The production of CAR-T cells suitable for therapeutic purposes required the development of an efficient technology for genetic modification of human T cells using viral vectors, such as lenti- or retro-virus based vectors, and the introduction of co-stimulatory signaling domains that, together with CD3ζ or CD3γ endodomains, promote full T-cell activation: intracellular domains of molecules such as CD28 or 4-1BB in tandem with the CAR allowed the optimal transmission of signals producing sustained activation, proliferation, and effector function of CAR-T cells.[2]
The overall process of preparation of CAR-T cell products (6-12 weeks) involves isolation of the starting cell population from the leukapheresis product, T-cell activation, genetic modification with the retrovirus or lentivirus vector, ex vivo expansion, final product formulation, and product release testing.[3]
Six CAR-T cell products have been approved for the treatment of hematological malignancies: CD19-specific CAR-T cell products, Tisagenlecleucel (Tisa-Cel), Axicabtagene ciloleucel (Axi-Cel), Brexucabtagene autoleucel (Brexu-Cel) and Lisocabtagene maraleucel (Liso-Cel); BCMA-specific CAR-T cell products, Idecabtagene Vicleucel (Ide-Cel) and Ciltacabtagene autoleucel (Cil-Cel). The two cell products targeting BCMA have been approved for the treatment of patients with relapsed/refractory multiple myeloma (MM).
MM is a disorder of the monoclonal plasma cells and is the second most common hematologic malignancy. Newer treatments have markedly improved the survival of MM patients; however, MM remains an incurable disease, and most patients experience multiple relapses.[4] The outcome of patients who have relapsed after three or more lines of standard treatment or who are refractory to current treatments is poor. The therapeutic options available for these patients are limited. However, CAR-T cell targeting membrane antigens highly expressed on the surface of malignant plasma cells is emerging as an effective therapeutic option of MM patients with R/R disease. The present review highlights recent studies involving the clinical evaluation of CAR-T cell products in the treatment of MM patients.
B Cell Maturation Antigen (BCMA) Targeting with CAR-T Cells in Multiple Myeloma
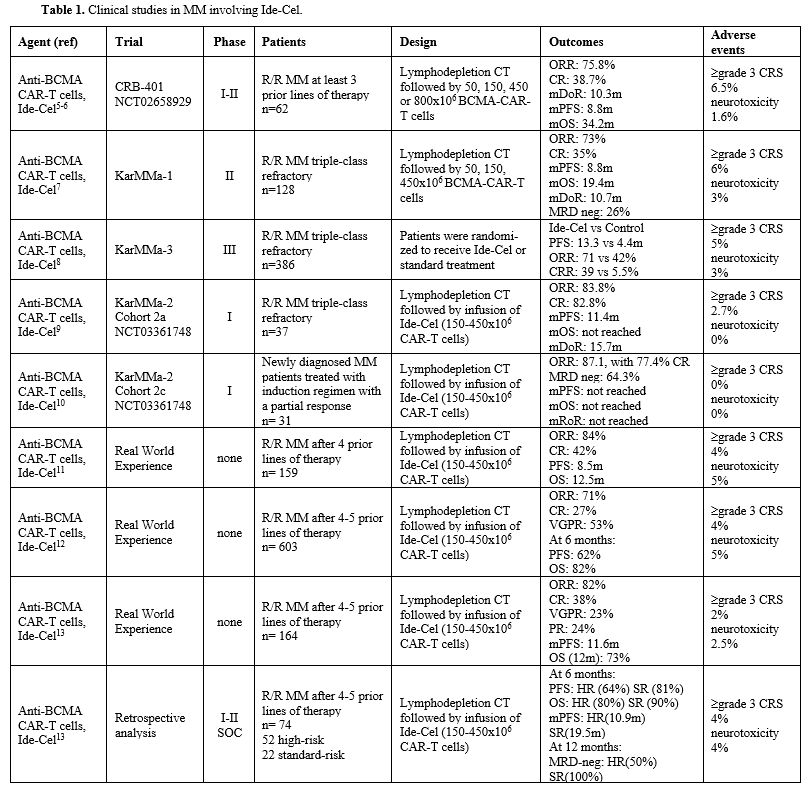
BCMA, also known as tumor necrosis receptor superfamily member 17 (TNFRSF 17) is a cell surface receptor of the TNFR superfamily which interacts with its ligand, B-cell activating factor (BAFF). This receptor is preferentially expressed in mature B lymphocytes and particularly in plasma cells (PCs); since its expression is rarely observed in other tissues, it represents a potentially important target for the treatment of patients with PC disorders.Idecabtagene violence (Ide-Cel) for treatment of multiple myeloma. Two main series of studies have been carried out in MM using Ide-Cel: CRB-401 study; KarMMa studies. (Table 1) The phase I CRB-401 study involved 62 R/R MM patients: the median follow-up was 18.1 months and 11 of 62 patients displayed ongoing responses, while 61% of patients displayed progressive disease.[5-6] The ORR was 75.8%, with 64.5% of patients achieving a very good partial response (VGPR) or better and 38.7% achieving a CR; response rate and depth increased with higher CAR-T cell dose.[6] With a median follow-up of 18 months, the mPFS and mOS were 88 and 34.2 months, respectively.[6] The decrease of BCMA levels in serum correlates with response to therapy; greater CAR-T expansion was observed in peripheral and bone marrow compartments in responding patients compared to nonresponding patients; correlates of long-term response included higher proportion of naïve and early memory CD4 T cells and a lower percentage of senescent CD3 and CD8 T cells in PBMC starting material for CAR-T cell generation.[6]
The KarMMA-1 study enrolled a total of 140 refractory MM patients: 73% of patients achieved a response (81% at the final target dose of 450x106 CAR-T cells), with 35% of CR and 26% of MRD negativity; the median PFS was 8.8 months (12.2 months at the final target dose of 450x106 CAR-T cells), the median OS was 19.4 months (24.8 months at the final target dose of 450x106 CAR-T cells), and the median DOR was 10.7 months (21.5 months for patients achieving a CR).[7] Based on these results, FDA approved Ide-Cel for the treatment of RR/MM patients who have received at least 4 prior lines of therapy.[7]
More recently, the phase III trial KarMMA-3 trial reported the results on 386 R/R MM patients who had received two to four regimens of previous treatments and who were refractory to the last treatment and who were randomly assigned in a 2:1 ratio to receive a treatment based either on Ide-Cel or one of five standard treatments.[8] At a median follow-up of 18.6 months, consistent and significant differences were observed in the group of patients treated with Ide-Cel compared to standard treatment: mPFS 13.3 vs 4.4 months; ORR 71% vs 42%; CRR 39% vs 5%.[8]
KarMMA-2 is a multicohort, phase II, multicenter trial evaluating the safety and the efficacy of Ide-Cel in MM patients with clinical high-risk newly diagnosed MM patients: cohorts 2a and 2b evaluated MM patients with early relapse after frontline autologous HSCT, while cohort 2c evaluated MM patients with inadequate response after frontline autologous HSCT. 37 patients were enrolled in the cohort 2a with an ORR of 83% (46% of CR), a 12-month MRD negativity in 70% of patients, a mPFS of 11.4 months and a mOS not reached, and a median DOR of 15.7 months.[9]
