Paola Stefanoni1, Laura Paris1, Nicola Pittalis1, Chiara Pavoni1, Giancluca Cavallaro1, Alessandro Rambaldi1,2 and Monica Galli1.
1 Department of Oncology and Hematology, ASST Papa Giovanni XXIII, Bergamo, Italy.
2 Department of Oncology and Hematology, Università degli Studi di Milano, Italy.
Correspondence to: Monica
Galli, MD, PhD. Dept. of Oncology and Hematology, ASST Papa Giovanni
XXIII, p.zza OMS, 1, 24121 Bergamo, Italy. Tel: +39 035 267 4652.
E-mail:
monicagalli@asst-pg23.it
Published: September 01, 2024
Received: June 27, 2024
Accepted: August 05, 2024
Mediterr J Hematol Infect Dis 2024, 16(1): e2024066 DOI
10.4084/MJHID.2024.066
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
To the editor
Most
randomised clinical studies (RCTs) place 65 years as the age limit for
patients with newly diagnosed multiple myeloma (NDMM) to receive
autologous stem cell transplantation (ASCT)-based treatments. There
are, however, patients who, despite exceeding this age, have a degree
of fitness adequate to receive these intensive treatments. Therefore,
we performed a monocentric, retrospective real-world analysis (RWA) to
evaluate the feasibility and the main results of an ASCT-based program
in a cohort of 90 NDMM patients aged > 65 years who were deemed
eligible for such a program on the basis of simplified frailty score.
Seventy-six patients received at least one ASCT, and 14 patients
received the second ASCT. No ASCT-related mortality was observed.
During a median follow-up of 46 months, median progression-free
survival (mPFS) was 42 months and overall survival (mOS) was estimated
at 84 months. These results compare well with those of other RWAs and
pave the way to further improvements upon incorporation of an
anti-CD38+ monoclonal antibody in the ASCT programs.
Based on the results of several RCTs, ASCT still represents a
cornerstone of NDMM patients’ treatment, as stated also by the last
EHA-ESMO guidelines.[1] These trials, in fact, have
shown that the incorporation of ASCT into the first-line treatment
improves the overall response rate (ORR), mPFS and, although not
consistently, the mOS of the patients. However, most RCTs place 65
years as the age limit to receive ASCT-based treatments. There are,
however, patients who, despite exceeding this age, have a degree of
fitness adequate to receive these intensive treatments, which may be
administered, in general, up to the age of 75 years. RWAs are the main
source of information on the prevalence and the main clinical outcomes
of the patients treated outside of RCTs.[2] When
compared to RCTs, RWAs have advantages and disadvantages. Among the
former, the inclusion of studies with heterogenous designs, the
possibility to capture the patients’ heterogeneity in terms of age,
comorbidities and performance status (PS) and information about the
different treatment patterns (i.e., duration, dosing, efficacy,
tolerability and safety profile). Among the latter, there is the
heterogeneity of datasets arising from heterogeneous clinical practice
and documentation, the possibility of patients’ selection biases and
the potential lack of uniformly defined endpoints. Taking all these
issues into account, we performed an RWA to evaluate the prevalence and
clinical outcomes of symptomatic NDMM patients aged > 65-75 years
treated from January 1, 2010, to December 31, 2021, in our Unit with an
ASCT-based program, according to the experimental arm of the
GIMEMA-MMY-3006 study.[3] Their degree of "fitness"
was judged according to a simplified frailty score3 that takes age
(<75 years, score 0), Charlson Comorbidity Index (CCI) (CCI <1,
score 0; CCI >1, score 1) and ECOG Performance Status (PS) (PS 0,
score 0; PS 1, score 1; PS >2, score 2) as variables to discriminate
between nonfrail (score 0-1) and frail (score >2) patients.[4]
Data were collected using an ad hoc specifically developed database,
which was implemented over time. Since we performed a monocentric,
retrospective study on consecutive and uniformed selected patients who
were treated with a uniform program, we could maximise the strengths
and minimise the weaknesses of our RWA. PFS was calculated as the time
from starting treatment to the time of first MM progression or death,
whichever occurred first. OS was calculated as the time from starting
treatment to the date of last contact or death for any cause. PFS and
OS were estimated until the last contact or February 15, 2023, using
the Kaplan-Meier method. All patients provided informed consent. Ninety
patients (24%) were deemed eligible for ASCT-based treatments. They had
a median age of 67.9 years (range 65.2-72.9 years); according to the
simplified frailty score, 79 patients (88%) were non-frail, and 11
(12%) were frail before starting the treatment. Because their frailty
status was a direct consequence of MM-related disabilities, also these
11 patients were enrolled in the ASCT-based program. During the
induction phase, which consisted of 4
bortezomib-thalidomide-dexamethasone (VTD) cycles in 84 patients (93%)
and 4 bortezomib-cyclophosphamide-dexamethasone cycles in the other 6
(7%), the dose of one or more of these drugs was reduced in 82 patients
(91%): bortezomib (n=13), thalidomide (n=71), cyclophosphamide (n=2),
dexamethasone (n=53). The patients’ disposition through the treatment
program is detailed in Figure 1.
