Ugo
Testa1, Elvira Pelosi1, Germana
Castelli1 and Patrizia Chiusolo2,3.
1
Istituto Superiore di Sanità,
2 Section of Hematology, Department of
Radiological and Hematological Sciences, Catholic University, Rome,
Italy.
3 Department of Laboratory and Hematological
Sciences, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome,
Italy
Published: September 01, 2024
Received: August 07, 2024
Accepted: August 12, 2024
Mediterr J Hematol Infect Dis 2024, 16(1): e2024070 DOI
10.4084/MJHID.2024.070
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Blinatumomab, a
CD19-CD3 bispecific T cell engager (BiTE), has two recombinant
single-chain variable fragments that temporarily link CD3+ T cells and
CD19+ B cells, leading to the T cell-mediated lysis of neoplastic B
cells. Improved minimal residual disease (MRD)-negative response rates
and long-term overall survival have been observed in B-ALL patients who
received this drug. These therapeutic successes have led to FDA
approval for refractory/relapsed and MRD-positive B-ALL patients.
Furthermore, recent studies in newly diagnosed B-ALL patients have led
in Philadelphia chromosome-positive patients to the development of
chemotherapy-free regimens based on tyrosine kinase inhibitors plus
Blinatumomab and in Philadelphia chromosome-negative patients to
improvement in outcomes using chemotherapy regimens that have
incorporated Blinatumomab in the consolidation phase of treatment.
|
Introduction
The development
of bispecific antibodies (bsAbs) has represented an area of
considerable interest in the past decade, related to their unique
properties compared to traditional monospecific monoclonal
antibodies.[1-2] Thus, a consistent number of bsAbs has been approved
for cancer therapy, thus showing the rapid evolution of these
antibodies as a novel category of therapeutic agents.[1-2]
T cell engagers (TCEs) are bsAbs that specifically bind to a tumor cell
surface antigen and to the CD3 chain of the TCR and have the property
of specifically activating an immune response T-cell mediated at the
level of sites of tumor development.[1,2] Blinatumomab, an example of
next-generation TCE, requires simultaneous spatial binding of the
targets: CD19 on the surface of leukemic cells and CD3 on the surface
of T cells.[1,2]
It is designed to lack an Fc portion, composed of the tandem scFv-based
CD19xCD3 antibody, which was first approved in 2014 for the treatment
of relapsed/refractory B-ALL and which is giving growing contributions
to the treatment of these leukemic patients in various clinical
settings.[3]
Blinatumomab in relapsed/refractory
B-ALL
Blinatumomab
was approved for use in patients with relapsed/refractory B-ALL based
on single-group trials showing enhanced efficacy and acceptable safety
profile. A large phase III trial explored a consistent group of 376
adult Ph- R/R B-ALL patients randomly assigned to treatment with
Blinatumomab or chemotherapy. Blinatumomab significantly improved OS
with respect to chemotherapy (7.7 months vs 4.0 months, respectively)
and event-free survival, as well as median duration of remission[4] (Table 1).
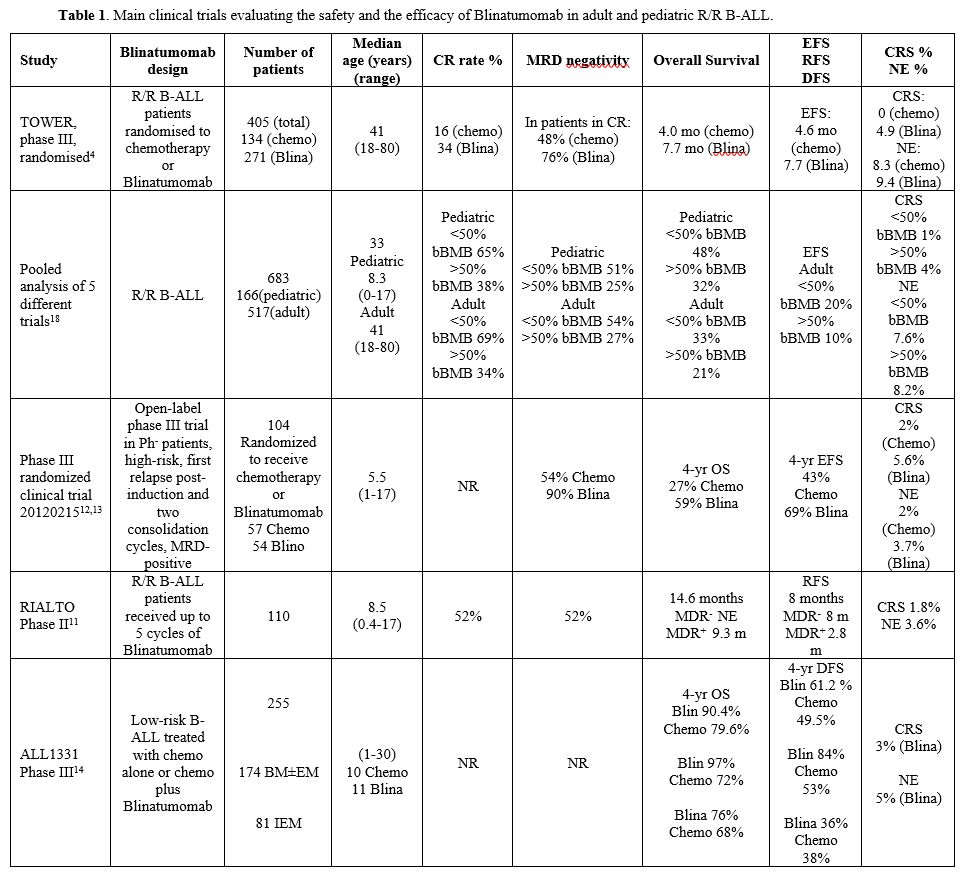 |
- Table
1. Main clinical trials evaluating the safety and the efficacy of Blinatumomab in adult and pediatric R/R B-ALL.
|
In another
study, Gokbuget et al. evaluated the prognostic impact of MRD status
after Blinatumomab treatme
The analysis of long-term survival of a large group of R/R,
Philadelphia-negative, B-ALL patients enrolled in two phase II studies
involving the treatment with Blinatumomab showed a mOS of 7.5 months;
importantly, both OS and RFS plateaued with 3-year.[5] For patients who
achieved a CR with Blinatumomab, followed by allogeneic HSCT while in
remission, the mOS was 18.1 months.[5] About 17% of R/R B-ALL patients
treated with Blinatumomab survived >36 months, including 55% of
patients who underwent aHSCT and 45% without transplantation. The
retrospective analysis of 532 R/R B-ALL patients treated with
Blinatumomab who received this drug as first salvage had a longer mOS
and RFS and higher rates of remission, MRD response, and aHSCT in
continuous remission compared to those who received Blinatumomab as
second or later salvage.[6]
The retrospective observational study (NEUF) explored the safety and
efficacy of Blinatumomab in R/R patients evaluated in the context of
real-world use of this drug in current clinical practice; 140 R/R B-ALL
patients were evaluated (106 Ph- and 34 Ph+).[6] This real-world data
set of adult R/R B-ALL patients treated with Blinatumomab confirms the
efficacy outcomes observed in randomized clinical trials.[7]
A phase I clinical study evaluated the safety and efficacy of
Blinatumomab in combination with PD1 and CTLA4 inhibitors in R/R B-ALL
patients [8]. Among 22 evaluable patients, the CR was 68%, and all
achieved MRD negativity: at 1 year, the RFS was 27%, and the OS was
63%.[8] These observations showed that combination therapy with
Blinatumomab and immune checkpoint inhibitors (ICIs) in R/R B-ALL
patients was safe and was associated with a high rate of MRD-negative
responses. However, these results are only preliminary and required to
be confirmed.
An open-label, single-arm, phase II, multicentre ALCANTARA study
explored the response of 45 B-ALL Ph+ patients who had relapsed or were
refractory to at least one TKI to Blinatumomab.[9] 16/45 patients
achieved a CR within the first two cycles of Blinatumomab therapy; mOS
was longer in patients who achieved a CR than in those without CR;
14/16 patients in CR achieved complete MRD response; the median
duration of complete MRD response was 9.7 months.[9]
The efficacy of Blinatumomab retreatment after relapse was evaluated.
Thus, Topp et al. evaluated 11 B-ALL patients who received Blinatumomab
retreatment after initial response and relapse. 4/11 patients responded
to the retreatment with a mOS of 9.4 months.[10] Grade ≥3 neurologic
events were observed in 3 patients.[10] These observations suggest that
Blinatumomab retreatment may represent a reasonable treatment for
relapse in patients who have responded initially to
Blinatumomab.
In the pediatric setting, Locatelli and coworkers reported the results
observed on 110 R/R pediatric B-ALL patients treated with 5 cycles of
Blinatumomab, showing a good safety profile with a low incidence of
grade 3 or 4 of cytokine release syndrome and adverse neurologic
events; a response rate not affected by the presence of adverse
cytogenetic/molecular abnormalities; mOS was significantly better for
patients achieving a CR with MRD negative status compared to those who
remained MRD-positive (not estimable vs 9.3 months, respectively); the
1-year probability was significantly better for patients who received
aHSCT after Blinatumomab compared to those without aHSCT.[11]
In a phase III randomized clinical trial, pediatric high-risk,
first-relapse B-ALL patients received Blinatumomab as consolidation
therapy, administered before allo-HSCT, resulting in improved EFS and
MRD remission rate compared to chemotherapy, with EFS benefit being
observed in all subgroups of patients, including those with
extramedullary disease and very early relapse (<18 months).[12]
A longer follow-up of these patients showed a markedly better OS among
patients treated with Blinatumomab compared to chemotherapy,
independently of the MRD status before treatment.[13]
The Children's Oncology Group ALL 1331 phase III trial compared the
survival of patients with low-risk first-relapse of B-ALL treated with
chemotherapy alone or chemotherapy plus Blinatumomab.[14] For children,
adolescents, and young adults with B-ALL in first relapse, there was no
statistically significant difference in DFS and OS between the
Blinatumomab and standard chemotherapy arms in an analysis performed
considering the whole population of patients enrolled in the study.
