotension,
resistant fever, detection of fungal microorganisms) or the development
of complicated catheter infections such as tunnel infection. The
treatment algorithm for patients with catheter-related infections is
summarized in Figure 1.
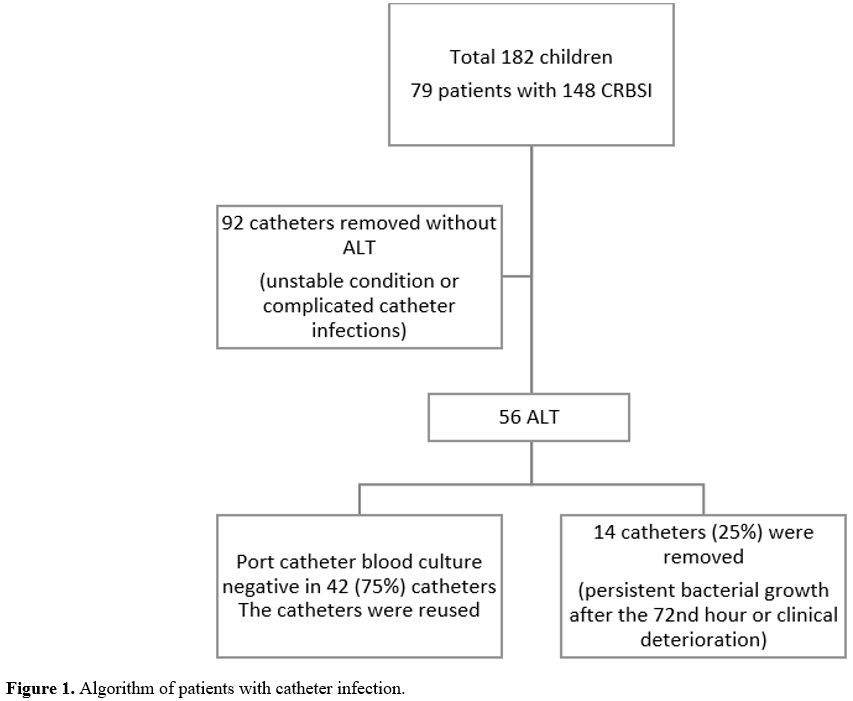 |
- Figure 1. Algorithm of patients with catheter infection.
|
Microorganisms growing on the catheters are listed in Table 2. The most frequently isolated pathogens were Staphylococci (46%), E. coli (19.6 %), and Klebsiella spp.
(14.2 %). ALT was started against 56 catheter-related infections.
Microorganisms growing in the catheter cultures are listed in Table 2.
Upon identification of gram-positive bacteria in 28 and gram-negative
bacteria in 28 catheters, vancomycin (n:28), amikacin (n:21),
gentamicin (n:5), colistin (n:1), TMP-SMX (n:1) were used in the
respective patients for the treatment of ALT. All patients also
received appropriate systemic intravenous antibiotics together with ALT.
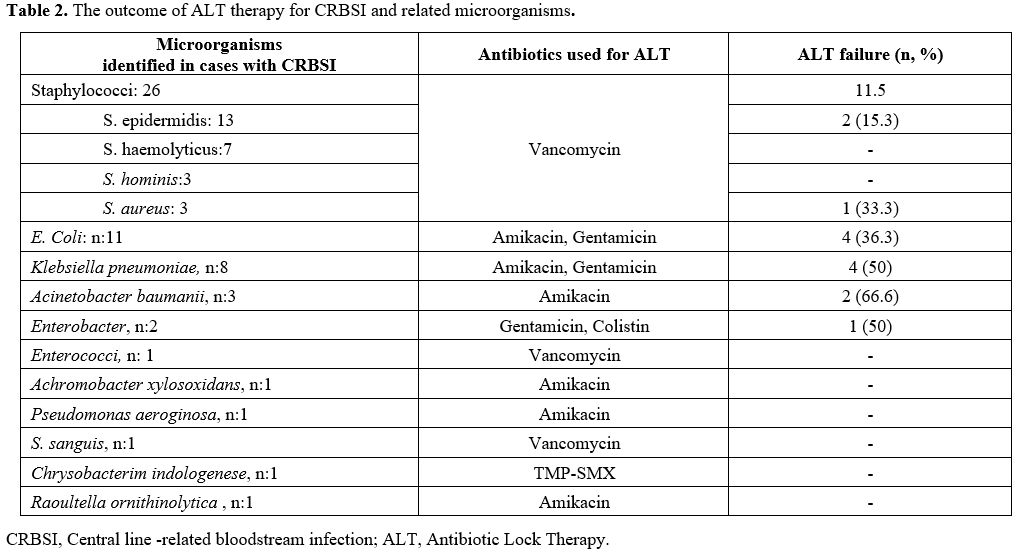 |
- Table 2. The outcome of ALT therapy for CRBSI and related microorganisms.