In cohort 2c, 31 patients were enrolled, and with a median follow-up of 27.9 months, Ide-Cel induced deep and durable responses, with an ORR of 87%, with 74% CR, with a 24-month PFS of 83%.[10] At a median follow-up of 39.4 months, all treated patients were alive, with 9.7% of patients having discontinued due to disease progression.[10] The ORR was 87%, with 77% of complete responses; the mDOR, mPFS, and mOS were not reached.[10]
The Myeloma CAR T Consortium reported the real-world experience in the treatment of R/R MM patients with Ide-Cel; 159 patients were evaluated, 75% of whom were considered ineligible for participation in the KarMMa trial.[11] Grade 3 CRS or neurotoxicity occurred in 3% and 6% of patients, respectively.[11] ORR and CRR was 84% and 42%, respectively; at a median follow-up of 6.1 months, the mPFS was 8.5 months and the mOS was 12.5 months; patients with previous BCMA-targeted therapy and with high-risk cytogenetics had a shorter PFS and OS in response to Ide-Cel.[11]
A retrospective analysis on 603 R/R MM patients infused with Ide-Cel reported an ORR of 71%, with 53 VGPR and 27% of CR; 6-month PFS was 62% and OS 82%.[12] The presence of extramedullary disease and a CAR-T cell dose <400x106 cells was associated with a lower CR rate.[12] Grade ≥3 CRS and neurotoxicity events were observed in 3% and 4% of cases, respectively.[12] Another real-world study confirmed these observations.[13]
Pesvolsky et al. have retrospectively analyzed 74 patients with high-risk features (HR-MM), including del(17p), t(4;14), t(14;16), 1q gain/amplification, extramedullary disease, plasma cell leukemia and R-ISS stage 3, treated in with Ide-Cel in a single cancer center.[14] For the HR-MM, the overall response rate was 85%, with 51% achieving a CR; the 6-month PFS for the HR-MM group was 64%, compared to 81% for the standard-risk group (SR-MM); the 6-month OS was 80% for HR-MM group compared to 90% for SR-MM group.[14] mPFS was 10.9 months for HR-MM and 19.5 months for SR-MM.[14]
Recent studies have explored biomarkers that could be associated with response to Ide-Cel-based therapy in KarMMa studies. Thus, Piasecki et al. have shown that in patients enrolled in the KarMMa-3 study, lower soluble BCMA levels were associated with higher ORR and higher CRR; furthermore, lower sBCMA levels were associated with lower-grade CRS and neurotoxicity events.[15]
Increased expression of inflammatory biomarkers, such as fibrinogen, ferritin, and protein C reactive, are associated with inferior OS, even after adjusting for covariates.[16]
Ciltacabtagene autoleucel (Cilta-Cel) in multiple myeloma. Cilta-Cel is CAR-T cell therapy based on a CAR encoding two anti-BCMA scFv antibodies. Several clinical studies defined as CARTITUDE have assessed the safety and efficacy of Cilta-Cel in MM patients (Table 2).
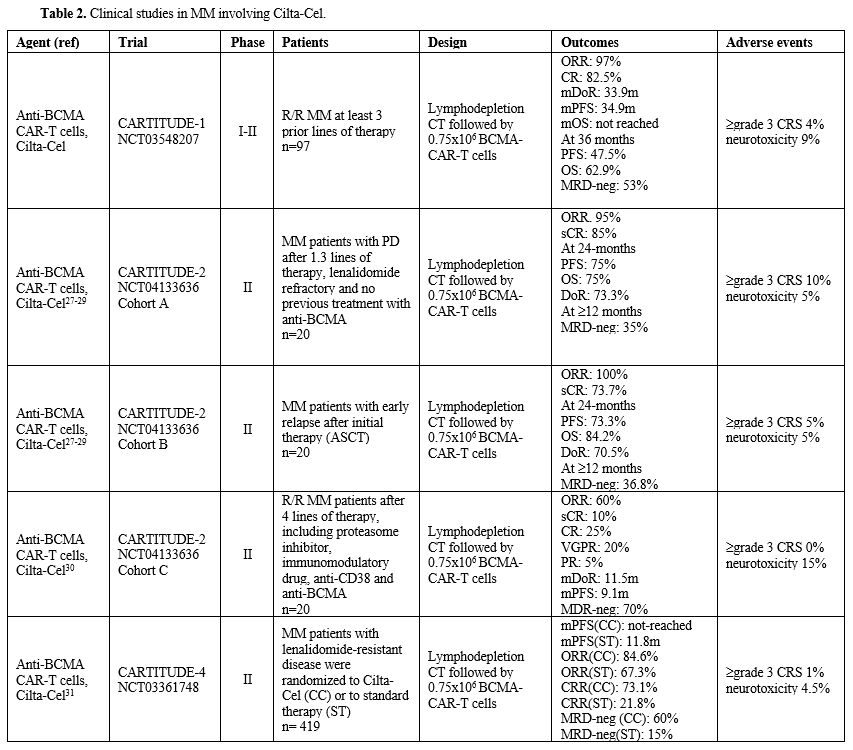
CARTITUDE-1. The CARTITUDE, phase I/II study evaluated Cilta-Cel in R/R MM patients. A first report on these studies evaluated 97 R/R MM patients (29 in phase Ib and 68 in phase II) who received three or more previous lines of therapy and were treated with a single infusion of Cilta-Cel (recommended dose 0.75x106 CAR-T cells/Kg).[17-18]
The final results of CARTITUDE-1 study showed the following results: mDoR 33.9 months, mPFS 34.9 months with 47.5% PFS at 36 months, mOS not reached, with 62.9% OS at 36 months, 53% of MRD negativity; six cases of secondary primary malignancy were reported; a total of 35 deaths occurred, mostly due to progressive disease and in only six cases related to Cilta-Cel treatment.[19] 85% of patients in cohort A and 73.7% in cohort B displayed a stringent complete response with a median follow-up of 29.9 months for cohort A and 27.9 months for cohort B.[20] Importantly, 35 patients in cohort A and 36.8% in cohort B had maintained MRD negativity at 10-5.[20]
Based on the results of this trial, Cilta-Cel was approved to treat R/R MM patients with at least three lines of prior therapy.
Seven patients enrolled in the CARTITUDE-1 study received prior allo-HSCT; the ORR in these patients was 85.7%, comparable to the rest of patients.[21] Seventy-eight patients who were enrolled in phase II of the CARTITUDE-1 study showed an improved quality of life.[22]
Patients treated with Cilta-Cel demonstrate improved efficacy compared to those enrolled in the LocoMMotion study and treated with immunomodulatory drugs and anti-CD38 antibodies.[23]
Infused CAR-T cells expanded, reaching a median peak concentration in the blood between days 12-14 post-infusion, and persisted in circulation for about 100 days; high efficacy and mDoR were not apparently related to the level CAR-T cell expansion and persistence of CAR-T cells in circulation over time.[24] A high CD8+ stem-like and a low CD4+ Treg-like phenotypes were associated with longer response to Cilta-Cel therapy. Interestingly, a high response rate was observed among patients with high-risk cytogenetics and high tumor burden.[26] Finally, the presence of a systemic inflammatory response (ferritin, C-reactive protein, and pro-inflammatory cytokines) was correlated with shorter DoR.[24]
CARTITUDE-2. The phase II CARTITUDE-2 study assesses Cilta-Cel in patients with MM in various clinical settings and evaluates the suitability of outpatient administration.