However, when the analysis was restricted to patients relapsing either
at the level of bone marrow with or without extramedullary disease, a
significant improvement in mOS was observed in the group of patients
treated with Blinatumomab compared to chemotherapy alone (4-year OS
rate 97.1% vs 84.8%, respectively).[14]
Inotozumab ozogamicin is an antibody anti-CD22-drug conjugate approved
for the treatment of R/R B-ALL. A recent study showed that Inotuzumab,
as well as Blinotumomab, may be used for clearing MRD in patients with
B-ALL in remission after induction chemotherapy.[15] However, a part of
patients treated with Inotuzumab or with Blinatumomab relapsed, and
there is a rationale to treat these patients with both these two
antibodies. Concerning R/R B-ALL patients, Fracchiolla and coworkers
reported the study of 71 patients treated for different relapses with
Blinatumomab and Inotuzumab; Blinatumomab represented the first
treatment for 54 patients and Inotuzumab for 14 patients.[16] In the
Blinatumomab/Inotuzumab group, after Blinatumomab, 65% of patients
achieved a CR, with 42% of MRD negativity; in the
Inotuzumab/Blinatumomab after Inotuzumab, 93% achieved a CR, with 46%
of MRD negativity.[16]
A recent study by Jabbour et al. demonstrated that subcutaneous
Blinatumomab displayed an efficacy comparable to that observed in
studies involving intravenous Blinatumomab administration.[17] In this
study, 29 R/R B-ALL patients were treated with two different schedules
of subcutaneous Blinatumomab: using two cycles of subcutaneous
Blinatumomab at 250-500 ug dose, 85% of patients achieved a CR,
including 75% with MRD-negativity; Blinatumomab at 500-1000 ug dose,
92%% of patients achieved a CR, including 100% with MRD-negativity.[17]
No treatment-related grade 4 CRS or neurologic events were reported.
In the study from Queudeville et al., through the analysis of five
different trials involving the treatment of both adult and pediatric
R/R B-ALL patients, patients were subdivided into two groups according
to the number of bone marrow leukemic blasts (<50% and
>50%). The proportion of patients achieving MRD negativity was
significantly higher in patients with baseline lower tumor burden
(<50% bBMB). OS and RFS were also significantly higher among
patients with baseline lower leukemic burden[18] (Table 1). Adverse
events related to grade 3 or more CRS are more frequent among patients
with higher tumor burden (Table
1). In conclusion, a high leukemia burden before therapy
limits the efficacy of Blinatumomab and lowering leukemic blast levels
by <50% before starting Blinatumomab therapy is required to
improve its efficacy.
The treatment of patients who are resistant or relapse after
Blinatumomab therapy is a great challenge. One possible salvage therapy
for these patients is represented by anti-CD19 CAR-T cells. Several
studies have explored the sensitivity of these refractory/relapsing
patients to CD19 CAR-T cells. An initial study by Pillai et al. based
on the retrospective analysis of 166 patients who have undergone
CD19-directed CAR-T cell therapy showed that prior therapy with
Blinatumumab observed in a part of these patients, was associated with
a higher rate of failure to achieve MRD negativity or subsequent
relapse with antigen escape.[19] A subgroup analysis performed in the
context of the phase II ZUMA-3 trial involving the treatment of R/R
B-ALL patients with CD19 CAR-T cells (Brexucabtagene autocel) showed in
patients, who had prior Blinatumomab treatment, an overall CR of 60%
compared to 80% observed in the patients without prior Blinatumomab.[20]
Myers and coworkers have performed a retrospective study on 420
pediatric B-ALL patients who received CD19-CART cells (mostly
Tisagenglecleucel for R/R B-ALL); CR rates, EFS, RFS and OS, were
comparable in blinatumomab-naïve (BLN) and blinatumomab-exposed
patients who responded to Blinatumomab (BLR), thus indicating that
Blinatumomab treatment does not preclude a response to CD19-CAR-T;
however, CR, RFS, EFS and OS were reduced in blinatumomab-exposed
patients who did not respond or did not achieve a CR following
Blinatumomab treatment (BLNR).[21] An additional exploration of these
patients showed that prior Blinatumomab nonresponse was associated with
an increased frequency of CD19-negative relapses after CD19 CAR-T cell
therapy.[22]
Gupta et al. have explored 157 R/R B-ALL adult patients
treated with autologous CD19-directed Brexu-Cel (CAR-T cells); 88 of
them received Blinatumomab prior to CAR-T cell therapy: 70% of these
patients initially responded to Blinatumomab but then relapsed (BLR)
and 30% did not respond to Blinatumomab (BLNR); the rest of patients
was Blinatumomab-naïve (BLNV).[23] Rates of CR to CAR-T cell therapy
were similar following therapy among BLR, BLNR and BLNV patients [23].
However, the 1-year OS was significantly better in BLNV and BLR
compared to BLNR.[23] Furthermore, PFS was significantly higher in the
BLNV group compared to BLR and BLNR.[23]
These observations, which need to be confirmed in larger, prospective
clinical trials, support the conclusion that (i) CD19-targeted CAR-T
cells represent an effective therapy for patients relapsing after an
initial response to Blinatumomab; B-ALL patients who did not respond to
Blinatumomab display shorter overall survival following CD19 CAR-T cell
therapy compared to those who responded or did not receive
Blinatumomab.
The possible therapeutic options for R/R B-ALL patients involving
Blinatumomab are shown in Figure
1.
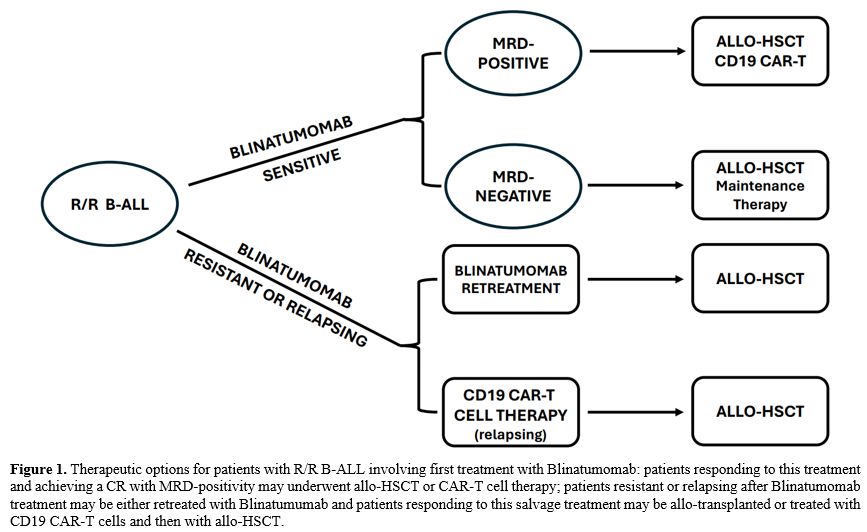 |
- Figure
1. Therapeutic options for patients with R/R B-ALL involving first
treatment with Blinatumomab: patients responding to this treatment and
achieving a CR with MRD-positivity may underwent allo-HSCT or CAR-T
cell therapy; patients resistant or relapsing after Blinatumomab
treatment may be either retreated with Blinatumumab and patients
responding to this salvage treatment may be allo-transplanted or
treated with CD19 CAR-T cells and then with allo-HSCT.
|
Blinatumomab for B-ALL in remission
MDR-positive
Monitoring
measurable residual disease (MRD) is a standardized and universally
accepted method for measuring disease status in B-ALL patients, and it
has become part of diagnostic patient care. MRD is a key independent
predictor of the risk of relapse and long-term survival in both
pediatric and adult B-ALL.[24] For virtually all B-ALL patients, it is
possible to evaluate MRD, either using multi-colour flow cytometry
(MCFC), quantitative polymerase chain reaction (PCR) to detect
immunoglobulin gene rearrangements or specific fusion transcripts, and
more recently, next-generation sequencing (NGS).[24] NGS is associated
with high sensitivity and allows the detection of very low MRD levels
(<10-4).[24]
A first pilot study from the GMALL group evaluated whether Blinatumomab
monotherapy could improve leukaemia-free survival in B-ALL patients
with MRD persistence after induction and consolidation therapy.[24] 20%
of patients were enrolled in the study, and 80% of them displayed a
conversion from MRD positivity to MRD negativity.[20] In most patients,
MRD negativity was achieved after 1 cycle of Blinatumomab. The
probability of relapse-free survival was 78% at a median follow-up of
405 days.[25] In a single-arm study, after treatment with Blinatumomab
in a population of 116 adult B-ALL patients predominantly Ph-negative,
with MRD-positive (≥10-3) disease, the median OS and RFS were
significantly longer among patients achieving a complete MRD response,
compared to those who remained MRD-positive[26] (Table 2) After a
longer follow-up, mOS in all patients was 36.5 months, and it was not
reached in MDR-negative patients, compared to 16.5 months in
MDR-positive patients.[27] The 5-year survival showed a 43% survival
for the whole population of patients and 50% for those achieving MRD
negativity. Future studies will be required to identify patients who
may benefit from Blinatumomab without HSCT, including older patients
and those without a related or matched donor, and to identify
therapeutic strategies that could improve outcomes further.
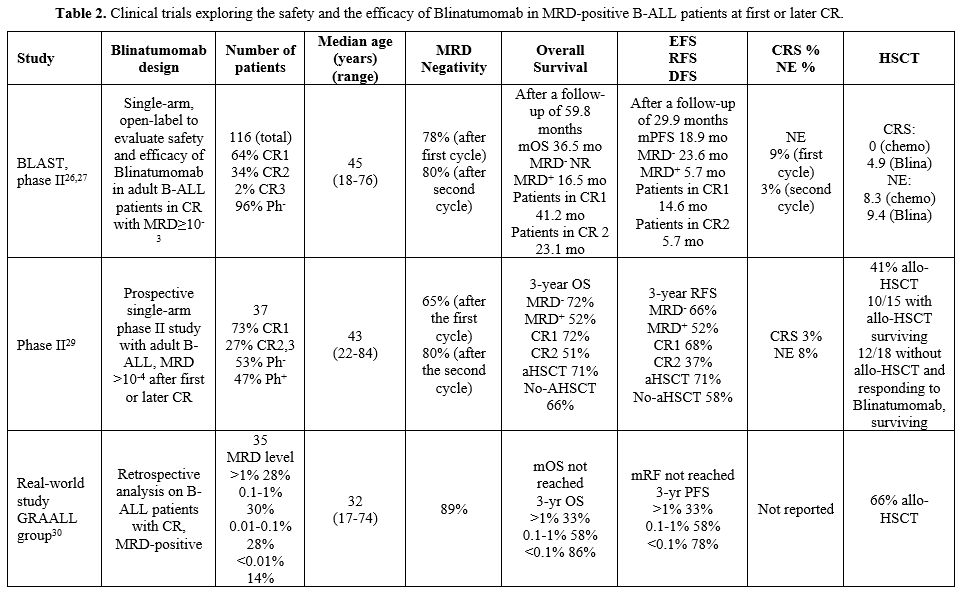 |
- Table 2. Clinical
trials exploring the safety and the efficacy of Blinatumomab in
MRD-positive B-ALL patients at first or later CR.
|
In another
study, Gokbuget et al. evaluated the prognostic impact of MRD status
after Blinatumomab treatment in 90 R/R B-ALL patients achieving a CR:
patients with a CR and MRD negative status displayed a significantly
longer OS and RFS compared to those with MRD positivity.[28] This study
supported the predictive value of MRD evaluation in B-ALL patients
treated with Blinatumomab.