|
Fourteen catheters (25%) were removed due to persistent bacterial growth after the 72nd
hour of ALT or clinical deterioration of the patients while receiving
ALT. A number of patients, indicated in bracket, infected with K. pneumonia (n:5), Staphylococcus spp (n:3), and P. aeruginosa (n:2), Achromobacter spp. (n:1), E. coli
(n:1) required intensive care treatment due to hemodynamic instability.
Seven of these patients died due to sepsis. Antibiotic lock treatment
was applied to one patient in whom pseudomonas growth was detected in
the catheter, and the port catheter was removed directly because the
other patient had clinical instability. Port catheter blood culture
became negative in 42 (75%) patients treated with ALT, and these
catheters were reused. Recurrent infection with S. epidermidis
was observed in one (1.7 %) patient. As a result, the therapeutic
success rate of ALT was 75 percent. The outcomes of CRBSI and
identified microorganisms are shown in Table 2. ALT was successful in the treatment of CoNS infections but failed in the treatment of infections caused by Acinetobacter spp.
Discussion
Antibiotic
lock therapy has been described in the prevention of CRBSI, and
treatment guidelines to sterilize the catheter lumen so as to prevent
its removal have been stated.[10] However, a limited number of studies have investigated the efficacy and safety of ALT' in children with leukemia.[11] In most guidelines, eradication of CRBSI that persists despite 72 hours of appropriate antimicrobial therapy, infections with S. aureus, P. aeruginosa,
fungi, or mycobacteria, and tunnel infections, port abscesses has been
recommended. Salvage of the catheter is essential in many children who
need continuous intravenous chemotherapies, antibiotics, and fluids and
for whom it is difficult to create a new peripheral venous access.
However, guidelines specify that for children affected with CRBSI under
“unusual extenuating circumstances," the attempt to salvage the CVC is
allowed. Therefore, the decision to salvage the catheter must be
assessed on a case-by-case basis.[8] There are also reports of successful antibiotic lock therapy in cases of CRBSI caused by the pathogens mentioned above.[1,13] In our study, we also obtained successful results with antibiotic lock therapy in infections caused by S. aureus, Pseudomonas aeruginosa, and Acinetobacter baumanii.
In our study, CoNS, E. coli, and Klebsiella spp.
were the most frequently isolated pathogens. Gram-positive and
gram-negative microorganisms were identified in equal percentages (50%)
of patients receiving ALT. S. epidermidis
and other CoNSs were reported as the most common causative
microorganisms in CRBSI due to skin contamination during catheter
manipulations.[1,2,9,14]
In
a meta-analysis involving adult and pediatric patients with CRBSI,
coadministration of systemic antibiotic therapy and ALT achieved better
outcomes than single systemic antibiotic therapy.[15]
Catheter removal rate was higher (33%) in patients receiving the sole
systemic antibiotic therapy than in those treated with sole ALT
(10%) and the recurrence rates in patients in the ALT group were
significantly lower than those in the non-ALT group (30% vs 20%).[15]
In a pediatric study, Kara et al. reported CoNS as the most common
organism, and the therapeutic success rate of the ALT was 81 percent.[16]
In Lafaurie et al. prospective adult study, continuous vancomycin
lock therapy was administered for 88 patients and intermittent
vancomycin lock therapy for 9 patients due to coagulase-negative
staphylococci infection. This therapy was successful in 44 patients.
