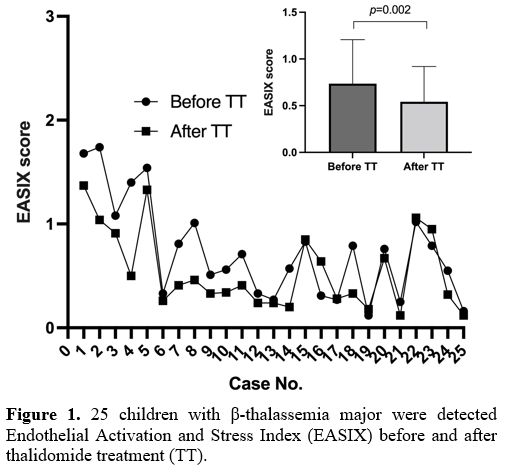
We retrospectively assessed children with TM who received thalidomide for more than three months between May 2021 and June 2024. The study included 25 patients (15 males and 10 females) aged 14-18 years. The EASIX score was calculated using LDH (U/L) × creatinine (mg/dL) / platelet count (×109/L) from laboratory data recorded after approximately three months of thalidomide treatment. A significant reduction in EASIX was observed in TM patients post-treatment (p=0.002) (Figure 1). We investigated the correlations between EASIX and baseline indicators. In addition to platelets (r=-0.601, p=0.001), LDH (r=0.536, p=0.006), and creatinine (r=0.595, p=0.002), EASIX was inversely correlated with white blood cell count (r=-0.450, p=0.024) and positively correlated with total bilirubin (r=0.615, p=0.001) and indirect bilirubin (r=0.561, p=0.004). No other significant correlations were found between EASIX and other parameters.
 |
|
Patients with thalassemia undergoing transplantation suffer a unique spectrum of complications, particularly those related to vascular endothelial injury, such as hepatic venular occlusive disease/sinusoidal obstruction syndrome (VOD/SOS), transplant-associated thrombotic microangiopathy, and graft-versus-host disease.[7] VOD/SOS incidence in thalassemia transplants can reach 10.4%.[8] Increased markers of endothelial cell damage in thalassemia patients are closely linked to post-transplantation thrombotic microangiopathy.[9,10] Therefore, understanding and addressing endothelial injury in these patients is crucial for improving transplant outcomes.
Although endothelial dysfunction is associated with various transplant complications, clear biomarkers are limited. EASIX, first reported in 2017 by Luft et al.,[3] is strongly correlated with transplant outcomes. In patients with SOS/VOD, EASIX was significantly higher on the day of transplantation than in those without SOS/VOD.[4] Elevated EASIX at different time points during transplantation is associated with higher mortality and poorer overall survival.[5] In TM patients, median EASIX is significantly higher in those with day +100 transplant-related mortality.[6] Consequently, EASIX can serve as a valuable indicator of vascular endothelial injury during transplantation, guiding early interventions to reduce complications and improve prognosis.
Thalidomide has emerged as a treatment option for β-thalassemia, with demonstrated benefits in reactivating fetal hemoglobin production and reducing transfusion needs.[11] Additionally, it has been shown to ameliorate erythropoiesis and iron homeostasis, reduce spleen size, and treat thrombocytopenia in hypersplenism.[12,13] In this study, we evaluated the effects of thalidomide on EASIX and analyzed the correlations between baseline indicators and EASIX to explore the support of the effectiveness of this drug. After thalidomide treatment, a significant reduction in EASIX was found in patients with TM, which may affect transplant outcomes and provide supporting evidence for pre-transplant use. In these 25 TM patients, treatment with thalidomide resulted in a significant reduction in LDH levels (291.0±97.7 vs. 236.4±51.7 U/L, p=0.003). In contrast, no significant changes were observed in creatinine (0.50±0.15 vs. 0.48±0.16 mg/dL, p=0.451) or platelet count (257±150 vs. 271±138 ×10^9/L, p=0.376). Thus, we believe that the improvement in EASIX is attributable to the alleviation of anemia and hemolysis, reducing iron overload, which is the main risk factor for thalassemia transplants.[14]
In summary, this study is the first to assess thalidomide's impact on EASIX in children with TM, offering new insights into its potential role as a pretransplantation therapy. Thalidomide treatment is a potential way to bridge patients to transplantation.
Ethics Statement
The study protocol was approved by the Medical Ethics Committee of the First People’s Hospital of Zigong.References
- Kattamis A, Kwiatkowski JL, Aydinok Y. Thalassaemia. Lancet. 2022;399:2310-24. https://doi.org/10.1016/S0140-6736(22)00536-0
- Liang
H, Pan L, Xie Y, Fan J, Zhai L, Liang S, Zhang Z, Lai Y. Health-related
quality of life in pediatric patients with beta-thalassemia major after
hematopoietic stem cell transplantation. Bone Marrow Transplant.