 |
- Figure 1. Patients’ disposition.
|
Seventy-eight
patients (87%) underwent the peripheral blood autologous stem cell
mobilisation procedure: 40 of them mobilised with granulocyte-colony
stimulating factor (G-CSF) alone, and the other 38 with
cyclophosphamide 2gr/sm plus G-CSF. Seven patients also required
Plerixafor. A median of 6.77x106 CD34+ cells/kg body weight were
harvested (range 2.56-44.85x106). No harvesting failure was recorded.
Seventy-six patients (84%) received at least one ASCT, following ev
melphalan administration at doses ranging from 100 to 200 mg/sm. Eight
of the 11 frail patients received one ASCT; none of them underwent the
second procedure. Although 48 patients collected enough CD34+ for 2
ASCTs, only 14 of them were in less than very good partial response
(VGPR) 3 months after the first ASCT: for this reason, they also
received the second ASCT, following ev melphalan administration, again
at different doses. All patients were hospitalised for the ASCT
procedures, which were characterised by Grade 3-4 haematological
toxicities in all cases, as expected. During the first ASCT procedure,
37 patients experienced Grade > 2 extra-haematological toxicities:
worsening of peripheral neuropathy (n=18), gastro-intestinal mucositis
(n=11), fever (n=3), cardiac complications (n=2), upper respiratory
infections (n=2), sepsis (n=1). During the second ASCT procedure, 3
patients experienced Grade > 2 extra-haematological toxicities:
peripheral neuropathy worsening (n=2) and gastro-intestinal mucositis
(n=1). No ASCT-related mortality was observed. The choice to omit
cyclophosphamide during the mobilisation phase and/or to reduce the
dose of pre-ASCT melphalan was driven by the patients’ fitness, which
was re-assessed before each phase of the treatment program. Forty-nine
patients received post-ASCT maintenance with the following drugs:
Lenalidomide (n=41) (approved in Italy since 2018), Dexamethasone
(n=5), and Thalidomide (n=3). The prevalence of good-quality MM
responses increased after each step of treatment (Figure 2).
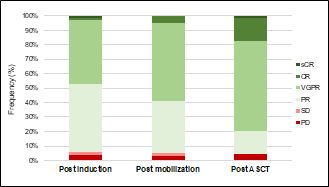 |
- Figure 2. Multiple myeloma responses during the treatment program.9
sCR = stringent complete response; CR = complete response; VGPR = very
good partial response; PR = partial response; SD = stable disease; PD =
progressive disease.
|
During a median follow-up of 46 months (range, 1-156 months), mPFS was 42 months (Figure 3A).
MM relapsed or progressed in 44 out of the 76 patients who received at
least one ASCT: 43 of them started the second line of treatment. As
expected, the number of patients receiving > 3 lines of therapy
approximately halved with each further line. The patients’mOS was
estimated at 84 months (Figure 3B).
At the last follow-up, 55 patients are still alive. Our RWA on NDMM
patients aged > 65 years confirmed that the application of a simple
frailty score allowed us to correctly identify the elderly MM patients
who can benefit from an ASCT-based treatment. In fact, most of our
patients enrolled in such a program, indeed, completed it. Furthermore,
the need to reduce and/or omit some drugs during each phase of
treatment did not compromise the results, which are very close to the
RWA reported by the Australian and New Zealand Myeloma and Related
Diseases Registry in a cohort of 65-70 years-old MM patients treated
with ASCT-based programs, which reported a mPFS of 46.7 months and a
mOS of 76.9 months.[2] Our data must, however, be
critically viewed in light of the results obtained by MM patients
enrolled in non-ASCT-based first-line treatments containing the
anti-CD38 monoclonal antibody daratumumab. In fact, the patients
enrolled in the MAIA study,[5] who received
daratumumab, lenalidomide and dexamethasone cycles until progression or
unacceptable toxicity, showed an mPSF of 61.9 months and have not yet
reached their mOS after a median follow-up of 64.5 months. Similarly,
fit patients enrolled in the ALCYONE study,[6-7] who
received 9 daratumumab, bortezomib, melphalan and dexamethasone cycles,
followed by daratumumab and dexamethasone maintenance until progression
or unacceptable toxicity, experienced an mPSF of 45.7 and a mOS of 82.7
months. Of note, both RCTs and our present study used the same criteria
to define patients' frailty.[4] On the other side, we
cannot exclude the possibility that the incorporation of daratumumab
into ASCT-based treatments – which is possible in Italy from the
beginning of 2022 - will further improve the results obtained in
patients eligible for ASCT-based programs. In fact, the patients
enrolled in the experimental arm of the CASSIOPEIA study,[8]
who received 4 pre-ASCT induction and 2 post-ASCT consolidation cycles
of daratumumab-VTD, reported a 29% rate of stringent complete response
(sCR) and a 39% rate of CR or better at day 100 after ASCT. Their mPFS
from the first randomisation has not been reached. Although this RCT
also enrolled patients up to the age of 65 years, it showed that the
addition of daratumumab did not increase toxicity when compared to the
conventional VTD arm. Therefore, we do not expect a higher rate of
adverse events in an elderly fit MM population.