They recommended that continuous lock therapy should be preferred to
intermittent therapy because of recurring infection risk.[17]
In the Okur et al. study, teicoplanin lock therapy was administered for
port-related Coagulase-negative Staphylococci in pediatric oncology
patients, and the overall port survival rate was found to be 72.7%. The
author suggested that this therapy is effective and safe for
catheter-related infections caused by methicillin-resistant
coagulase-negative staphylococcus.[18]
In our
study, 42 of 56 catheters of patients who received ALT were reused, and
our ALT success rate was 75 percent. ALT might be given without
systemic antibiotics for patients with multiple positive catheter blood
cultures demonstrating the growth of CoNS and concurrent negative
peripheral blood cultures.[19] ALT was used with
systemic antibiotics in our patients with CoNS infection. The
therapeutic success rate of ALT was 70% for CoNS infection; however,
for gram-negative infections, the ALT was successful in 57 of the
cases. In a retrospective pediatric study, 37 febrile attacks in 28
patients were examined. Gram-negative bacilli were determined to be the
most common causative agent, and the therapeutic success rate of ALT
was 67.6 percent.[20] In that study conducted by Signorino et al., the most common causative gram-negative agent was K. pneumoniae
(n:6), and 3 catheters infected with this agent were saved. In our
study, the most frequently detected gram-negative pathogen was E. coli (n:11), and we successfully eradicated E. coli from 7 contaminated catheters with ALT.
In
our study, the catheter removal rate was 53 percent. In a pediatric
study, Adler et al. reported that catheters were removed in
46% of their patients during 207 CRBSI episodes.[3] Another study reported that removal of the catheters was necessitated in 56.4% of CRBSI episodes.[12] The mortality rate has been reported to range from 1.9% to 11% in children with CRBSI.[2,21,22] In our study, the mortality rate was 7.6 percent.
Our
study's limitations were its retrospective nature and small sample
size. However, the most powerful aspect of this study is that we
included only pediatric patients with hematological malignancies to
ensure the homogeneity of the patient population.
Conclusions
CRBSI
is a significant cause of morbidity and mortality in pediatric patients
with acute leukemia. ALT is a safe strategy and helps prevent
unnecessary catheter removals when administered with systemic therapy
in pediatric patients with acute leukemia. Multi-center randomized
controlled trials are required to support the available data.
References
- Wolf J, Allison KJ, Tang L, Sun Y, Hayden RT, Flynn
PM: No evidence of benefit from antibiotic lock therapy in pediatric
oncology patients with central line-related bloodstream infection:
results of a retrospective matched cohort study and review of the
literature. Pediatric blood & cancer 2014, 61(10):1811-1815. https://doi.org/10.1002/pbc.25101 PMid:24923808
- Adler
A, Yaniv I, Solter E, Freud E, Samra Z, Stein J, Fisher S, Levy I:
Catheter-associated bloodstream infections in pediatric
hematology-oncology patients: factors associated with catheter removal
and recurrence. Journal of pediatric hematology/oncology 2006,
28(1):23-28.