The cohort A of the CARTITUDE-2 study enrolled MM patients with progressive disease after 1-3 prior lines of therapy, lenalidomide-refractory and with no previous exposure to BCMA-targeting agents.[25] The results on the first 20 patients treated showed an ORR of 95%, with a CRR of 82%; PFS at six months was 90%, and at 12 months, 75%.[25] Of 13 patients evaluable for MRD status, 92% were MRD-negative.[25]
In cohort B of the CARTITUDE-2 study, the safety and the efficacy of Cilta-Cel are being evaluated in MM patients with early relapse after initial therapy; these patients have early relapse after autologous HSCT.[26] The results on 19 treated patients with a median follow-up of 12.4 months showed an ORR of 100%, 90% CR, 93% MRD-negativity, mDoR not reached, the 12-month event-free survival rate of 88.9%, and PFS of 90%.[26] A more extended follow-up of these two studies up to 27.9 months confirmed and extended the results previously reported with a 24-month PFS of 75% and 73.3% in cohorts A and B, respectively, and with a 24-month OS of 75% and 84.2% in cohort A and B, respectively.[27]
The cohort C of the CARTITUDE-2 trial enrolled MM patients who progressed despite prior treatments. The results on the first 20 patients enrolled, 80% refractory to prior anti-BCMA therapy, showed an ORR of 60%, an mDoR of 11.5 months, and a PFS of 9.1 months.[28] Seventy-five percent of patients evaluable for MRD status achieved an MRD-negative condition.[28]
CARTITUDE-4. In the phase II, randomized, open-label, CARTITUDE-4 trial, 419 patients with lenalidomide-refractory MM were randomized to receive Cilta-Cel or the physician’s choice of effective standard of care.[29] At a median follow-up of 15.9 months, the mPFS was not reached in the Cilta-Cel group and was 11.8 months in the standard care group; PFS at 12 months was 75.9% in the Cilta-Cel group and 48.5% in the standard-care group; ORR was 84.6% in the Cilta-Cel group compared to 67.3% in the standard-care group, CRR 73.1% vs 21.8% and MRD negativity 60.6% vs 15.6%, respectively.[29] Grade 3-4 CRS and neurotoxicity events were observed in 1% and 4.5%, respectively, of Cilta-Cel-treated patients.[29] The analysis of the CAR-treated patients of this trial showed at 16 month an ORR of 99.4%, with 86.4% of complete responses; 77% of patients achieved MRD negativity; mDoR was not reached, 12-month PFS was 89%.[30]
The phase I/II LEGEND 2 study reported the long-term effects of Cilta-Cel in 74 Chinese R/R MM patients; the 5-year PFS and OS rates were 21% and 49%, respectively; importantly, 16% of patients remained relapse-free, irrespective of baseline high-risk cytogenetic abnormalities.[31] 83.8% of patients suffered progressive disease; 61% of these patients could well respond to subsequent treatments.[31]
Interestingly, the long-term results of 49 Chinese R/R MM patients treated with the anti-BCMA CAR-T cells HDS269B were recently reported, showing a mPFS and mOS of 9.5 months and 20.0 months, respectively; the 5-year PFS and OS rates were 21% and 34%, respectively.[32] Patients with a good performance score [Eastern Cooperative Oncology Groups (ECOG) scores 0 to 2] had markedly longer survival, with mPFS of 12.0 months and mOS of 41.8 months.[32]
Indirect comparison of the efficacy of Ide-Cel versus Cilta-Cel. Martin and coworkers have made a matching-adjusted comparison of efficacy outcomes for Cita-Cel in the CERTITUDE-1 trial versus Ide-Cel in the KarMMa-1 trial: Cilta-Cel was associated with statistically improved ORR, CRR, DoR, PFS, and OS compared with Ide-Cel.[33] Cilta-Cel provides a clinical benefit over Ide-Cel across response outcomes and PFS for triple-class exposed R/R-MM patients treated with 2-4 prior lines of therapy.[34]
Mechanisms of resistance and relapse in BCMA-targeted CAR-T cell therapy in multiple myeloma. Baseline pretreatment BCMA levels on malignant plasma cells are not associated with response to BCMA CAR-T cell therapy.[35] Changes in BCMA expression over time may affect the effectiveness of CAR-T cell treatment. Following BCMA CAR-T cell infusion, BCMA expression decreased on residual MM cells (67% of patients), and in some of these patients, BCMA levels were restored at later time points.[36] The mechanism through which CAR-T cell therapy reduces BCMA expression is related to the selection of cells with lower BCMA expression and the elimination of those with higher BCMA expression.
Munshi et al., in the KarMMa-1 trial, observed that at baseline, 98% of the enrolled patients clearly expressed BCMA, with at least 50% BCMA-positive cells; at disease progression, 97% of patients displayed increased levels of soluble BCMA (sBCMA); loss of tumor BCMA expression was observed in 3 of 71 (4%) patients evaluated at progression.[7] Interestingly, in one of these three patients showing loss of BCMA expression, biallelic BCMA gene deletion was observed.[37] Importantly, in this study, it was also reported heterozygous BCMA gene loss or monosomy 16 in 37 of 168 patients with MM, including 28 of 33 patients with hyperdiploid MM, not previously treated with BCMA-targeting therapies.[37] A biallelic BCMA gene loss was observed also in another MM patient with hypoploidy relapsing 9 months after CAR-T cell therapy with Ide-Cel.[38] Truger et al. confirmed the frequent heterozygous deletions of the BCMA gene (4%) observed in MM patients, as well as heterozygous mutations of other immunotherapy-related genes, such as GPRC5D (15%), CD28 (10%), and SDC1 (5%).[39] A homozygous BCMA gene deletion was observed in a heavily pretreated MM patient after immunotherapy with BCMCA targeting T cell-redirecting bispecific antibody.[39]
A recent study based on the analysis of 2883 MM patients showed that monoallelic del 16p occurs in 8.58% of patients and is responsible for BCMA heterozygous gene loss.[40] High-risk deletion events, such as del 1p and del 17p, are frequently observed in patients with BCMA loss compared with those in other patients; however, high-risk CN gains were not significantly different between patients with and without 16p loss.[40] BCMA loss frequently co-occurs with other deletions, including TP53 and CDKN2C losses.[40] BCMA reflects an increased genomic instability.[40]
A study of whole genome sequencing confirmed the occurrence of biallelic BCMA gene deletion in some patients relapsing after BCMA-directed CAR-T cell therapy.[39] Furthermore, in patients relapsing after therapy with bispecific T cell engagers targeting BCMA, missense mutations or in-frame deletions in the extracellular domain of BCMA interfering with the efficiency of anti-BCMA TCE therapies were observed.[41]