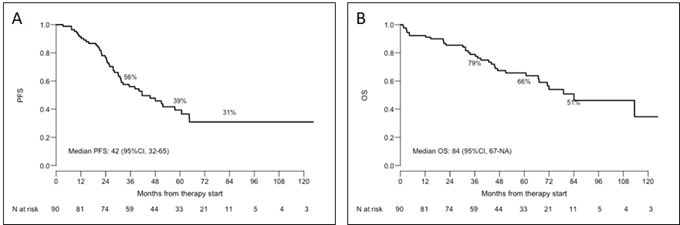 |
- Figure 3. Outcomes of the 90 patients. A) Progression-Free Survival (PFS); B) Overall Survival (OS).
|
Conclusions
The
dynamic evaluation of the degree of fitness helps to tailor the most
appropriate treatment of elderly NDMM patients, paving the way for
further improvements in their outcomes upon incorporation of an
anti-CD38+ monoclonal antibody in the ASCT programs.
References
- Dimopoulos
MA, Moreau P, Terpos E et al. Multiple myeloma: EHA-ESMO clinical
practice guidelines for diagnosis, treatment and follow-up. Ann Oncol
2021;32:309-322. https://doi.org/10.1016/j.annonc.2020.11.014 PMid:33549387
- Bergin
K, Wellard C, Augustson B et al. Real-world utilisation of ASCT in
multiple myeloma (MM): a report from the Australian and New Zealand
myeloma and related disease registry (MRDR). Bone Marrow Transplant
2021;56:2533-2543. https://doi.org/10.1038/s41409-021-01308-8 PMid:34011965
- Cavo
M, Tacchetti P, Patriarca F et al. Bortezomib with thalidomide plus
dexamethasone compared with thalidomide plus dexamethasone as induction
therapy before, and consolidation therapy after, double autologous
stem-cell transplantation in newly diagnosed multiple myeloma: a
randomised phase 3 study. Lancet 2010;376:2075-85. https://doi.org/10.1016/S0140-6736(10)61424-9 PMid:21146205
- Facon
T, Dimopoulos MA, Meulman N et al. A simplified frailty scale predicts
outcomes in transplant-ineligible patients with newly diagnosed
multiple myeloma treated in the FIRST (MM-20) trial. Leukemia
2020;34:224-233. https://doi.org/10.1038/s41375-019-0539-0 PMid:31427722 PMCid:PMC7214253
- Kumar
S, Moreau P, Bahlis BJ et al. Daratumumab plus lenalidomide and
dexamethasone (D-Rd) vs lenalidomide and dexamethasone (Rd) alone in
transplant-ineligible patients with newly diagnosed multiple myeloma
(NDMM): updated analysis of the phase 3 MAIA study. Blood 2022;140
(suppl.1):10150-10153. https://doi.org/10.1182/blood-2022-163335
- Mateos
MV, Dimopoulos M, Cavo M et al. Daratumumab plus bortezomib, melphalan,
and prednisone versus bortezomib, melphalan, and prednisone in
transplant-ineligible newly diagnosed multiple myeloma: frailty
subgroup analysis of ALCYONE. Clin Lymph Myel, Leuk 2021;21:785-791. https://doi.org/10.1016/j.clml.2021.06.005 PMid:34344638
- Mateos
MV, San-Miguel J, Cavo M et al. Daratumumab plus bortezomib, melphalan,
and prednisone (D-VMP) versus bortezomib, melphalan, and prednisone
(VMP) alone in transplant-ineligible patients with newly diagnosed
multiple myeloma (NDMM): updated analysis of the phase 3 ALCYONE study.
Blood 2022;140 (suppl.1):10157-10159. https://doi.org/10.1182/blood-2022-163347
- Moreau
P, Attal M, Hulin C et al. Bortezomib, thalidomide, and dexamethasone
with or without daratumumab before and after autologous stem-cell
transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a
randomised, open-label, phase 3 study. Lancet 2019;394:29-38.https://doi.org/10.1016/S0140-6736(19)31240-1 PMid:31171419
- Durie
BG, Harousseau JL, Miguel JS et al. International uniform response
criteria for multiple myeloma. Leukemia 2006;20:1467-73. https://doi.org/10.1038/sj.leu.2404284 PMid:16855634