In a phase II study, Jabbour et al. evaluated 37 B-ALL patients (27 in
first complete remission and 10 in second complete remission) in CR
with MRD-positive status (≥10-4) and then treated with Blinatumomab:
73% of these patients achieved an MRD-negative remission.[29]
Importantly, the 3-year RFS and OS rates were 51% and 61% in patients
with baseline MRD≥10-3 and 83% and 77% in patients with baseline
MRD<10-3[29] (Table
2).
Based on these studies, the FDA granted accelerated approval and final
approval for Blinatumomab in 2018 and 2023 for the treatment of adults
and children with B-ALL in first or second complete remission with MRD
greater than or equal to 0.1%.
Cabanes-Hamy et al. retrospectively evaluated 73 patients who received
treatment with Blinatumomab either in the first CR with MRD positivity
or at relapse; high pre-Blinatumomab MDR levels were associated with
shorter RFS and OS.[30] In relapsed patients, those who directly
received Blinatumomab had shorter RFS and OS than patients bridged to
Blinatumumab after chemotherapy treatment.[30]
Two other retrospective studies have further supported the efficacy of
Blinatumomab in MRD-positive B-ALL patients. In the NEUF retrospective
observational study, 109 adult MRD-positive B-ALL patients were
included (83 Ph- and 26 Ph+); in this group of patients, within the
first cycle of Blinatumomab treatment, 93% of Ph- and 64% of Ph+
patients achieved an MRD response (MRD<0.01%).[7]
A recent real-world study reported the outcome of adult patients who
received Blinatumomab in first or second complete remission.[26]
Patients in CR1 received Blinatumomab mostly for MRD persistence or for
the inability to receive standard consolidation therapy. A complete MRD
response was achieved after one Blinatumomab cycle in 83% of CR1 and
86% of CR2; after a median follow-up of 3.1 years, the 3-yr cumulative
incidence of relapse was 23% for CR1 and 26% for CR2.[31]
Blinatumomab in
B-ALL patients in remission MRD-negative
Patients
with newly diagnosed B-ALL frequently relapse even when achieving
complete remission and MRD negativity after chemotherapy treatment.
Litzow et al. have explored a group of 220 B-ALL patients achieving CR
with MRD negativity (defined as MFC-MRD <0.01%) after induction
chemotherapy; these patients were randomized either to receive
consolidation therapy based on chemotherapy alone or chemotherapy plus
Blinatumomab.[32] Patients undergoing consolidation therapy plus
Blinatumomab displayed a mOS significantly lower than those treated
with chemotherapy alone.[32] The benefit deriving from Blinatumomab
administration was more pronounced in patients <55 years, and
the improvement of OS induced by Blinatumomab was observed both in the
group of patients MRD-negative and those with MRD levels between
undetectable and 0.01%.[28] The RFS in MRD-negative patients favored
the Blinatumomab arm vs the chemotherapy arm.[33] Another subgroup
analysis of these patients showed that the OS of patients who received
1-2 cycles of Blinatumomab displayed no significant difference compared
with the controls.[34]
Gu and coworkers have explored the effectiveness of Blinatumomab in
clearing NGS-measurable MRD in pediatric B-ALL patients.[35] To this
end, 19 B-ALL pediatric patients, bearing at least one
unfavorable genetic abnormality, such as KMT2A rearrangement, in
hematological CR with MRD <10-4 after induction or consolidation
chemotherapy; however, all these patients were identified as
MRD-positive by NGS.[35] After Blinatumomab treatment, MRD negativity
by MFC was 95%, and the NGS-MRD negativity rate at 10-6 was 68%.[37]
The possible therapeutic options involving Blinatumomab for patients
achieving a CR after induction chemotherapy with either MRD-positive or
MRD-negative condition are shown in Figure
2.
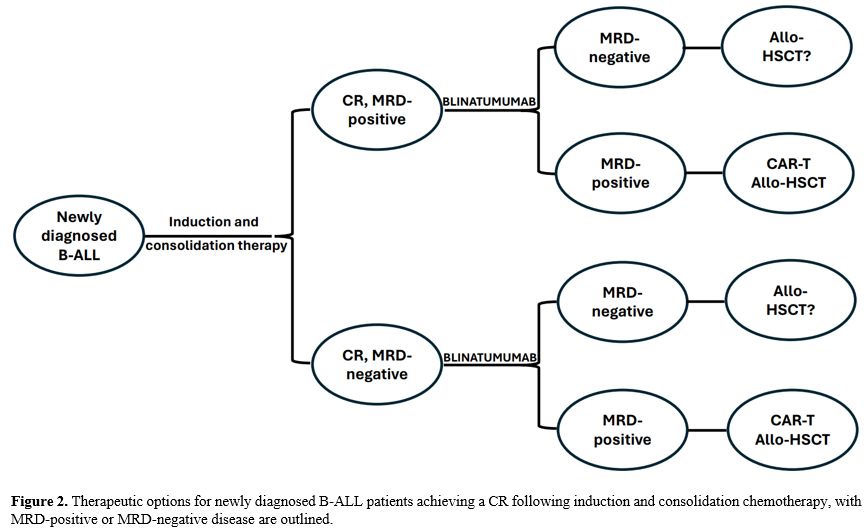 |
- Figure
2. Therapeutic options for newly diagnosed B-ALL patients achieving a
CR following induction and consolidation chemotherapy, with
MRD-positive or MRD-negative disease are outlined.
|
Blinatumomab
and hematopoietic stem cell transplantation
Allogeneic
hematopoietic stem cell transplantation (a-HSCT) represents a
potentially curative approach for B-ALL patients, as well as for other
hematologic malignancies. However, a significant proportion of B-ALL
patients relapses after a-HSCT. These relapsing patients have poor
prognosis and some studies have evaluated their response to
Blinatumomab. Stein et al. evaluated the response of 64 B-ALL patients
who relapsed after aHSCT and investigated the safety and efficacy of
Blinatumomab.[36] 45% of these patients achieved a CR within two cycles
of Blinatumomab treatment and 30% with a complete MRD response.[33]
These observations have supported Blinatumomab as a valuable salvage
therapy in relapsing B-ALL patients after aHSCT.[36]
Gaballa and coworkers have reported the result of a single center phase
II study evaluating the administration of Blinatumomab during the first
year after a-HSCT, with the main aim to mitigate relapse in high-risk
B-ALL patients.[39] A comparison with a contemporary group of 57 B-ALL
patients suggests no benefit from Blinatumomab administration.[39]
Correlative studies suggested the classification of patients into
responders and non-responders according to specific T-cell
profiles.[37] It is important to note that in this study, B-ALL
patients remained on immunosuppression during Blinatumomab
treatment.[37] A more recent phase Ib/II trial evaluated the
tolerability and the efficacy of Blinatumomab as post-aHSCT remission
maintenance in B-ALL (19 patients) and NHL patients off
immunosuppression.[38] The results showed that post-aHSCT maintenance
therapy is feasible with minimal toxicity in patients off
immunosuppression with 18% of relapses, all occurring at the level of
the central nervous system.[38]
Chauvet et al. evaluated 72 B-ALL patients relapsing after aHSCT: 50
patients received Blinatumomab alone, and 22 patients Blinatumomab plus
donor lymphocyte infusion (DFI).[39] Two-year OS was not significantly
different between these two groups of patients; PFS and adverse events
were similar in the two groups of patients.[39] The observations
suggest that the DLI with Blinatumomab administration to B-ALL patients
relapsing after aHSCT is safe but does not seem to improve outcomes.
Other studies have evaluated the administration of Blinatumomab
pre-transplantation. There is a strong rationale for using Blinatumomab
before aHSCT as a tool to clear MRD. In fact, residual MRD before HSCT
is predictive of recurrence and thus, achieving MRD negativity before
HSCT is a key strategy to improve and optimize the curative capacity of
transplantation. Two randomized, phase III trials have shown the
superiority of Blinatumomab compared to conventional chemotherapy as a
consolidation treatment before aHSCT in pediatric patients with
high-risk or intermediate-risk B-ALL, showing an improved disease-free
survival, lower incidence of disease relapse post-HSCT and
significantly reduced toxicity.[12,40] Furthermore, a post-hoc analysis
of the study of Locatelli et al.[12] showed that a higher proportion of
patients with high-risk first-relapse B-ALL with MRD positivity at the
time of randomization achieved an MRD negative status after treatment
with Blinatumomab compared with patients treated with intensive
chemotherapy.[43] OS showed a strong benefit with Blinatumomab vs
chemotherapy. Furthermore, Blinatumomab also improved outcomes for
patients who had already achieved an MRD-negative condition prior to
randomization.[41]
Sayyed and coworkers have explored 177 adult B-ALL patients undergoing
aHSCT: 26.5% of these patients received Blinatumomab before HSCT, while
the rest of the patients received chemotherapy.[42] Pretransplant
Blinatumomab has been associated with improved OS and lower risk of
non-relapse mortality in B-ALL patients undergoing HSCT, seemingly
reflecting a lower burden of treatment-related toxicity in the
Blinatumomab-treated population.[42] Multivariate analysis confirmed
the association between pretransplant Blinatumomab and improved OS and
NRM.[44] A similar study was performed on pediatric B-ALLs.[43]
Blinatumomab is Used in the Frontline Treatment of Philadelphia-Positive B-ALL
Several
recent studies have shown that it is possible to replace the
chemotherapy backbone with Blinatumomab in association with TKIs.