Samur et al. have subdivided patients undergoing Ide-Cell CAR-T cell therapy into three different groups: those with no response to therapy and then relapsing shortly (within 12 months) and those with a durable response to CAR-T cell therapy.[42] Single-cell sequencing studies on plasma cells showed that non-responders displayed upregulated expression of genes involved in oxidative phosphorylation and proteasome degradation and decreased expression of apoptosis-related genes, as well as high expression of CD38, SLAMF7, Bcl-2, and FGFR3 genes; patients initially responding to therapy, at relapse exhibited an RNA expression profile comparable to that observed in non-responders.[42] Finally, in this study, a monoallelic BCMA loss was observed in 4% of pretreatment samples and 12% in relapsed samples, with 6% of biallelic BCMA gene loss.[42]
Many MM patients relapse after an initial response to BCMA-directed CAR-T cell therapy. A recent study analyzed the outcomes of 79 MM patients relapsing after BCMA-directed CAR-T cell therapy; these patients received a median of 2 treatment lines. The overall response rate to the first salvage treatment was 43.4%, with a mean PFS of 3.5 months; 44% of these patients received a T-cell engaging therapy (bispecific antibody or subsequent CAR-T) as salvage treatment, and their OS was not reached after a median follow-up of 21.3 months.[43]
Dual-targeting CAR-T cells (targeting BCMA and another antigen expressed on malignant plasma cells) may represent a strategy to overcome BCMA antigen loss in MM cells.[44-45]
Fischer et al. recently reported a detailed longitudinal analysis of cellular dynamics correlating with response, resistance, and/or toxicity in 27 heavily pretreated R/R MM patients undergoing treatment with Ide-Cel (10 CR, 6 VGPR/PR, and 11PD).[46] Evaluation of CAR-T cell dynamics post-infusion showed that the peak of CD3+ CAR-T cells was observed two weeks post-infusion and then declined in the following weeks, with no detectable circulating CAR-T cells 100 days post-infusion; the longitudinal analysis of cellular dynamics showed a similar profile in patients exhibiting CR, VGPR and PR; the peak of CD3+ CAR-T cells was markedly lower in patients with PD compared to those with CR or PR.[46] In MM patients with PD, the number of circulating CD3+ CAR-T cells always remained low post-infusion (days 7, 14, and 30). The majority (at least 80%) of CD3+ CAR-T cells are represented by CD8+ T lymphocytes.[46] The absolute number of CD8+ T cells was significantly lower in non-responders than in responders; responders have significantly higher levels of CD8+ than CD4+ lymphocytes; non-responders have an increased proportion of Treg and PD1+ T lymphocytes.[46]
Strategies to improve the efficacy of BCMA-directed CAR-T cell therapy. Target antigen density may limit the efficacy of BCMA-targeted CAR-T cell therapy in MM patients. Surface BCMA expression is modulated by γ-secretase (GS). This protease mediates the cleavage of some proteins, including BCMA, resulting in the generation and release of soluble BCMA (sBCMA), corresponding to the extracellular domain of BCMA and part of the transmembrane region. G-secretase inhibitors can be used to increase membrane expression of BCMA on MM cells, thus improving their recognition by CAR-T cells.[47] In vitro and in vivo studies have shown that γ-secretase inhibition increases the efficiency of BCMA-specific CAR-T cells in MM.[47]
These observations have provided the rationale for a clinical trial investigating the safety and efficacy of a γ-secretase inhibitor in association with BCMA CAR-T cells. In this phase I study, the γ-secretase inhibitor Crenicagestat was administered before and after CAR-T cell infusion to 18 R/R MM patients. The administration of this inhibitor prior to CAR-T cell infusion resulted in a 12-fold increase in BMCA expression in MM cells.[48] In this phase I study (NCT 03502577) fully human BCMA-targeting CAR-T cells were used. The ORR was 89%, with 44% of CR; with a median follow-up of 20 months, the PFS is 11 months.[48] Frequent non-hematological adverse events of grade 3 or more were represented by hypophosphatemia (78%), fatigue (61%), hypercalcemia (50%), and hypertension (39%); two deaths related to treatment were observed.[49]
Fully human BCMA CAR-T. Fully human CAR-T cells targeting BCMA were based on a lentiviral vector encoding a fully human BCMA scFv. Green et al. reported the evaluation of fully human CAR-T cells obtained through transduction of CD4+: CD8+ T cells in a 1:1 ratio with a lentivirus encoding a fully human BCMA scFv.[50] 7 R/R MM patients with a median of 8 prior treatments were treated with fully human BCMA-targeted CAR-T cells: ORR was 100% and all patients were surviving after a median of 16 weeks; one patient relapsed at day +60.[50]
Two studies have explored another fully human CAR-T product (CT103A) targeting BCMA in 18 R/R MM patients, reporting a 100% ORR; at 1 year, the PFS was 58% for all patients and 79% for patients without extramedullary disease.[51] The median persistence of the CAR transgene in vivo was 307 days.[51] Recently, a long-term evaluation (median follow-up 41.47 months) on these patients was reported: the rate of PFS and OS at 2 year was 50% and 72%; the patients with extramedullary disease had shorter survival than those without extramedullary disease (PFS 12 months vs 27.9 months, respectively; OS 28.5 months vs 41.9 months).[52] At the latest time of follow-up, 50% of patients are still alive, and 39% remained in CR.[52] Using these CAR-T cells, a phase I/II clinical trial (FUMANBA-1) was carried out, enrolling a total of 103 R/R MM who received a median of 4 lines of prior therapy; with a median follow-up 13.8 months, the ORR was 96%, with 74% of CR; median DoR and median PFS were still not reached; the 12-month PFS rate was 78.8%.[53] For patients without prior BCMA CAR-T therapy, ORR was 99%, with 79% CR; for patients with prior BCMA CAR-T cell therapy, 4/5 achieved a CR, maintained over 18 months post-infusion.[53] 95% of treated patients achieved an MRD-negative status and 82% maintained MRD negativity over 12 months.[53]
Juno Therapeutics developed a CAR-T product (JCARH125, orvacabtagene autoleucel) comprised of autologous CD4+ and CD8+ cells transduced with a lentiviral vector encoding a BCMA-specific CAR.[54] A phase I clinical study was carried out using BMS-986354, a next-generation CAR-T cell product containing the same fully human BCMA-targeted CAR construct as orva-cel and manufactured using the NEXT-T process allowing the generation of a less differentiated CAR-T cell product with improved potency and tumor control compared to orva-cel.[55] 55 patients have been treated with BMS-986354, with a very good safety profile.[55] After a follow-up of 4-9 months, the ORR was 98%, with 57.4% of patients achieving a VGPR and 29.6% a CR.[55]
Sperling et al. have evaluated the safety and efficacy of PHE885 fully human BCMA-targeted CAR-T cells manufactured using the T-ChargeTM platform, which takes <2 days to manufacture the final CAR-T cell product.[56] A phase I study evaluated 15 R/R MM with at least two prior lines of therapy.[56] In the dose-escalation phase, the patients were treated at fixed doses. 2 patients had grade 3 CRS. ORR was 93%, with 33% of CRs; 33% of patients were MRD-negative at 10-6.[56] An updated report of phase I study showed the results obtained in the first 46 enrolled patients.[57] 11% of patients had grade 3 CRS, and 7% had grade 3 neurologic events.[56] For all-treated patients, the ORR was 98%; for patients treated at the 10x106 cells, the ORR was 100%, and the CRR was 42%; 60% of these patients were MRD-negative.[57]
