The pathogenesis of DS is not fully understood. Still, ATRA/ATO treatment is thought to cause the production of proinflammatory cytokines and changes in blasts' adhesive behavior as they differentiate into mature myeloid cells, leading to a systemic inflammatory state with increased vascular permeability and endothelial damage.[2]
Common clinical manifestations can include weight gain, hypotension, unexplained fever, pulmonary infiltrates, pleural and pericardial effusion, and acute renal failure.[3] With this report, we present an atypical incipient manifestation of DS, outlining how early recognition and fast initiation of effective treatments are of utmost importance in this rare but potentially fatal complication.
A 39-year-old man with no medical history presented in the emergency department with weakness, fever, and epistaxis. Routine blood tests showed pancytopenia (leukocytes 2.6x109/L, hemoglobin 7.7 g/dL, platelets 21x109/L) and coagulopathy, with a highly increased d-dimer (93109 ng/ml) and elongated prothrombin time (PT ratio 1.43). Blast cells were detected in peripheral blood.
Thus, the patient was admitted to the hematology unit, and ATRA 45 mg/m2/day was promptly started along with transfusion support.[4]
Peripheral blood and bone marrow morphology revealed blast cells with typical hypergranular patterns, Auer rods, and folded, convoluted nuclei. Fluorescent in situ hybridization (FISH) and molecular biology confirmed the suspected diagnosis of APL by detecting chromosomal translocation t(15;17)(q24;q21) and the corresponding PML::RARA fusion transcript, bcr1 subtype.
According to blood count at onset, this case was classified as intermediate risk based on Sanz criteria.[5] Hence, ATO 0.15 mg/kg/day was added to upfront therapy, and methylprednisolone 0.5 mg/kg was introduced to prevent DS, as per local policy and ELN guidelines.[2,6] Moderate leukocytosis developed after treatment initiation and was successfully controlled with low doses of hydroxyurea. After ten days of ATRA/ATO therapy, the patient complained of blurred vision, more emphasized on the left side of the visual field, and bilateral phosphenes. No other symptoms suggestive for DS were present; physical examination showed no indirect signs of fluid overload, and neurological examination was negative for acute events. The coagulation profile was in range, and transfusion support was appropriately given.
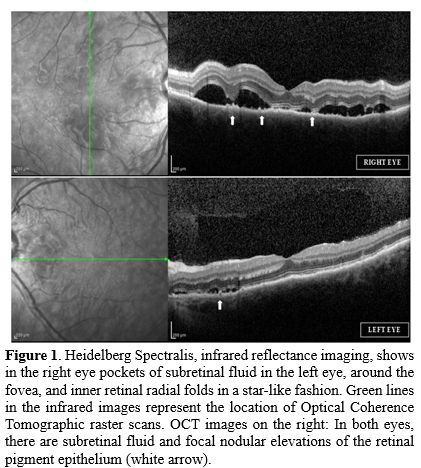
Thus, an ophthalmologist's counseling was required. The best corrected visual acuity (BCVA) in the left eye was 20/20 and 20/25 in the right eye. Pupils were round and reactive, without a relative afferent pupillary defect (RAPD); slit lamp examination was negative for any signs of inflammation of the anterior segment. Fundus examination revealed in both eyes retinal hemorrhages in the peripapillary region, consistent with the underlying coagulopathy, without papillary edema and multifocal areas of serous retinal detachment in the macular region. Optical coherence tomography (OCT) demonstrated pockets of subretinal fluid (SRF) and focal retinal pigment epithelium (RPE) changes with nodular elevations (Figure 1). This exudative retinal detachments' pattern, clinically similar to the cases described by Newman et al.,[8] combined with the other clinical features, was consistent with the diagnosis of DS. Indeed, the inflammatory state and the increased vascular permeability[7] involving the choroidal stroma, choriocapillaris, and RPE can lead to the serous retinal detachment (RPE-Bruch complex’s alteration disrupts the external blood-retina barrier) observed in our case.
This finding was further investigated with brain magnetic resonance, and no signs of edema, hemorrhages, or thrombosis were found.
Since DS was suspected, dexamethasone 10 mg BID was started, and ATRA/ATO treatment was continued. Rapid improvement in visual acuity was obtained, although scotomas persisted.
After seven days of steroid therapy, Enhanced Depth Imaging (EDI) Optical Coherence Tomography demonstrated persistent choroidal thickening, incomplete subretinal fluid reabsorption, and residual flat neuroretinal detachment. Heidelberg Spectralis showed multiple nodular reflectances of the RPE in the macular area at infrared imaging and multifocal hyperautofluorescent spots around the foveal area at autofluorescence imaging (AF). OCT was followed by fluorescein angiography (FA) and indocyanine green angiography (ICGA) (Figure 2A and 2B). In the early phase, FA showed focal pinpoints of leakage in the macular region, corresponding to focal hypofluorescent spots in the late phase of ICGA, which may be due to choriocapillary hypoperfusion or masking effect from inflammatory nodular deposits above the RPE. ICGA did not show stromal choroidal infiltration by a cluster of immune cells, but an angiographic pattern similar to the convalescent phase of MEWDS (multiple evanescent white dot syndrome), a primary inner capillary choroidopathy, self-limiting, considered as an RPE inflammation.
Dexamethasone therapy was then tapered and discontinued. At the end of treatment, visual acuity was completely restored, and bilateral phosphenes, related to retinal hemorrhages, were reduced.
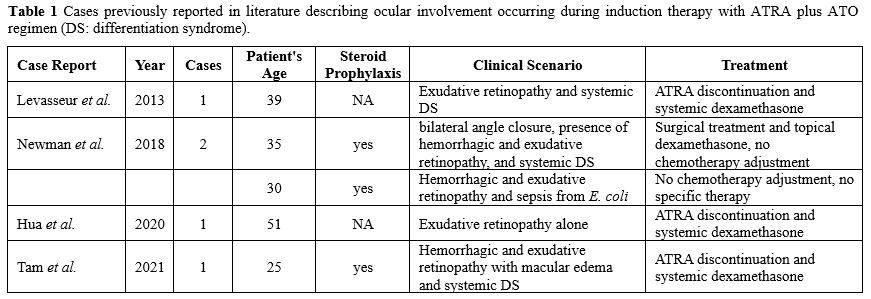
Only a few cases of visual complications characterized by acute vision loss during APL induction have been reported in literature. They all share superimposable visual manifestations and optimal response to steroid IV therapy and are generally accompanied by an entourage of systemic symptoms suggestive of DS.[8-10] Other cases were more consistent with a hemorrhagic retinopathy rather than an exudative DS-like pattern (Table 1).[11]
 |
|
Our patient experienced isolated vision loss during APL induction therapy while on steroid prophylaxis, but common neurological and visual manifestations such as headache and papilledema were absent. Moreover, no other typical signs or symptoms of DS were initially observed nor developed in the subsequent days. Whether this manifestation was a prodromic event of an incipient DS that was successfully avoided or just an isolated manifestation of a mild DS cannot be firmly established. The early shift from methylprednisolone to high-dose dexamethasone interrupted a cascade of events that would have resulted in the full development of the syndrome. Also, methylprednisolone prophylaxis already in place for several days may have mitigated the intensity of the symptoms.
With this report, we emphasize the importance of early recognition of DS when presenting with typical but also when mimicked by atypical clinical manifestations, and the consequent relevance of starting a specific therapy as soon as possible.