In
the LAL 0216 (D-ALBA) trial, the GIMEMA group explored the safety and
efficacy of a chemotherapy-free regimen based on Blinatumomab plus
Dasatinib in newly diagnosed Ph+ B-ALL patients (Table 3 and Figure 3).[46]
In this study, 63 newly diagnosed B-ALL patients were treated first
with an induction therapy based on Dasatinib plus glucocorticoids and
then with two to five cycles of Blinatumomab and Dasatinib and 12 doses
of intrathecal chemotherapy.[44] In an initial report of this study, at a median follow-up of 18 months, OS was 95% and DFS 88%.[44]
Patients with IKZF1 deletion plus additional genetic abnormalities
displayed a lower rate of DFS compared to patients without these
genetic aberrations.[44] A recent update of the study reported the long-term results of this study with a median follow-up of 53 months.[45]
After induction therapy, there was a difference in DFS and OS between
patients with MRD-positivity and MDR-negativity. However, after two
cycles of Blinatumomab, no significant differences in DFS and OS
between molecular and nonmolecular responders were observed, thus
suggesting that Blinatumomab is effective in preventing a relapse also
in patients with MRD-positive disease after induction therapy.[47] Patients with MRD-positivity received an aHSCT.[45]
These observations support the capacity of a chemotherapy-free regimen
based on Dasatinib and Blinatumomab to induce durable long-term
hematologic and molecular responses in adult Ph+ B-ALL patients.[45]
Advani and coworkers have reported the results of a clinical trial carried out in 24 older (65 years of age or older) Ph+
B-ALL with newly diagnosed disease or R/R disease, treated with
induction therapy based on Dasatinib/Prednisone: patients achieving a
CR continued this treatment up to day 84, while those not achieving a
CR after day 56 attempted a re-induction treatment plus one cycle of
Blinatumomab, followed by 3 cycles of post-remission therapy based on
Blinatumomab and Dasatinib and maintenance therapy always based these
two drugs (Table 3).[46] This study showed that this therapeutic regimen was safe and feasible.[46]
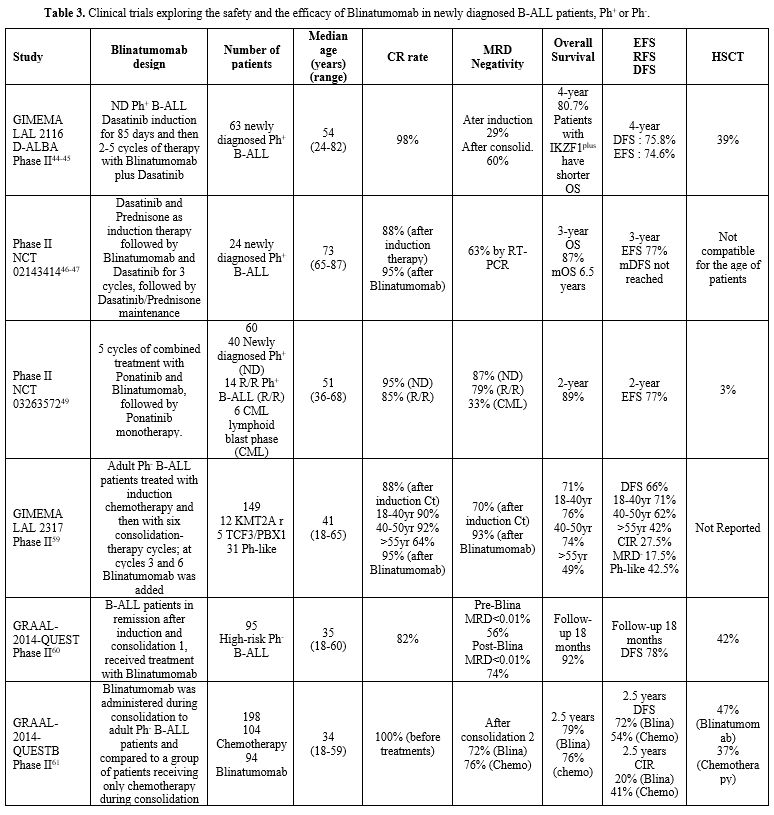 |
- Table 3. Clinical trials exploring the safety and the efficacy of Blinatumomab in newly diagnosed B-ALL patients, Ph+ or Ph-.
|
A phase II study designed Blinatumomab as a chemotherapy-sparing strategy in patients with Ph+
B-ALL (BLISSPHALL). For patients in CR, Blinatumomab was used as early
as 6 weeks into treatment, with the aim of accelerating MRD clearance
and suppressing resistant clones early in the disease course. A
maintenance phase based on Blinatumomab plus Dasatinib was included for
patients in molecular complete response (CMR).[48] A
strategy to suppress T315I clones and reduce their recurrence in the
CNS could be achieved by combining third-generation TKIs or
chemotherapy with Blinatumomab.
A single-centre, single-arm,
phase II study enrolled 60 B-ALL patients with newly diagnosed or R/R
B-ALL or chronic myeloid leukemia in the lymphoid blast phase and
received treatment with the combined administration of Ponatinib and
Blinatumomab for up to five cycles of treatment.[49] (Table 3 and Figure 3)
With a median follow-up of 16 months, 87% of patients with newly
diagnosed B-ALL had a complete molecular response, and 79% of patients
with R/R Ph+ B-ALL had a complete molecular response.[49]
In an updated analysis, 62 patients with newly diagnosed B-ALL were
included, of whom 55 patients were available for molecular response;
84% achieved a complete molecular response; the 2-year OS was 89%, and
EFS was 77%.[50]
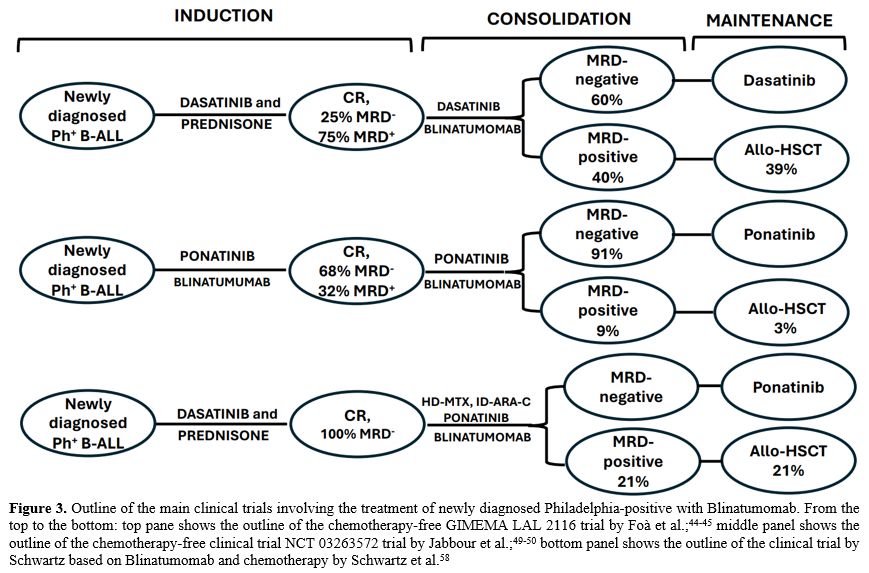 |
- Figure 3. Outline of
the main clinical trials involving the treatment of newly diagnosed
Philadelphia-positive with Blinatumomab. From the top to the bottom:
top pane shows the outline of the chemotherapy-free GIMEMA LAL 2116
trial by Foà et al.;[44-45] middle panel shows the outline of the chemotherapy-free clinical trial NCT 03263572 trial by Jabbour et al.;[49-50] bottom panel shows the outline of the clinical trial by Schwartz based on Blinatumomab and chemotherapy by Schwartz et al.[58]
|
In
the GIMEMA ALL2820, whose preliminary results were recently reported,
Dasatinib was replaced by Ponatinib, which was administered for 70 days
together with steroids, followed by ≥2 cycles of Blinatumomab
consolidation and 15 doses of intrathecal chemotherapy.[51] The maintenance therapy consisted either of Ponatinib or aHSCT for MRD-positive or IKZF1plus
patients. At the end of the induction therapy, 95% of patients achieved
complete hematological remission; with a median follow-up of 6.1
months, only one relapse was observed (IKZF1plus with T315I mutation).[51]
Olvermbatinib
is a novel third-generation TKI that has been demonstrated to
effectively target a wide range of BCR-ABL1 kinase mutations,
particularly T315I, in CML patients and B-ALL patients.[52] Olvermbatinib, in association with chemotherapy[53] or with Blinatumomab,[52,54]
was active in treating B-ALL patients who have failed TKI-based
regimens, including T315I-mutated cases. Zhang et al. have reported the
results on the treatment of 13 B-ALL patients (11 Ph+
and 2 Ph-like) with Olvermbatinib and Blinatumomab for one cycle of
treatment (both administered during the induction phase), with 72.7% of
patients achieving a CMR.[55]
The combination of Ponatinib with hyper-CVAD chemotherapy resulted in high rates of complete molecular remission and survival.[56] A recent phase II study explored in Ph+
B-ALL patients the safety and the efficacy of a therapeutic regimen
based on the sequential combination of low-intensity chemotherapy
mini-Hyper-CVD and Ponatinib followed by Blinatumomab and Ponatinib.[45]
Twenty patients were enrolled in this study, including 12 newly
diagnosed B-ALL, 4 R/R B-ALL, and 4 lymphoid crises of CML. A high rate
of complete molecular remission was observed.[57]
With a median follow-up of 25 months, the rates of 2-year remission
duration and OS in the newly diagnosed cohort were 90% and 82%,
respectively.[57]
Schwartz et al. have reported
the first results of a phase II clinical study involving the evaluation
of consolidation with Ponatinib and sequential Blinatumomab and
Chemotherapy after low-intensity Dasatinib-based induction in patients
with newly diagnosed B-ALL (Figure 3).[58]
14 patients received initial induction treatment with Dasatinib plus
prednisone until CMR was achieved; this treatment was followed by up to
four cycles of consolidative chemotherapy (high-dose methotrexate and
intermediate-dose cytarabine) plus Ponatinib and Blinatumomab; patients
not proceeding to aHSCT continue Ponatinib monotherapy up to 5 years
and 12 doses in intrathecal chemotherapy.[58] 21% of
the patients underwent aHSCT at CR1. The results obtained in this study
were compared to those observed in a historical group of patients
treated in the same institution with Dasatinib induction treatment and
aHSCT, and they showed a better RFS rate and a comparable OS rate.[58]
The studies on therapy of newly diagnosed Ph+
B-ALL patients with either Dasatinib plus Blinatumomab or Ponatinib
plus Blinatumomab indicate that these patients could be spared the
toxicities associated with chemotherapy and the need for aHSCT in first
response.