The phase I trial LUMMICAR STUDY1 explored zevoircabtagene autoleucel (Zevor-Cel), a fully human autologous BCMA CAR-T, and showed in 14 R/R MM patients that Zevor-Cel is well tolerated, with an ORR of 100%.[58] In phase II, 102 R/R MM patients were treated with Zevor-Cel with an ORR of 92.8% and a 6-month PFS of 90%; 92% of patients with a CR achieved an MRD-negative status.[58] The evaluation of patients reported in the phase I study with a median follow-up of 37.7 months showed a PFS of 25 months and a mDoR of 24 months.[59]
Allogeneic BCMA-targeting CAR-T cells. Preclinical studies have shown that allogeneic anti-BCMA CAR-T cells generated from the T cells of healthy controls are superior to autologous anti-BCMA CAR-T cells derived from MM T cells.[60] In fact, healthy donors showed increased T cell counts, higher CD4/CD8 ratio, and expanded naïve T lymphocyte population compared to patients with MM.[60] Compared to healthy controls, patients with R/R MM have lower frequencies of CAR-T cells, decreased control memory phenotype, and increased expression of inhibitory checkpoint markers.[60] CAR-T cells derived from healthy donors efficiently kill MM cells within the BM microenvironment of different MM genomic subgroups, and their cytotoxic activity can be improved with gamma-secretase inhibitors.[60]
Lin et al. have described the design of inducible chimeric cytokine receptors that mimic the signaling of the native receptors and whose activity can be controlled by pharmacological agents.[61] Ligand-independent, constitutively active chimeric cytokine receptors (called turbodomains) can be introduced into the structure of a CAR, potentiating CAR-T cell function; using this approach, allogeneic anti-BCMA CAR-T cells, engineered to express a turbodomain mimicking IL-15, were generated and their use in MM was proposed.[62]
ALLO-715 contains an integrated, self-inactivating, third-generation, recombinant lentiviral vector that expresses a second-generation anti-BCMA CAR containing a scFv derived from a human anti-BCMA antibody and intracellular domains of 4-1BB and CD3ζ; the extracellular domain of the BCMA CAR also contains two mimitope conferring susceptibility to anti-CD20 antibody; furthermore, two additional changes were introduced through gene editing using nuclease TALEN technology: knockout of T-cell receptor alpha constant and knockout of CD52 (to reduce the risk of GvHD and to protect CAR-T cell destruction through via host-versus graft reaction).[61] Escalating doses of ALLO-715 after lymphodepletion with an anti-CD52 antibody-containing regimen was evaluated in 43 patients with relapsed/refractory MM as part A of the phase I UNIVERSAL trial.[61] The ORR was 55%; in patients treated at 320x106 CAR-T cells, the ORR was 70%, with 45.8% of VGPR and 25% of CR; the mDoR was 8.3 months.[63]
P-BCMA-ALLO1 CAR-T cells are manufactured from healthy donor T-cells using non-viral transposon-based integration (piggyBac DNA delivery system), introducing an anti-human BCMA VH-based CAR and an iCas 9 safety-switch gene-editing system to eliminate endogenous TCR expression via knockout of the TCR beta chain 1 gene and the beta 2 microglobulin gene (to improve the immunological tolerance of allogenic CAR-T cells).[64] Using these CAR-T cells, phase I is ongoing in R/R MM patients; an ORR of 82% was observed in the first two arms of patients (P1 and P2); in the P2 arm, 40% of patients achieved a CR; the treatment was well tolerated with no GvHD at any dose; analysis of P-BCMA-ALLO1 cellular kinetics in two patients showed a CAR-T persistence up to 6 weeks.[64]
GPRCD5 targeting using CAR-T cells. The orphan G protein-coupled receptor, class C, group member D (GPRC5D) is normally expressed only in the hair follicle. However, this membrane receptor is expressed on CD138+ MM cells from primary bone marrow samples, with a distribution independent of BCMA.[65] GPRC5D-specific CAR-T cells showed potent anti-MM activity in preclinical xenograft models.[65]
In a phase II clinical study, Xia and coworkers evaluated 33 R/R MM patients the safety and the efficacy of anti-GPRC5D CAR-T cells; at a median follow-up of 5.2 months, the ORR was 91%, including 33% of CR; VGPR or PR were observed in 100% of patients with previous anti-BCMA treatment.[66] The safety profile was favorable, with no grade 3 CRS and 1 event of grade 3 neurotoxicity.[66]
Zhang et al. have developed and clinically evaluated autologous GPRC5D-directed CAR-T cells (OriCAR-017) containing an Ori element to improve CAR-T expansion and durability.65 In a phase I study 9 R/R MM patients received a single infusion of OriCAR-017 (3 at 1x106/Kg; 3 at 3x106/Kg; 3 at 6x106/Kg); ORR was 100%, with 60% of CR and 40% of VGPR; no grade 3 CRS or neurotoxicity were observed.[67]
Mailankody and coworkers reported the development of autologous MCARH109 T-cell therapy anti-GPRC5D.[66] These CAR-T cells were evaluated in a phase I study involving the enrollment of 17 patients with R/R MM; MCARH109 was administered at various doses and the maximum tolerated dose was 150x106 cells; 81% of ORR was observed in the entire cohort and in 58% of patients receiving doses from 25x106 to 150x106 cells; major toxicity events were observed in patients treated at the highest dose of 450x106 cells.[68]
Bal et al. initially reported the results of a BMS-986393 (CC-95266) trial phase I GPRC5D-targeted CAR-T cell therapy in 14 patients with R/R MM; in this heavily pretreated population, the initial ORR was 86%, including 4/6 patients treated previously with anti-BCMA-targeted therapies.[69] An updated report of this study showed the results observed in 70 R/R MM patients treated with different doses of BMS-986393 from 25x106 cells to 450x106 cells; the overall response rate was 85.9%, with 37.5% of CR (this high rate of responses was observed both at low, intermediate and high CAR-T cell doses); in patients, refractory to prior BCMA therapies, ORR was 85%, and CR was 46%; patients with CR resulted to be MRD-negative; safety profile was good for patients treated from 25x106 to 150x106 CAR-T cells and toxicity events were mostly observed in patients treated with 300x106 or 450x106 CAR-T cells.[70]
A recent study reported the first evaluation of anti-GPRC5D/BCMA bispecific CAR-T cells in 21 R/R MM patients at four dose levels (0.5, 1, 2, and 4x106 cells/kg); MTD was reached at the 2x106 dose level; ORR was 86%, with 62% of CR and 81% of patients achieving MRD-negativity.[71] The rate of response appeared encouraging, but the follow-up was limited to 5-8 months.