- Sievert
DM, Ricks P, Edwards JR, Schneider A, Patel J, Srinivasan A, Kallen A,
Limbago B, Fridkin S: Antimicrobial-resistant pathogens associated with
healthcare-associated infections: summary of data reported to the
National Healthcare Safety Network at the Centers for Disease Control
and Prevention, 2009-2010. Infection control and hospital epidemiology
2013, 34(1):1-14. https://doi.org/10.1086/668770 PMid:23221186
- Flynn
PM: Diagnosis and management of central venous catheter-related
bloodstream infections in pediatric patients. The Pediatric Infectious
Disease Journal 2009, 28(11):1016-1017. https://doi.org/10.1097/INF.0b013e3181bf7bfc PMid:19859018
- Yacobovich
J, Ben-Ami T, Abdalla T, Tamary H, Goldstein G, Weintraub M, Yaniv I,
Toren A, Kenet G, Revel-Vilk S: Patient and central venous catheter
related risk factors for blood stream infections in children receiving
chemotherapy. Pediatric blood & cancer 2015, 62(3):471-476. https://doi.org/10.1002/pbc.25281 PMid:25327811
- Ceri
H, Olson ME, Stremick C, Read RR, Morck D, Buret A: The Calgary Biofilm
Device: new technology for rapid determination of antibiotic
susceptibilities of bacterial biofilms. Journal of clinical
microbiology 1999, 37(6):1771-1776. https://doi.org/10.1128/JCM.37.6.1771-1776.1999 PMid:10325322 PMCid:PMC84946
- De
Sio L, Jenkner A, Milano GM, Ilari I, Fidani P, Castellano A, Gareri R,
Donfrancesco A: Antibiotic lock with vancomycin and urokinase can
successfully treat colonized central venous catheters in pediatric
cancer patients. The Pediatric Infectious Disease Journal 2004,
23(10):963-965. https://doi.org/10.1097/01.inf.0000141740.82420.e6 PMid:15602201
- Mermel
LA, Allon M, Bouza E, Craven DE, Flynn P, O'Grady NP, Raad, II,
Rijnders BJ, Sherertz RJ, Warren DK: Clinical practice guidelines for
the diagnosis and management of intravascular catheter-related
infection: 2009 Update by the Infectious Diseases Society of America.
Clinical infectious diseases: an official publication of the Infectious
Diseases Society of America 2009, 49(1):1-45. https://doi.org/10.1086/599376 PMid:19489710 PMCid:PMC4039170
- Tsai
HC, Huang LM, Chang LY, Lee PI, Chen JM, Shao PL, Hsueh PR, Sheng WH,
Chang YC, Lu CY: Central venous catheter-associated bloodstream
infections in pediatric hematology-oncology patients and effectiveness
of antimicrobial lock therapy. Journal of microbiology, immunology, and
infection = Wei mian yu gan ran za zhi 2015, 48(6):639-646. https://doi.org/10.1016/j.jmii.2014.07.008 PMid:25311403
- Justo
JA, Bookstaver PB: Antibiotic lock therapy: review of technique and
logistical challenges. Infection and drug resistance 2014, 7:343-363. https://doi.org/10.2147/IDR.S51388 PMid:25548523 PMCid:PMC4271721
- Castagnola
E, Ginocchio F: Rescue therapy of difficult-to-treat indwelling central
venous catheter-related bacteremias in cancer patients: a review for
practical purposes. Expert review of anti-infective therapy 2013,
11(2):179-186. https://doi.org/10.1586/eri.12.160 PMid:23409823
- Ford
WJH, Bundy DG, Oyeku S, Heo M, Saiman L, Rosenberg RE, DeLaMora P,
Rabin B, Zachariah P, Mirhaji P et al: Central Venous Catheter Salvage
in Ambulatory Central Line-Associated Bloodstream Infections.