Blinatumomab in Frontline Treatment of Philadelphia-Negative B-ALL Patients
The
outcome of adult B-ALL patients with Ph-negative B-ALL markedly
improved following the introduction therapy of a chemotherapy regimen
inspired by pediatric protocols associated with the evaluation of MRD
at various time points during treatment, thus allowing a rational
stratification of each patient or aHSCT, if required.
This
treatment strategy inspired the phase II GIMEMA LAL2317 trial, and the
study evaluated whether the introduction of Blinatumomab may improve
the rate of patients achieving an MRD-negative status (Table 3).[59]
In this study, adult Ph-negative B-ALL patients were treated with a
pediatric chemotherapy backbone, with the introduction of two
treatments with Blinatumomab after early consolidation cycle 3 and late
consolidation cycle 6.[46] One hundred forty-nine
patients were enrolled in this study. At the end of the induction
period, 88% of patients achieved a CR, with a pronounced difference
according to the age of the patients.59 After early consolidation, 70%
achieved an MRD-negative condition; the rate of MRD negativity
increased to 93% after the first cycle of Blinatumomab treatment.[59] The OS was significantly better for patients achieving MRD-negativity. The cumulative incidence of relapse was 27.5%.[59]
For patients achieving MRD-negativity, the cumulative incidence of
relapse was 42.5% in Ph-like cases, compared to 17.5% in the remaining
patients.[59] Factors affecting OS were the age of patients, CR achievement and MRD status observed after the first cycle of Blinatumomab.[59-60]
The
GRAAL-2014-Quest study evaluated Blinatumomab in first-line in B-ALL
defined as high-risk for one of these three conditions, including KMT2A rearrangements, IKZF1 intragenic deletion or MRD-positivity post-induction.[61]
High-risk B-ALL patients responding to induction treatment were
enrolled in this study: patients with an aHSCT indication and a stem
cell source received Blinatumomab until transplant for a minimum of 4
weeks; these patients received Blinatumomab during consolidation and
maintenance therapy (Table 3).[61]
MRD response was lower in patients with high pre-Blinatumomab MRD
levels, while not impacted by age, WBC, or oncogenic subgroup; with a
median of 20 months, 18-month DFS and OS were 78.8% and 92.1%,
respectively.[61] Patients with a very high-risk condition (i.e., MRD <0.1% at 6 weeks or <0.01% at 12 weeks) displayed a worse DFS.[61]
After an amendment, the modified study GRAALL-2014/B study included a
group of patients receiving, when in remission with MRD>10-3 (or with >10-4
post-consolidation) Blinatumomab and a group of control patients
undergoing only chemotherapy treatment as consolidation therapy (Table 3).[62] The median age of these patients was 34 years; 17% of them bear KMT2A rearrangements, and 40% have IKZF1
deletion. Patients treated with Blinatumomab achieved a rate of MRD
negativity higher than patients treated with chemotherapy alone.[62]
A sub-analysis of this study showed that among high-risk B-ALL
Ph-negative patients who benefit from Blinatumomab, there is a
consistently heterogeneous landscape of response among genetic
entities, with patients with IKZF1 deletion exhibiting the most significant benefit from Blinatumomab of DFS.[63]
Several
studies have associated the early use of Blinatumomab with a reduction
of chemotherapy intensity and burden. A phase II Australian Leukemia
and Lymphoma Group (ALLG) evaluated reduced-intensity chemotherapy in
combination with Blinatumomab. Thirty patients received debulking
low-intensity chemotherapy with cyclophosphamide, vincristine and
dexamethasone, followed by 7 days of Blinatumomab; the patients then
received three alternating cycles of Blinatumomab and part B cycles of
hyper-CAVD, followed by two years of maintenance therapy in patients
and proceeding to aHSCT.[64] All treated patients achieved a CR.[64] 4 patients proceeded to aHSCT. The results appeared encouraging for older patients.[64]
The
ALLG study group developed a trial to evaluate Blinatumomab in sequence
with chemotherapy in a population of older, newly diagnosed B-ALL
patients; in this study, Blinatumomab replaced three cycles of standard
consolidation therapy.[65] Overall, the tolerability
and efficacy of this regimen were very promising, with a high rate of
hematologic and molecular responses.[53] In
comparison with the current standard therapy, the MRD response rates
were significantly better, and OS was superior to standard treatment.[65]
The
multicentre, single-arm, phase II trial (NCT055557110) enrolled adult
patients (15-59 years) with newly diagnosed Ph-negative B-ALL; the
induction regimen comprised reduced-intensity chemotherapy, followed by
two weeks of Blinatumomab.[54] The MRD negativity rate was 90.5% after 2 weeks of Blinatumomab.[66]
Adverse events were rare, with 1/21 patients exhibiting grade 3 CRS and
no patient displaying grade 3 or more neurologic events.[66]
A
phase II study explored in 75 newly diagnosed Ph- B-ALL patients the
safety and the efficacy of a therapeutic regimen based on hyperCVAD
alternating with high-dose methotrexate and cytarabine for up to 4
cycles, followed by 4 cycles of Blinatumomab at standard doses, in 37
patients Inotuzumab was added to 2 cycles of MTX/Ara-C.[55]
In the whole population of patients (38 treated with HCVAD+Blina and 37
with HCVAD+Ino+Blina), the 3-yr OS was 88% and RFS 89%; the 3-year OS
rate for patients without or with high-risk features was 93% and 83%,
respectively.[67]
Two recent studies have
explored the use of Blinatumomab in newly diagnosed pediatric B-ALL
patients. In this context, van der Sluis and coworkers have evaluated
the safety and the efficacy of Blinatumomab added to induction
chemotherapy in 30 infants affected by KMT2A-rearranged
B-ALLs. All 30 patients received the full course of Blinatumomab. 28/30
(93%) patients displayed either MRD-negativity (16 patients) or very
low MRD levels (<5x10-4) (12
patients) after Blinatumomab infusion. Two-year DFS and OS were 81.6
and 93.3% in this study and compared very favorably with the
corresponding rates observed in the Interinfant-06 study based on
chemotherapy alone.[68] In conclusion, this study
showed that Blinatumomab added to the chemotherapy backbone used in the
study Interinfant-o6 appeared to be safe and displayed high efficacy
compared with historical controls.[68]
Schrappe
and coworkers reported data on the safety profile of pediatric patients
with high-risk B-ALL in first complete remission; after a 4-drug
induction phase and two weeks of consolidation treatment, the patients
were randomized to receive either two additional courses of
consolidation chemotherapy or two 28-day courses of Blinatumomab.[69] The toxicity profile of Blinatumomab was more favorable to the intensive chemotherapy approach.[69] Outcome data are expected to demonstrate that the Blinatumomab arm is not inferior to chemotherapy.
Conclusions
The
introduction of the bispecific CD19-CD3 antibody Blinatumomab in the
treatment of B-ALL patients has significantly improved their outcomes,
particularly those of adult B-ALL patients.
Studies carried out in
R/R B-ALL patients in combination with chemotherapy improve RFS and OS
and increase the frequency of patients who underwent an HSCT when in
complete remission. Future studies will be required to define
therapeutic strategies, such as a decrease of tumor burden before
immunotherapy, to increase the number of patients responding to
Blinatumomab. Importantly, patients relapsing after an initial response
to Blinatumomab are clearly responsive to CD19 CAR-T treatment, while
patients refractory to a previous Blinatumomab treatment are less
responsive to CAR-T cells. Ongoing studies are evaluating the capacity
of other drugs, such as Inotuzumab or ICIs, that, in combination with
Blinatumomab, could improve its efficacy in R/R B-ALL patients.
Other
studies have shown that Blinatumomab could represent an efficient
therapeutic tool for clearing residual disease in B-ALL patients
achieving a CR after induction chemotherapy with an MRD. In line with
these observations, several studies have shown that there is a strong
rationale for using Blinatumomab as a consolidation treatment before
aHSCT. The consolidation therapy pre-transplantation with Blinatumomab
improves RFS and OS compared to consolidation therapy based on
chemotherapy only.
Several studies have explored the use of Blinatumomab in the first line of treatment for newly diagnosed Ph+ or Ph- B-ALL patients. The introduction of Blinatumomab in the first-line treatment of Ph+
patients allowed to develop a chemotherapy-free approach based on the
combination of Blinatumomab with a TKI (either Dasatinib or Ponatinib),
resulting in high rates of complete molecular responses and long-term
survival; the use of Ponatinib or other third-generation TKIs seems to
reduce the rate of relapses related to generation/selection of
resistant ABL1 mutations.
These studies have shown that these chemotherapy-free treatments can
spare the toxicities related to chemotherapy and reduce the need for
aHSCT. The clinical studies carried out in newly diagnosed Ph-
B-ALL patients showed a benefit of adding Blinatumomab, as supported by
the achievement of high rates of MRD negativity, OS and DFS, better
than those observed in historical controls. Future randomized clinical
studies will be required to demonstrate the real improvement related to
the addition of Blinatumomab to the chemotherapy treatment of Ph- newly diagnosed B-ALL.
References
- Klein C, Brinkmann U,
Reichert JM, Kontermann RE. The present and future of bispecific
antibodies for cancer therapy. Nat Rev Drug Discov 2024 https://doi.org/10.1038/s41573-024-00896-6
PMid:38448606
- Surowka
M, Klein C. A pivotal decade for bispecific antibodies? MABS 2024; 16:
2321625. https://doi.org/10.1080/19420862.2024.2321635
PMid:38465614 PMCid:PMC10936642
- Bargou
R, Leo E, Zugmaier G, Klinger M, Goebeler M, Knop S, Noppeney R,
Viardot A, Hess G, Schuler M, Einsele M, et al. Tumor regression in
cancer patients by very low doses of a T cell-engaging antibody.