CAR-T-mediated CD19 targeting in MM patients. CD19 is typically absent in the dominant MM cell population but may be present in minor cell subsets with unique stem cell properties (myeloma propagating cells) (Table 3).
 |
|
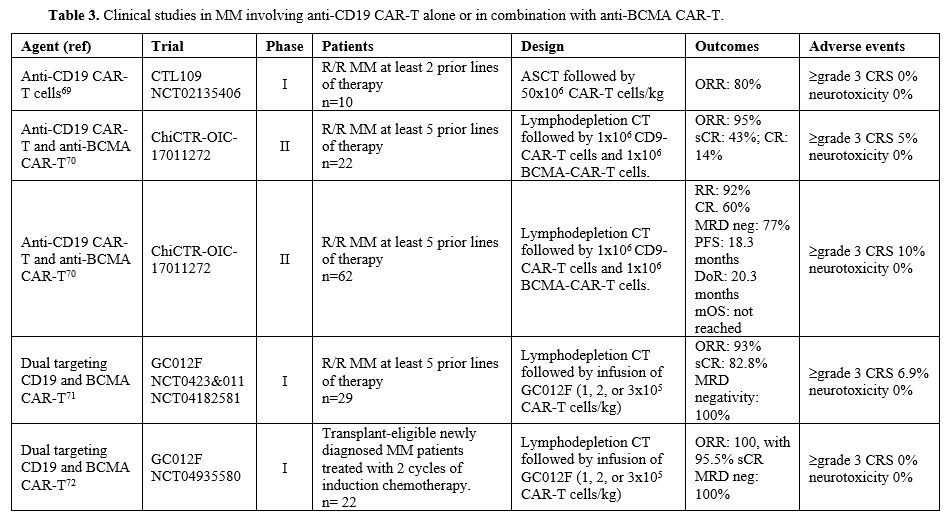
Garfall et al. evaluated autologous CD19 CAR-T cells (CTL-109) in 10 R/R MM patients; the patients received CTL109 following salvage high-dose melphalan and ASCT.[72] This treatment was safe and feasible, with most toxicities attributable to ASCT and no to severe CRS.[72] Two of the ten treated patients displayed significantly longer PFS after ASCT+CT109 compared with prior ASCT (479 vs. 181 days; 249 vs. 127 days).[72]
Yan et al. reported the study of 21 R/R MM patients undergoing treatment with humanized anti-CD19 and anti-BCMA CAR-T cells; 95% of patients had a response to treatment: 43% stringent CR (sCR), 14% CR, 24% VGPR, and 14% PR.[73] 90% of the patients had CRS, and in 4% was of grade 3.[73] Wang et al. reported the extension and the long-term analysis of this study involving 62 R/R MM patients with a median follow-up of 21.3 months; the ORR was 92%, with 60% of CR and 77% of MRD negativity; mDoR was 20.3 months, mPFS was 18.3 months and mOS was not reached.[74]
Du et al. reported the results of two different clinical studies involving the use of GC012F, a BCMA, and CD19 dual-targeting CAR-T cells developed in the novel FasTCAR-T platform, enabling manufacturing of CAR-T cells within 22-36 hours. A first phase I study was carried out in 29 R/R MM patients (90% high-risk, 27% with extramedullary disease, and 34% pretreated with anti-BCMA agents) and reported an ORR of 93%, with 83% of stringent CR; mDoR was 37 months and mPFS was 38 months.[76]Grade 3 CRS was observed in 6.9% of cases.[75] The second study was carried out in 22 high-risk MM patients treated in first-line with GC012F (either at 1x105 cells/kg or 2x105 cells/kg or 3x105 cells/kg); with a median follow-up of 13.6 months, ORR was 100%, with 12-month PFS, OPS, and DoR not reached and with 100% of patients achieving a MRD-negative status.[76] None of the treated patients developed grade 3 or more CRS or neurotoxicity events.[76]
Another study carried out in 50 Chinese R/R MM patients confirmed a high ORR (92%) following treatment with bispecific BCMA/CD19 (BC19) CAR-T cells; mPFS and mOS were 19.7 months.[77]
Garfall and coworkers have carried out a phase I clinical trial in which anti-BCMA and anti-CD19 CAR-T cells were administered to MM patients with low tumor burden, including patients with >2 prior lines of therapy who responded to the third or later line of therapy and patients with high-risk disease responding to first-line therapy.[78] Among 15 patients with measurable disease, 10 exhibited PR or better; among 26 subjects responding to prior therapy, 9 improved their response category, and 4 converted to MRD-negativity/sCR.[78]
CAR-T-mediated SLAMF7 (CS1) targeting in MM patients. SLAMF7 (also known as CS1 or CD319), a member of the signaling lymphocyte activation molecule family of receptors is highly expressed in malignant plasma cells and represents a potential target for immunotherapy. Several groups of investigators have developed anti-SLMF47 CAR-T cells and tested them in preclinical models, reporting an efficient killing of malignant plasma cells.
Clinical trials are now evaluating anti-SLAMF7 CAR-T cells in MM patients. In this context, a recent study reported the first results of a phase I clinical study reporting the evaluation of the bispecific CS1-BCMA autologous CAR-T cells in 16 R/R MM patients: 13 patients with BM disease responded to treatment, while 3 patients with extramedullary disease were refractory; among the 13 responding patients, 6 achieved a CR, 3 a very good partial response and a partial response; 1 year PFS and OS were 72.7% and 56.5%, respectively.[79]
A recent study reported the evaluation of allogeneic anti-SLAMF7 CAR-T cells (UCARTCS1) in mouse xenograft models, which displayed both in vitro and in vivo activity.[80] UCARTCS1 cells also exerted some cytolytic activity against normal immune cells expressing SLAMF7 but to a lower extent compared to MM cells.[80] These observations warrant UCARTCS1 cell evaluation in patients with advanced MM.
Conclusions
CAR-T cells, as well as other T-cell therapies, were developed to address the need for more effective therapies for the treatment of MM patients who have been heavily pretreated.Outcomes after CAR-T cell therapy are affected by both factors intrinsic to CAR-T cells, such as the type of CAR-T cell product or endogenous T cells, and extrinsic to CAR-T cells, such as the genetic features of MM cells, the tumor microenvironment, and host characteristics.
Anti-BCMA CAR-T cell therapies currently approved by the FDA and EMA have consistently improved the clinical outcomes of heavily pretreated R/R MM patients. However, despite this consistent progress, MM remains an incurable disease.
Although MM patients with high-risk diseases, including ISS stage III, high-risk cytogenetic abnormalities, or extramedullary disease, display a poor response to standard treatments, their outcome was improved by CAR-T cell-based treatments.
Despite these significant improvements in the response of R/R MM patients, outcomes for these patients following CAR-T cell therapy remain highly variable, with some patients exhibiting either no response or only brief responses and with other patients with long-term PFS following treatment. Before the start of treatment, there are no clear criteria to predict the response to CAR-T cell therapy. A deeper understanding of the mechanisms underlying durable remission after CAR-T cell therapy is needed.
A significant proportion of MM patients relapse after CAR-T cell treatment, and there is a need to define optimal salvage therapeutic strategies for these patients. The use of CAR-T cells targeting membrane antigens different from BCMA and expressed on the membrane of MM cells, such as GPRCD, may represent a potentially important strategy for the improvement of the efficacy of BMCA CAR-T cell therapy or for the treatment of patients relapsing after BCMA CAR-T cell therapy.
It is interesting to note that BCMA loss is lower after CAR-T cell therapy than after therapy with bispecific anti-BCMA antibodies (BsAbs), suggesting that the efficacy of BsAbs is most likely retained if CAR-T cell therapy is used before treatment with BsAbs.
Some studies (KarMMa-3 and CARTITUDE-4) have explored the therapeutic efficacy of BCMA-CAR-T cells in earlier lines of therapy and have provided evidence in favor of a superiority of CAR-T cell therapies compared to standard treatments in this patient population. Additional future clinical trials will be required to define the role of CAR-T cell therapy in early treatment settings.