Pediatrics 2021, 148(6). https://doi.org/10.1542/peds.2020-042069 PMid:34814175
- Buonsenso
D, Salerno G, Sodero G, Mariani F, Pisapia L, Gelormini C, Di Nardo M,
Valentini P, Scoppettuolo G, Biasucci DG: Catheter salvage strategies
in children with central venous catheter-related or -associated
bloodstream infections: a systematic review and meta-analysis. The
Journal of Hospital Infection 2022, 125:1-20. https://doi.org/10.1016/j.jhin.2022.03.010 PMid:35390396
- Celebi
S, Sezgin ME, Cakır D, Baytan B, Demirkaya M, Sevinir B, Bozdemir SE,
Gunes AM, Hacimustafaoglu M: Catheter-associated bloodstream infections
in pediatric hematology-oncology patients. Pediatr Hematol Oncol 2013,
30(3):187-194. https://doi.org/10.3109/08880018.2013.772683 PMid:23458064
- O'Horo
JC, Silva GL, Safdar N: Anti-infective locks for treatment of central
line-associated bloodstream infection: a systematic review and
meta-analysis. American journal of nephrology 2011, 34(5):415-422. https://doi.org/10.1159/000331262 PMid:21934302
- Kara
TT, Özdemir H, Erat T, Yahşi A, Aysev AD, Taçyıldız N, Ünal E, İleri T,
İnce E, Haskoloğlu Ş et al: Is antibiotic lock therapy effective for
the implantable longterm catheter-related bloodstream infections in
children? The Turkish journal of pediatrics 2019, 61(6):895-904. https://doi.org/10.24953/turkjped.2019.06.011 PMid:32134584
- Lafaurie
M, Montlahuc C, Kerneis S, de Lastours V, Abgrall S, Manceron V,
Couzigou C, Chabrol A, de Raigniac A, Lescure X, Longuet P, Lesprit P,
Vanjak D, Lepeule R; Groupe des référents en Infectiologie d'Ile de
France (GRIF). Efficacy of vancomycin lock therapy for totally
implantable venous access port-related infection due to
coagulase-negative staphylococci in 100 patients with cancer. J
Antimicrob Chemother. 2023 May 3;78(5):1253-1258. https://doi.org/10.1093/jac/dkad083 PMid:37014800
- Okur
Acar, Sultan MD*; Tahta, Neryal MD*; Böncüoğlu, Elif MD†; Odaman Al,
Işik MD*; Kiymet, Elif MD†; Gözmen, Salih MD*; Demirağ, Bengü MD*;
Karapinar, Tuba H. MD*; Oymak, Yeşim MD*; Vergin, Canan MD*; Devrim,
İlker MD†. Efficacy of Teicoplanin Lock Therapy in the Treatment of
Port-related Coagulase-negative Staphylococci Bacteremia in Pediatric
Oncology Patients. Journal of Pediatric Hematology/Oncology 45(1):p
e17-e20, January 2023. https://doi.og/10.1097/MPH.0000000000002502 PMid:35700426
- Tural
Kara T, Erat T, Yahşi A, Özdemir H, İleri T, İnce E, Taçyıldız N, Ünal
E, Çiftçi E, İnce E: Bloodstream infections in pediatric
hematology/oncology patients: Six years' experience of a single center
in Turkey. Turkish journal of medical sciences 2019, 49(4):1157-1164. https://doi.org/10.3906/sag-1812-101 PMid:31342734 PMCid:PMC7018311
- Signorino
C, Fusco E, Galli L, Chiappini E: Effectiveness of Antimicrobial Lock
Therapy for the Treatment of Catheter-Related and
Central-Line-Associated Bloodstream Infections in Children: A Single
Center Retrospective Study. Antibiotics (Basel, Switzerland) 2023,
12(5). https://doi.org/10.3390/antibiotics12050800 PMid:37237703 PMCid:PMC10215690
- Eggimann
P, Pittet D: Overview of catheter-related infections with special
emphasis on prevention based on educational programs. Clinical
microbiology and infection : the official publication of the European
Society of Clinical Microbiology and Infectious Diseases 2002,
8(5):295-309. https://doi.org/10.1046/j.1469-0691.2002.00467.x PMid:12047407
- van
de Wetering MD, Poole J, Friedland I, Caron HN: Bacteraemia in a
paediatric oncology unit in South Africa. Medical and pediatric
oncology 2001, 37(6):525-531. https://doi.org/10.1002/mpo.1246 PMid:11745891