Science 2008; 321: 974-977. https://doi.org/10.1126/science.1158545
PMid:18703743
- Kantarjian
H, Stein A; Gokbuget N, Fielding AK, Schuh AC, Ribera JM, Wei A;
Dombret H, Foa R, Bassan R, et al. Blinatumomab versus chemotherapy for
advanced acute lymphoblastic leukemia. N Engl J Med 2017; 3: 836-847. https://doi.org/10.1056/NEJMoa1609783
PMid:28249141 PMCid:PMC5881572
- Topp
MS, Gokbuget N, Zugmaier G, Stein AS, Dombret H, Chen Y, Ribera
JM,Bargou RF, Horst HA, Kantarjian H. Long-term survival of patients
with relapsed/refractory acute lymphoblastic leukemia treated with
Blinatumomab. Cancer 2021; 127: 554-559. https://doi.org/10.1002/cncr.33298
PMid:33141929 PMCid:PMC7894150
- ToppMS,
Stein AS, Gokbuget N, Horst HA, Boissel N, Martinelli G, Kantarjian H,
Bruggemann M, Chen Y, Zugmaier G. Blinatumomab as first salvage versus
second or later salvage in adults with relapsed/refractory B-cell
precursor acute lymphoblastic leukemia: results of a pooled analysis.
Cancer Med 2021; 1: 2601-2610. https://doi.org/10.1002/cam4.3731
PMid:33734596 PMCid:PMC8026950
- Boissel
N, Chiaretti S, Papayannidis C, Ribera JM, Bassan R, Sokolov AN, Alam
N, Brescianini A, Pezzani I, Kreuzbauer G, et al. Real-world use of
Blinatumomab in adult patients with B-cell acute lymphoblastic leukemia
in clinical practice: results from the NEUF study. Blood Cancer J 2023;
13:2. https://doi.org/10.1038/s41408-022-00766-7
PMid:36599847 PMCid:PMC9813344
- Webster
JA, Luskin MR, Rimando J, Blackford A, Zeidan AM, Sharon E, Stericher
H, DeAngelo DJ, Uznik L, Gojo I, et al. Blinatumomab in combination
with immune checkpoint inhibitors (ICIs) of PD-1 and CTLA-4 in adult
patients with relapsed/refractory (R/R) CD19 positive B-cell acute
lymphoblastic leukemia (ALL): results of a phase I study. Blood 2023;
142(suppl1): 966. https://doi.org/10.1182/blood-2023-191109
- Martinelli
G, Boissel N, Chevalier, Ottmann O, Gokbuget N, Rambaldi A, Ritchie EK,
Papayannidis C, Tuglus CA, Morris JD, et al. Long-term follow-up of
Blinatumomab in patients with relapsed/refractory Philadelphia
chromosome-positive B-cell precursor acute lymphoblastic leukemia:
final analysis of ALCANTARA study. Eur J Cancer 2021; 146: 107-114. https://doi.org/10.1016/j.ejca.2020.12.022
PMid:33588145
- Topp
MS, Stelljes M, Zugmaier G, Barnette P, Heffner LT, Trippett T, Duell
J, Bargou RC,Holland C, Benjamin JE, et al. Blinatumomab retreatment
after relapse in patients with relapsed/refractory B-precursor acute
lymphoblastic leukemia. Leukemia 2018; 32: 562-565. https://doi.org/10.1038/leu.2017.306
PMid:28990581 PMCid:PMC5808068
- Locatelli
F, Zugmaier G, Mergen N, Bader P, Jeha S, Schlegel PG, Bouquin JP,
Handgretinger R, Brethon B, Rossig C, et al. Blinatumomabv in pediatric
relapsed/refractory B-cell acute lymphoblastic leukemia: RIALTO
expanded access study final analysis. Blood Adv 2022; 6: 1004-1013. https://doi.org/10.1182/bloodadvances.2021005579
PMid:34979020 PMCid:PMC8945309
- Locatelli
F, Zugmaier G, Rizzari C, Morris JD, Gruhn B, Klingbiel T, Parasole R,
Linderkamp C, Flotho C, et al. Effect of Blinatumomab vs chemotherapy
on event-free survival among children with high-risk first-relapse
B-cell acute lymphoblastic leukemia: a randomized clinical trial. JAMA
2021; 325: 843-854. https://doi.org/10.1001/jama.2021.0987
PMid:33651091 PMCid:PMC7926287
- Locatelli
F,Zugmaier G, Rizzari C, Morris JD, Gruhn B, Klingbiel T, Parasole R,
Linderkamp C, Flotho C, et al. Improved survival and MRD remission with
Blinatumomab vs. chemotherapy in children with first high-risk relapse
B-ALL. Leukemia 2023; 37: 222-225. https://doi.org/10.1038/s41375-022-01770-3
PMid:36482128 PMCid:PMC9883152
- Hogan
LE, Brown PA, Ji L, Xu X, Devidas M, Bhatla T, Borwitz MJ, Raetz EA,
Carroll A, Heerema NA, et al. Children's oncology group AALL1331: phase
III trial of Blinatumomab in children, adolescents, and young adults
with low-risk B-cell ALL in first relapse. J Clin Oncol 2023; 41:
4118-4129. https://doi.org/10.1200/JCO.22.02200
PMid:37257143
- Jabbour
E, Haddad PG, Short NJ, et al. Phase 2 study of inotuzumab ozogamicin
for measurable residual disease in acute lymphoblastic leukemia in
remission. Blood 2024; 143: 417-421. https://doi.org/10.1182/blood.2023022330
PMid:37879077
- Fracchiolla
NS, Sciumé M, Papayannidis C, Vitale A, Chiaretti S, Annunziata M,
Giglio F, Salutari P, Forghieri F, Lazzarotto D, et al. Blinatumomab
and inotuzumab ozogamicin sequential use for the treatment of
relapsed/refractory acute lymphoblastic leukemia: a real-life campus
A11 study. Cancers 2023; 15: 4623. https://doi.org/10.3390/cancers15184623
PMid:37760592 PMCid:PMC10526797
- Jabbour
E, Zugmaier G, Agrawal V, Martinez-Sanchez P, Rifon Roca J, Cassaday R,
Boll B, Rijneveld A, Abdul-Hay M, Huguet F, et al. Single agent
subcutaneous blinatumomab for advanced acute lymphoblastic leukemia. Am
J Hematol 2024; 99: 586-595. https://doi.org/10.1002/ajh.27227
PMid:38317420
- Queudeville
M, Stein AS, Locatelli F, Ebinger M, Handgretinger R, Gokbuget N, Gore
L, Zeng Y, Gokani P, Zugmaier G, et al. Low leukemia burden improves
Blinatumomab with relapsed/refractory B-cell acute lymphoblastic
leukemia. Cancer 2023; 129: 1384-1393. https://doi.org/10.1002/cncr.34667
PMid:36829303
- Pillai
V, Muralidharan K, Meng W, Bagashev A, Oldridge DA, Rosenthal J, Van
Arnam J, Melenhorst JJ, Mohan D, DiNofia AM, et al. CAR T-cell therapy
is effective for CD19-dim B-lymphoblastic leukemia but is impacted by
prior blinatumomab therapy. Blood Adv 2019; 3: 3539-3549. https://doi.org/10.1182/bloodadvances.2019000692
PMid:31738832 PMCid:PMC6880911
- Shah
RD, Cassaday RD, Park JH, Houot R, Oluwole OO, Logan AC, Boissel N,
Leguay T, Bishop MR, Topp MS, et al. Subgroup analyses of KTE-X19, an
anti-CD19 chimeric antigen receptor (CAR) T-cell therapy, in adult
patients with relapsed or refractory B-cell acute lymphoblastic
leukemia (R/R B-ALL) in ZUMA-3. HemaSphere 2022; 6: S3. https://doi.org/10.1097/01.HS9.0000844312.20412.89
- Myers
RM, Taraseciviute A, Steinberg SM, Lamble AJ, Sheppard J, Yates B,
Kovach AE, Wood B, Borowitz MJ, Stetler-Stevenson M, et al.
Blinatumomab nonresponse and high disease burden are associated with
inferior outcomes after CD19-CAR for B-ALL. J Clin Oncol 2022; 40:
932-944. https://doi.org/10.1200/JCO.21.01405
PMid:34767461 PMCid:PMC8937010
- Lamble
AJ, Myers RM, Taraseviciute A, John S, Yates B, Steinberg SM, Sheppard
J, Kovach AE, Wood B, Borowitz MJ, et al. Preinfusion factors impacting
relapse immunophenotype following CD19 CAR T cells. Blood Adv 2023; 7:
575-584. https://doi.org/10.1182/bloodadvances.2022007423
PMid:35482927 PMCid:PMC9979750
- Gupta
VK, Roloff GW, Muffly LS, Aldoss I, Kopmar NE, Lin C, Dekker SE,
Jeyakumar N, O'Connor TE, Zhang A, et al. Impact of prior response to
Blinatumomab on outcomes of brexucabtagene autoleucel (Brexu-cel) in
adult patients with relapsed or refractory (r/r) B-cell acute
lymphoblastic leukemia (B-ALL): results from the real-world outcomes
collaborative of CAR-T in adult ALL (ROCCA). Blood 2023; 142(suppl 1):
2119. https://doi.org/10.1182/blood-2023-182915
- Saygin
C, Cannova J, Stock W and Muffly L. Measurable residual disease in
acute lymphoblastic leukemia: methods and clinical context in adult
patients. Haematologica 2022; 107: 2783-2793. https://doi.org/10.3324/haematol.2022.280638
PMid:36453516 PMCid:PMC9713546
- Topp
MS, Kufer P, Gokbuget N, Goebele M, Klinger M, Neumann S, Horst HA,
Raff T, Viardot A, Schmidt M, et al. Targeted therapy with the T-cell
engaging antibody blinatumomab of chemotherapy-refractory minimal
residual disease in B-lineage acute lymphoblastic leukemia patients
results in high response rate and prolonged leukemia-free survival. J
Clin Oncol 2011; 29: 2493-2498. https://doi.org/10.1200/JCO.2010.32.7270
PMid:21576633
- Gokbuget
N, Dombret H, Bonifacio M,Reichle A, Graux C, Faul C, Diedrich H, Topp
MS, Bruggemann M, Horst HA, et al. Blinatumomab for minimal residual
disease in adults with B-cell precursor acute lymphoblastic leukemia.