A deeper understanding of the molecular mechanisms responsible for CAR-T cell resistance has provided the support for ongoing and for future clinical studies based on CAR-T cells.
Another area of development of future clinical studies will consist in the identification of other membrane targets of CAR-T cells with the aims of consolidating the therapeutic effects elicited by BCMA CAR-T cells or of providing a therapeutic opportunity for patients relapsing after BCMA CAR-T cell therapy.
An important area of investigation is represented by the exploration of a possible benefit induced by CAR-T cell therapy after ASCT. A recent retrospective analysis suggested that CAR-T cell therapy after ASCT improved outcomes compared with salvage ASCT alone in relapsed MM patients.[81] Furthermore, a single-arm exploratory clinical trial of sequential anti-CD19 and anti-BCMA CAR-T cell infusion after ASCT in 10 high-risk newly diagnosed MM patients supported the safety and the possible efficacy of this approach.[82] Finally, a few R/R MM patients treated with Celta-Cel after allogeneic SCT showed a safety profile consistent with patients without allo HSCT as prior therapy.[19] The good safety profile and the clinical benefits observed in these preliminary clinical observations warrant ongoing randomized controlled clinical trials.
Acknowledgment
We thank Professor Pellegrino Musto for the useful suggestions.References
- Eshhar Z, Waks T, Gross G, Schindler DG, Specific
activation and targeting of cytotoxic lymphocytes through chimeric
single chains consisting of antibody-binding domains and the gamma or
zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl
Acad Sci USA 1993; 90: 720-724. https://doi.org/10.1073/pnas.90.2.720
- Savoldo
B, Ramos CA, Liu E, Mims MP, Keating MJ, Carrum G, Kamble RT, Bollard
CM, Gee AP, Mei Z, et al. CD28 costimulation improves expansion and
persistence of chimeric antigen receptor-modified T cells in lymphoma
patients. J Clin Invest 2011; 121: 1822-1826. https://doi.org/10.1172/JCI46110
- Ceya
MA, Khericha M, Harris CM, Puig-Saus C, Chen YY. CAR-T cell
manufacturing: major process parameters and next-generation strategies.
J Exp Med 2024; 221: e20230903. https://doi.org/10.1084/jem.20230903
- Wiedmeier-Nutor
JE, Bergsagel PL. Review of multiple myeloma genetics, including
effects on prognosis, response to treatment, and diagnostic workup.
Life 2022; 12; 812. https://doi.org/10.3390/life12060812
- Raje
N, Berdeja J, Lin Y, Siegel D, Jagannath S, Madduri D, Liedtke M,
Rosenblatt J, Maus M, et al. Anti-BCMA CAR T-cell therapy bb2121 in
relapsed or refractory multiple myeloma. N Engl J Med 2019; 380:
1726-1737. https://doi.org/10.1056/NEJMoa1817226
- Lin
Y, Raje NS, Bardeja JG, Siegel DS, Jaganath S, Madduri D, Liedtke M,
Rosenblatt J, Maus MV, Massaro M, et al. Idecabtagene vicleucel for
relapsed and refractory multiple myeloma: post hoc 18-month follow-up
of a phase 1 trial. Nat Med 2023; 29: 2286-2294. https://doi.org/10.1038/s41591-023-02496-0
- Munshi
NC, Anderson LD, Shah N, Madduri N, Berdeja J, Lonial S, Raje N, Lin Y,
Siegel D, Oriol A, et al. Idecabtagene vicleucel in relapsed and
refractory multiple myeloma. N Engl J Med 2021; 384: 705-716. https://doi.org/10.1056/NEJMoa2024850
- Rodriguez-Otero
P, Allawadhi S, Arnulf B, Patel K, Cavo M, Nooka AK, Manier S,
Callander N, Costa LJ, Vij R, et al. Ide-cel or standard regimens in
relapsed and refractory multiple myeloma. N Engl J Med 2023; 388:
1002-1014. https://doi.org/10.1056/NEJMoa2213614
- Usmani
S, Patel K, Hari P, Berdeja J, Alsina M, Vij R, Raje N, Leleu X,
Dhodapakar M, Reshef R, et al. KarMMa-2 cohort 2a: efficacy and safety
of idecabtagene vicleucel in clinical high-risk multiple myeloma
patients with early relapse after frontline autologous stem cell
transplantation. Blood 2021; 140 (suppl.1): 875-877. https://doi.org/10.1182/blood-2022-162469
- Dhodapakar
M, Alsina M, Berdeja J, Patel K, Vij R, Leleu X, Truppel-Hartmann A,
Basudhar D, Thompson E, Zheng X, et al. KarMMa-2 cohort 2c: efficacy
and safety of idecabtagene vicleucel in patients with clinical
high-risk multiple myeloma due to inadequate response to frontline
autologous stem cell transplantation. Blood 2022; 14 (suppl.1):
7441-7443. https://doi.org/10.1182/blood-2022-162615
- Hansen
DK, Sidana S, Peres LC, Leitzinger CC, Shune L, Shrewsbury A, Gonzalez
R, Sborov DW, Wagner C, Dima D, et al. Idecabtagene vivleucel for
relapsed/refractory multiple myeloma: real-world experience from the
myeloma CAR T consortium. J Clin Oncol 2023; 41: 2087-2097. https://doi.org/10.1200/JCO.22.01365
- Cayla
S, Ferment B, Bigot N, Caillot D, Lafon I, Karlin L, Lazareth A,
Touzeau C, Leleu X, Moya N, et al. French early natiowide idecabtagene
vicleucel (Ide-Cel) chimeric antigen receptor (CAR) T-cell therapy
experience in patients with relapsed/refractory multiple/myeloma
(FENIX): update of the IFM study from the Descar-T registry. Blood
2023; 142( suppl.1): 2139. https://doi.org/10.1182/blood-2023-188655
- Sidana
S, Ahmed N, Akhtar OS, Heim M, Brazauyskas R, Hansen DK, Ferreri C,
Freeman CL, Afrough A, et al. Rel world outcomes with idecabtagene
vicleucel (Ide-Cel) CAR-T cell therapy for relapsed/refractory multiple
myeloma. Blood 2023; 142 (suppl.1): 705. https://doi.org/10.1182/blood-2023-181762
- Pasvolsky
O, Hildebrandt M, Subramanian N, Ferreri C, Lee H, Manasanch EE, Thomas
SK, Weber DM, Gaballa M, Dilalrd C, et al. Safety and efficacy outcomes
for patients with high-risk multiple myeloma receiving idecabtagene
vicleucel: the MD Anderson experience. Blood 2023; 142 (suppl.1): 4712.
https://doi.org/10.1182/blood-2023-187854
- Plasecki
J, Desai K, Courtney C, Thompson E, Pratt J, Raje NS, Patel KK, Raab
MS, Cook M, Simonsen KL, et al. Baseline and early post-infusion
biomarkers associated with optimal response to idecabtagene cicleucel
(Ide-cel) in the KarMMa-3 study at triple-class-exposed (TCE) relapsed
and refractory multiple myeloma (RRMM). J Clin Oncol 2023; 41(suppl.