2022;57:1108-15. https://doi.org/10.1038/s41409-022-01663-0
- Luft
T, Benner A, Jodele S, Dandoy CE, Storb R, Gooley T, Sandmaier BM,
Becker N, Radujkovic A, Dreger P, Penack O. EASIX in patients with
acute graft-versus-host disease: a retrospective cohort analysis.
Lancet Haematol. 2017;4:e414-e23. https://doi.org/10.1016/S2352-3026(17)30108-4
- Jiang
S, Penack O, Terzer T, Schult D, Majer-Lauterbach J, Radujkovic A, Blau
IW, Bullinger L, Muller-Tidow C, Dreger P, Luft T. Predicting
sinusoidal obstruction syndrome after allogeneic stem cell
transplantation with the EASIX biomarker panel. Haematologica.
2021;106:446-53. https://doi.org/10.3324/haematol.2019.238790
- Mariotti
J, Magri F, Giordano L, De Philippis C, Sarina B, Mannina D, Taurino D,
Santoro A, Bramanti S. EASIX predicts non-relapse mortality after
haploidentical transplantation with post-transplant cyclophosphamide.
Bone Marrow Transplant. 2023;58:247-56. https://doi.org/10.1038/s41409-022-01874-5
- Kulkarni
UP, Pai AA, Kavitha ML, Selvarajan S, Lionel S, Devasia AJ, Korula A,
Fouzia NA, Sindhuvi E, Abraham A, Srivastava A, Mathews V, George B,
Balasubramanian P. Endothelial Activation and Stress Index-Measured
Pretransplantation Predicts Transplantation-Related Mortality in
Patients with Thalassemia Major Undergoing Transplantation with
Thiotepa, Treosulfan, and Fludarabine Conditioning. Transplant Cell
Ther. 2022;28:356 e1- e6. https://doi.org/10.1016/j.jtct.2022.05.001
- Milone
G, Bellofiore C, Leotta S, Milone GA, Cupri A, Duminuco A, Garibaldi B,
Palumbo G. Endothelial Dysfunction after Hematopoietic Stem Cell
Transplantation: A Review Based on Physiopathology. J Clin Med.
2022;11. https://doi.org/10.3390/jcm11030623
- Lai
X, Liu L, Zhang Z, Shi L, Yang G, Wu M, Huang R, Liu R, Lai Y, Li Q.
Hepatic veno-occlusive disease/sinusoidal obstruction syndrome after
hematopoietic stem cell transplantation for thalassemia major:
incidence, management, and outcome. Bone Marrow Transplant.
2021;56:1635-41. https://doi.org/10.1038/s41409-021-01233-w
- Caprari
P, Profumo E, Massimi S, Buttari B, Rigano R, Regine V, Gabbianelli M,
Rossi S, Risoluti R, Materazzi S, Gullifa G, Maffei L, Sorrentino F.
Hemorheological profiles and chronic inflammation markers in
transfusion-dependent and non-transfusion- dependent thalassemia. Front
Mol Biosci. 2022;9:1108896. https://doi.org/10.3389/fmolb.2022.1108896
- Abusin
GA, Abu-Arja R, Bajwa RPS, Horwitz EM, Auletta JJ, Rangarajan HG.
Severe transplant-associated thrombotic microangiopathy in patients
with hemoglobinopathies. Pediatr Blood Cancer. 2017;64. https://doi.org/10.1002/pbc.26503
- Jian
X, Liu X, Peng W, Li L, Hua F, Chen K, Zhang J, Luo S, Yang K, Wu Y.
Long-term efficacy and safety of thalidomide treatment in children with
beta-thalassemia major. Pediatr Blood Cancer. 2023:e30391. https://doi.org/10.1002/pbc.30391
- Yang
K, Liu X, Peng W, Hua F, Li L, Chen K, Zhang J, Luo S, Li W, Ding Y,
Chen J, Xiao J. Effects of Thalidomide on Erythropoiesis and Iron
Homeostasis in Transfusion-Dependent beta-Thalassemia. Mediterr J
Hematol Infect Dis. 2024;16:e2024001. https://doi.org/10.4084/MJHID.2024.001
- Chen
Y, Cai N, Lai Y, Xu W, Li J, Huang L, Huang Y, Hu M, Yang H, Chen J.
Thalidomide for the Treatment of Thrombocytopenia and Hypersplenism in
Patients With Cirrhosis or Thalassemia. Front Pharmacol. 2020;11:1137. https://doi.org/10.3389/fphar.2020.01137
- Xu F., Tang C., Huang Y., Liang L., Huang F., Yang G., Peng P. Quantitative analysis of liver iron deposition based on dual-energy CT in thalassemia patients. Mediterr J Hematol Infect Dis 2023, 15(1): e2023020. https://doi.org/10.4084/MJHID.2023.020