Blood 2018; 131: 1522-1531. https://doi.org/10.1182/blood-2017-08-798322
PMid:29358182 PMCid:PMC6027091
- Gokbuget
N, Zugmaier G, Dombret H, Stein A, Bonifacio M, Graux C, Faul C,
Bruggemann M, Taylor K, Mergen N, et al. Curative outcomes following
Blinatumomab in adults with minimal residual disease B-cell precursor
acute lymphoblastic leukemia. Leukemia&Lymphoma 2020; 61 :
2665-2673. https://doi.org/10.1080/10428194.2020.1780583
PMid:32619115
- Gokbuget
N, Kantarjian H, Bruggemann M, Stein AS, Bargou RC, Dombret H, Fielding
AK, Heffner L, Rigal-Huguet F, Litzow M, et al. Molecular response with
Blinatumomab in relapsed/refractory B-cell precursor acute
lymphoblastic leukemia. Blood Adv 2019 ; 3 : 3033-3037. https://doi.org/10.1182/bloodadvances.2019000457
PMid:31648325 PMCid:PMC6849936
- Jabbour
EJ, Short NJ, Jain N, Jammal N, Jogersen J, Wang S, Ohanian M, Alvarado
Y, Kadia T, et al. Blinatumomab is associated with favorable outcomes
in patients with B-cell lineage acute lymphoblastic leukemia and
positive measurable residual disease at a threshold of 10-4 and higher.
Am J Hematol 2022; 97: 1135-1141. https://doi.org/10.1002/ajh.26634
PMid:35713551
- Cabannes-Hamy
A, Brissot E, Leguay T, Huguet F, Chevallier P, Hunault M,
Escoffre-Barbre M, Cluseau T, Balsat M, Nguyen S, et al. High tumor
burden before Blinatumomab has a negative impact on the outcome of
adult patients with B-cell precursor acute lymphoblastic leukemia. A
real-world study by the GRAAL. Haematologica 2022; 107: 2072-2080. https://doi.org/10.3324/haematol.2021.280078
PMid:35263986 PMCid:PMC9425331
- Urbino
I, Lengline E, Rabian F, Cerrano M, Kim R, Chevillon F, Ferrero D,
Sebert M, Dhédin N, Itzykson R, et al. Blinatumomab consolidation for
adult B-cell acute lymphoblastic leukemia in first and second complete
remission. Blood Adv 2024, in press. https://doi.org/10.1182/bloodadvances.2023012139
PMid:38507689 PMCid:PMC11112603
- Litzow
MR, Sun Z, Paietta E, Mattison RJ, Lazarus HM, Rowe JM, Arber DA,
Mullighan CG, Willman CI, Zhang Y, et al. Consolidation therapy with
Blinatumomab improves overall survival in newly diagnosed adult
patients with B-lineage acute lymphoblastic leukemia in measurable
residual disease negative remission; results from the ECOG-ACRIN E1910
randomized phase III national cooperative clinical trials network
trial. Blood 2022; 140(suppl.1): LBA-1. https://doi.org/10.1182/blood-2022-171751
- Litzow
M, Sun Z, Mattison R, Paietta E, Mullighan C, Roberts K, Zhang Y,
Racevskis J, Willman C, Wieduwilt M, et al. Consolidation with
Blinatumomab improves overall and relapse-free survival in patients
with newly diagnosed B-cell acute lymphoblastic leukemia: impact of age
and MRD level in ECOG-ACRIN E1910. Hemasphere 2023; 7(33): EHA 2023
Hybrid Congress S115. https://doi.org/10.1097/01.HS9.0000967372.19440.62
PMCid:PMC10428281
- Luger
SM, Sun Z, Mattison RJ, Paietta E, Roberts KG, Zhang Y, Racevckis J,
Lazarus HM, Rowe JM, Arber DA, et al. Assessment of outcomes of
consolidation therapy by number of cycles of Blinatumomab received in
newly diagnosed measurable residual disease negative patients with
B-lineage acute lymphoblastic leukemia: in the ECOG-ACRIN E1910
randomized phase III national clinical trials network trial. Blood
2023; 142(suppl.1): 2877. https://doi.org/10.1182/blood-2023-189648
- Gu
ME, Zhang JY, Tang YM, Xu WQ, Song H, Xu X. The effectiveness of
Blinatumomab in clearing next-generation sequencing measurable residual
disease in pediatric patients with B-cell acute lymphoblastic leukemia.
Blood 2023; 142(suppl.1): 6076. https://doi.org/10.1182/blood-2023-179796
- Stein
AS, Kantarjian H, Gokbuget N, Bargou R, Litzow MR, Rambaldi A, Ribera
JM, Zhang A, Zimnmerman Z,Zugmaier G, et al. Blinatumomab for acute
lymphoblastic leukemia relapse after allogeneic hematopoietic stem cell
transplantation. Biol Blood Marrow Transplant. 2019Aug;25(8):1498-1504.
doi: 10.1016/j.bbmt.2019.04.010. Epub 2019 Apr 17.
- Gaballa
MR, Banerjee P, Milton DR, Jiang X, Ganesh C, Khazal S, Nandivada V,
Islam S, Kaplan M, Daher M, et al. Blinatumomab maintenance after
allogeneic hematopoietic cell transplantation for B-lineage acute
lymphoblastic leukemia. Blood 2022; 139: 1908-1919. https://doi.org/10.1182/blood.2021013290
PMid:34914826 PMCid:PMC8952188
- Webster
JA, Jones RJ, Blackford A, Shedeck A, Ambinder RF, Swinnen LJ,
Wagner-Johnston N, Fuchs EJ, Bolanos-Meade J, Imus P, et al. A phase
IB/II study of Blinatumomab in patients with B-cell acute lymphoblastic
leukemia (ALL) and B-cell non-Hodgkin lymphoma (NHL) as post-allogeneic
blood or marrow transplant (alloBMT) remission maintenance. Blood 2023;
142(suppl.1): 3582. https://doi.org/10.1182/blood-2023-191047
- Chauvet
P, Paviglianiti A, Labopin M, Labussiere H, Boissel N, Robin M,
Maillard N, Ouachée-Chardin M, Forcade E, Poiré X, et al. Combining
blinatumomab and donor lymphocyte infusion in B-ALL patients relapsing
after allogeneic hematopoietic cell transplantation: a study of the
SFGM-TC. Bone Marrow Transplant 2023; 58: 72-79. https://doi.org/10.1038/s41409-022-01846-9
PMid:36261707
- Brown
PA, Ji L, Xu X, Devidas M, Hogan LE, Borowirtz MJ, Raetz EA, Zugmaier
G, Sharon E, Bernhardt MB, et al. Effect of post-reinduction therapy
consolidation with Blinatumomab vs chemotherapy on disease-free
survival in children, adolescents, and young adults with first relapse
of B-cell acute lymphoblastic leukemia: a randomized clinical trial.
JAMA 325: 833-842. https://doi.org/10.1001/jama.2021.0669
PMid:33651090 PMCid:PMC7926290
- LocatellI
F, Eckert C, Hrusak O, Buldini B, Sartor M, Zugmaier G, Zeng Y,
Pilankar D, Morris J, von Stackelberg A. Blinatumomab overcomes poor
prognostic impact of measurable residual disease in pediatric
high-.risk first relapse B-cell precursor acute lymphoblastic leukemia.
Pediatr Blood Cancer 2022; 69: e29715. https://doi.org/10.1002/pbc.29715
PMid:35482538
- Sayyed
A, Chen C, Gerbitz A. Pretransplant blinatumomab improves outcomes in B
cell acute lymphoblastic leukemia patients who undergo allogeneic
hematopoietic transplantation. Transplant Cell Ther 2024; in press. https://doi.org/10.1016/j.jtct.2024.03.004
PMid:38462215
- Llaurador
G, Shaver K, Wu M, Wang T, Gillispei A, Doherty E, Craddock J, Read J,
Yassine K; Morales E, et al. Blinatumomab therapy is associated with
favorable outcomes after allogeneic hematopoietic cell transplantation
in pediatric patients with B cell acute lymphoblastic leukemia.
Transplant Cell Ther 2024; 30: 217-227. https://doi.org/10.1016/j.jtct.2023.10.024
PMid:37931800
- Foa
R, Bassan R, Vitale A, Elia L, Piciocchi A, Puzzolo MC, Canichella M,
Viero P, Ferrara F, Lunghi M, Fabbiano F, et al. Dasatanib-Blinatumomab
for pH-posotive acute lymphoblastic leukemia in adults. N Engl J Med
2020; 383: 1613-1623. https://doi.org/10.1056/NEJMoa2016272
PMid:33085860
- Foa
R, Bassan R, Elia L, Piciocchi A, Soddu S, Messina M, Ferrara F,m
Lunghi M, Mulè A, Bonifacio M, Fracchiolla N, et al. Long-term results
of the dasatanib-blinatumomab protocol for adult Philadelphia-positive
ALL. J Clin Oncol 2024; 42: 8871-885. https://doi.org/10.1200/JCO.23.01075
PMid:38127722 PMCid:PMC10927329
- Advani
AS, Moseley A, O'Dwyer KM, Wood BL, Park J, Wieduwilt M, Jeyakumar D,
Yaghmour G, Atallah EL, Gerds AT, et al. Dasatanib/prednisone induction
followed by blinatumomab/dasatanib in Ph+ acute lymphoblastic leukemia.
Blood Adv 2023; 7: 1279-1285.
https://doi.org/10.1182/bloodadvances.2022008216
PMid:36322825 PMCid:PMC10090098
- Advani
AS, Moseley A, O'Dwyer KM, Wood BL, Park J, Wieduwilt M, Jeyakumar D,
Yaghmour G, Atallah EL, Gerds AT, et al. Long-term follow up for SWOG
1318: combination of dasatanib, prednisone, and Blinatumomab for older
patients with Philadelphia-chromosome (Ph) positive acute lymphoblastic
leukemia (ALL). Blood 2023; 142(suppl.1): 1499. https://doi.org/10.1182/blood-2023-180204
- Geyer
MB, Mascarenhas J, Smith M, pacsual S, shah A, Sivestrone MR,
Czaplinska T, Johnson K, Thompson MC, Park JH. Chemotherapy-sparing
induction followed by consolidation and maintenance with Blinatumomab
and concurrent tyrosine kinase inhibitor therapy for newly-diagnosed
Philadelphia chromosome-positive acute lymphoblastic leukemia: primary
endpoint results from the Bissphall study. Blood 2023; 142 (suppl.1):
1510. https://doi.org/10.1182/blood-2023-173551
- Jabbour
E, Short NJ, Jain N, Huang X, Montalban-Bravo G, Banerejee P, Rezvani
K, Jiang X, Kim KH, Kanagal-Shamanna R, et al. Ponatinib and
blinatumomab for Philadelphia chromosome-positive acute lymphoblastic
leukemia: a US, single-centre, single-arm, phase 2 trial. Lancet
Hematol 2023; 10: e24-e34. https://doi.org/10.1016/S2352-3026(22)00319-2
PMid:36402146
- Haddad
FJ, Jabbour E, Short NJ, Jain N, Huang X, Montalban-Bravo G, Kadia TM,
Daver N, Nasnas C, Major E, et al. Chemotherapy-free combination of
Blinatumomab and ponatinib in adults with newly diagnosed Philadelphia
chromosome-positive acute lymphoblastic leukemia: updates from a phase
II trial. Blood 2023; 142(suppl.1): 2827. https://doi.org/10.1182/blood-2023-188064
- Chiaretti
S, Leoncin M, Elia L, Piciocchi A, Matarazzo M, Di Trani M, Sica S,
Luppi M, Mancini V, Borlenghi E, et al. Comparison between
dasatinib-blinatumomab vs ponatinib-blinatumomab chemo-free strategy
for newly diagnosed Ph+ acute lymphoblastic leukemia patients.
Preliminary results of the Gimema ALLL2820 trial. Blood 2023;
142(suppl.1): 4249. https://doi.org/10.1182/blood-2023-189632
- Jabbour
E, Kantarjian HM, Koller PB, Jamy O, Oehler VG, Lomaia E, Hunter AM,
Uspenskaya O, Samarina S, Mukherjee S, et al. Update of olvermbatinib
(HQP1351) overcoming ponatinib and/or asciminib resistance in patients
(Pts) with heavily pretreated/refractory chronic myeloid leukemia (CML)
and Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+
ALL). Blood 2023; 142(suppl.1): 1798. https://doi.org/10.1182/blood-2023-187744
- Li
Z, Ting Z, Hu L, Duan W, Jiang Q. Olvermbatinib (HQP1351) combined with
chemotherapy is an effective and safe treatment in patients with
Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia
(ALL) and chronic myeloid leukemia in lymphoid blast phase (CMN-LBP)
that failed TKI-based regimens. Blood 2023; 142(suppl.1): 5895. https://doi.org/10.1182/blood-2023-185471
- Fan
S, Wang L, Lu Y, Li Z. Olvermbatinib combined with Blinatumomab in
treating T315I-mutated Philadelphia chromosome-positive acute
lymphoblastic leukemia: two-case report. Ann Hematol 2024; 103:
525-532. https://doi.org/10.1007/s00277-023-05519-5
PMid:37940719
- Zhang
T, Zhu K, Zihong C, Lin R, Liu Q, Zhou H. Frontline combination of 3rd
generation TKI Olvermbatinib and Blinatumomab for Ph+/Ph-like ALL
patients. Blood 2023; 142(suppl.1): 1504. https://doi.org/10.1182/blood-2023-186139
- Kantarjian
H, Short NJ, Jain N, Sasaki K, Huang X, Haddad FG, Khouri I, DiNardo
CD, Pemmaraju N, Wierda W, et al. Frontline combination of ponatinib
and hyper-CVAD in Philadelphia chromosome-positive acute lymphoblastic
leukemia: 80-months follow-up results. Am J Hematol 2023; 98: 493-501. https://doi.org/10.1002/ajh.26816
PMid:36600670
- Jen
WY, Jabbour E, Haddad FGF, Nasr L, Short NJ, Zoghbi M, Nasnas C, Issa
GC, Yilmaz M, Daver N, et al. A phase II study of low-intensity
chemotherapy (Mini-hyper-CVD) and ponatinib followed by Blinatumomab
and ponatinib in patients with Philadelphia-positive acute
lymphoblastic leukemia. Blood 2023; 142(suppl.1): 2868. https://doi.org/10.1182/blood-2023-182997
- Schwartz
M, McMahon CM, Amaya ML, Witkowski M, Pollkyea DA, Gutman JA,
Minajuddin M, Smith C, Jordan CT. Consolidation with ponatinib plus
sequential Blinatumomab and chemotherapy after low intensity
dasatinib-based induction in adults with Philadelphia
chromosome-positive acute lymphoblastic leukemia: outcomes from a
single institution. Blood 2023; 142(suppl.1): 4247. https://doi.org/10.1182/blood-2023-188118
- Chiaretti
S, Della Starza I, Santoro A, Spinelli O, Elia L, De Propris MS,
Piccini M, Chiusolo P, Ferrara F, Zappasodi P, et al. Sequential
chemotherapy and Blinatumomab to improve minimal residual disease in
dult Ph- B-lineage acute lymphoblastic leukemia. Final results of the
phase II GIMEMA LAL2317 trial. Blood 2023; 142(suppl.1): 826. https://doi.org/10.1182/blood-2023-174973
- Tasian
SK, Loh ML, Hunger SP. Philadelphia chromosome-like acute lymphoblastic
leukemia. Blood 2017; 130: 2064-2072.
https://doi.org/10.1182/blood-2017-06-743252 PMid:28972016
PMCid:PMC5680607
- Boissel
N, Huguet F, Graux C, Hicheri Y, Chevalier P, Kim R, Balsat M, Leguayt
T, Hunault M, Maury S, et al. Frontline consolidation with Blinatumomab
for high-risk Philadelphia-negative acute lymphoblkastic adult
patients. Early results from the Graall-2014-QUEST phase 2. Blood 2021;
138(suppl.1): 1232. https://doi.org/10.1182/blood-2021-146163
- Boissel
N, Huguet F, Leguay T, Hunault M, Kim R, Hicheri Y, Chevallier P,
Balsat M, Maury S, Thiebaut-Bertrand A, et al. Blinatumomab during
consolidation in high-risk Philadelphia chromosome (Ph)-negative B-cell
precursor (BCP) acute lymphoblastic leukemia (ALL) adult patients : a
two-cohorts comparison within the Graall-2014/B study. Blood 2022 ;
140(suppl.1) : 507-509. https://doi.org/10.1182/blood-2022-159397
- Boissel
N, Huguet F, Leguay T, Hunault M, Kim R, Hicheri Y, Passet M,
Chevallier P, Balsat M, Maury S, et al. Exploring the heterogeneity of
response to Blinatumomab in high-risk Philadephia-negative B-cell
precursor acute lymphoblastic leukemia: an analysis from the QUEST
sub-study of the Graall 2014/B trial. Blood 2023; 142(suppl.1): 499. https://doi.org/10.1182/blood-2023-177847
- Fleming
S, Reynolds J, Bajel A, Venn N, Kwan J, Moore J, Yeung D, Pati N, Lehy
M, Nkyekyer J, et al Sequential Blinatumomab with reduced intensity
chemotherapy in the treatment of older adults with newly diagnosed Ph
negative B-precursor acute lymphoblastic leukemia-Interim analysis of
the Australian Leukemia and Lymphoma group ALLo8 study. Blood 2021;
138(suppl.1): 1234. https://doi.org/10.1182/blood-2021-151826
- Fleming
S, Reynolds J, Bajel A, Venn N, Kwan J, Moore J, Yeung D, Pati N, Lehy
M, Kollipara S, et al. Sequential Blinatumomab with reduced intensity
chemotherapy for older adults with newly diagnosed Ph- B-precursor
acute acute lymphoblastic leukemia- Final results of the ALLG ALL08
study. Hemasphere 2023; 7(suppl.1): e811479d. https://doi.org/10.1097/01.HS9.0000968372.81147.9d
PMCid:PMC10428405
- Goekbuget
N, Schwartz S, Faul C, Topp MS, Subklewe M, Renzelmann A, Stoltefuss A,
Artenstein B, Wilke A; Raffel S, et al. Dose reduced chemotherapy in
sequence with Blinatumomab for newly diagnosed patients with Ph/BCR:ABL
negative B-cell precursor adult lymphoblastic leukemia 8ALL):
preliminary results of the GMALL bold trial. Blood 2023; 142(suppl.1):
964. https://doi.org/10.1182/blood-2023-180472
- Nguyen
D, Kantarjian HM, Short NJ, Jain N, Haddad FG, Yilmaz M,Ferrajoli A,
Kadia TM, Valero YA, Maiti A, et al. Updated results from a phase II
study of hyper-CVAD, with or without inotuzumab ozogamicin, and
sequential Blinatumomab in patients with newly diagnosed B-cell acute
lymphoblastic leukemia. Blood 2023; 142(suppl.1): 4245. https://doi.org/10.1182/blood-2023-190902
- Van
der Sluis I, De Lorenzo P, Kotecha RS, Attarbaschi A, Escherich G,
Nysom Stary J, Ferster A, Brethon B, Locatelli F, Schrappe M, et al.
Blinatumomab added to chemotherapy in infant lymphoblastic leukemia. N
Engl J Med 2023; 8: 1572-1581. https://doi.org/10.1056/NEJMoa2214171
PMid:37099340
- Schrappe
M, Locatelli F, Valsecchi MG, Cario G, Vossen-Gajcy M, Stary J,
Attarbaschi A, Bodmer N, Barbaric D, Elitzur S, et al. Pediatric
patients with high-risk B-cell LL in first complete remission may
benefit from less toxic immunotherapy with Blinatumomab - Results from
randomized controlled phase 3 trial AIEOP-BFM ALL 2017. Blood 2023;
142(suppl.1): 825. https://doi.org/10.1182/blood-2023-181524