16): 8031. https://doi.org/10.1200/JCO.2023.41.16_suppl.8031
- Pan
DD, Mouhieddine TH, Fu W, Moshier E, Parekh S, Jagannath S, Rossi AC,
Cho HJ, Richter J, Sanchez LJ, et al. Inflammatory biomarkers and
outcomes in multiple myeloma patients after CAR T-cell therapy. Blood
2023; 142 (suppl.1): 92. https://doi.org/10.1182/blood-2023-188310
- Berdeja
JG, Madduri D, Usmani SZ, Jakubowiak A, Agha M, Cohen AD, Stewart AK,
Hari P, Htut M, Lesokhin A, et al. Ciltacabtagene autoleucel, a B-cell
maturation antigen-directed chimeric antigen receptor T-cell therapy in
patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a
phase 1b/2 open-label study. Lancet 2021; 398: 314-324. https://doi.org/10.1016/S0140-6736(21)00933-8
- Martin
T, Usmani SZ, Berdeja JG, Agha M, Cohen AD, Hari P, Avigan D, Deol A,
Htut M, Lesokhin A, et al. Ciltacabtagene autoleucel, an anti-B-cxell
maturation antigen chimeric antigen receptor T-cell therapy, for
relapsed/refractory multiple myeloma: CARTITUDE-1 2-year follow-up. J
Clin Oncol 2023; 41: 1265-1274. https://doi.org/10.1200/JCO.22.00842
- Lin
Y, Martin T, Usmani SZ, Berdeja J, Jakubawiak A, Agha M, Cohen A, Deol
O, Htut M, Lesakhin A, Munshi N, et al. MM-309 CARTITUDE-1 final
results: phase 1b/2 study of ciltacabtagene autoleucel in heavily
pretreated patients with relapsed/refractory multiple myeloma. Clin
Lymphoma Myeloma Leukemia 2023; 23 (suppl.1): 5488-5489. https://doi.org/10.1016/S2152-2650(23)00862-5
- Cohen
AD, Parewkh S, Santomasso BD, Perez-Larraya JC, van de Donk N, Arnulf
B, Mateos MV, Lendvai N, Jackson CC, Braganca K, et al. Incidence and
management of CAR-T neurotoxicity in patients with multiple myeloma
treated with ciltacabtagene autoleucel in CARTITUDE studies. Blood
Cancer J 2022; 12: 32. https://doi.org/10.1038/s41408-022-00629-1
- Htut
M, Cohen AD, Martin T, Berdeja JG, Usmani SZ, Agha M, Jackson CC,
Madduri D, Deraedt W, Zudaire E, et al. Ciltacabtagene autoleucel in
patients with prior allogeneic stem cell transplant in the CARTITUDE-1
study. Clin Lymphoma Myeloma Leuk 2023; S2152. https://doi.org/10.1016/j.clml.2023.08.012
- Martin
T, Agha M, Cohen AD, Htut M, Stewart AK, Hari P, Berdejs JG, Usmani SZ,
Yeh TM, Olyslager Y, et al. Health-related quality of life in patients
given ciltacabtagene autoleucel for relapsed or refractory multiple
myeloma (CARTITUDE-1): a phase 1b-2, open-label study. Lancet Hematol
2022; 9: e897-e905. https://doi.org/10.1016/S2352-3026(22)00284-8
- Mateos
MV, Weisel K, Martin T, Berdeja JG, Jakubowiak A, Stewart AK, Jagannath
S, Lin Y, Diels J, Ghilotti F, et al. Adjusted comparison of outcomes
between patients from CARTITUDE-1 versus multiple myeloma patients with
prior exposure to proteasome inhibitors, immunomodulatory drugs and
anti-CD38 antibody from the prospective, multinational LocoMMotion
study of real-world clinical practice. Haematologica 2023; 108:
2192-2202. https://doi.org/10.3324/haematol.2022.280482
- Montes
de Oca R, Gu J, Zhao H, Zelinsky K, Wu D, Davis C, Patel J, Foulk B,
Boominathan R, Lau O, et al. Biomarker correlates of response to
ciltacabtagene autoleucel in patients with relapsed or refractory
multiple myeloma from CARTITUDE-1, a phase 1b/2 open-label study, al
the 3-year follow-up. Blood 2023; 142 (suppl.1): 2099. https://doi.org/10.1182/blood-2023-182298
- Cohen
EH, Delforge M, Hillengass J, Goldschmidt H, Weisel K, Raab MS, Scheid
C, Schecter JM, De Braganca KC, Varsos K, et al. CARTITUDE-2 update:
ciltacabtagene autoleucel, a B-cell maturation antigen-directed
chimeric antigen receptor T-cell therapy, in lenalidomide-refractory
patients with progressive multiple myeloma after 1-3 prior lines of
therapy. Hema-Sphere 2022; 6 (suppl.2): P08. https://doi.org/10.1097/01.HS9.0000829604.35383.e8
- Van
de Donk N, Agha ME, Cohen AD, Cohen YC, Anguille S, Kerra T, Roeloffzen
W, Scehter JM, De Braganca KC, Varsos H, et al. Biological correlative
analyses and updated clinical data of ciltacabtagene autoleucel
(cilta-cel), a BCMA-directed CAR-T cell therapy, in patients with
multiple myeloma (MM) and early relapse after initial therapy.
CARTITUDE-2, cohort B. J Clin Oncol 2022; 40 (suppl. 16): 8029. https://doi.org/10.1200/JCO.2022.40.16_suppl.8029
- Hillengass
J, Cohen AD, Agha ME, Delforge M, Kerre T, Roeloffzen W, Einsele H,
Goldschmidt H, Weisel K, Raab MS, et al. the phase 2 CARTITUDE-2 trial
updated efficacy amd safety of ciltacabtagene autoleucel in patients
with multiple myeloma and 1-3 prior lines of therapy (cohort A) and
with early relapse after first line treatment (cohort B). Blood 2023;
142 (suppl.1): 1021. https://doi.org/10.1182/blood-2023-178882
- Cohen
AD, Mateos MV, Cohen YC, Rodriguez-Otero P, Pavia B, van de Donk N,
Martin T, Suvannsankha A, De Braganca KC, Corsale C, et al. Efficay and
safety of cilta-cel in patients with progressive multiple myeloma after
exposure to other BCMA-targeting agents. Blood 2023; 141: 219-230. https://doi.org/10.1182/blood.2022015526
- San-Miguel
J, Dhakal B, Yong K, Spencer A, Anguille S, Mateos MV, de Larrea CF,
Matinez-Lopez J, Moreau P, Touzeau C, et al. Cilta-cel or standard care
in lenalidomide-refractory multiple myeloma. N Engl J Med 2023; 389:
335-347. https://doi.org/10.1056/NEJMoa2303379
- Sidiqi
MH, Corradini P, Putill D, Einsele H, Dhakal B, Karlin L, Manier S,
Lida S, Giebel S, Harrison SJ, Lipe B, et al. Efficay and safety in
patients with lenalidomide-refractory multiple myeloma after 1-3 prior
lines who received a single infusion of ciltacabtagene autoleucel as
study treatment in the phase 3 CARTITUDE-4 trial. Blood 2023; 142
(suppl.1): 4866. https://doi.org/10.1182/blood-2023-178778
- Martin
T, Usmani SZ, Schecter JM, Roccia T, Jackson CC, Deraedt W, Yeh T,
Usmani SZ, Pacaud L, Garrett A, et al. Updated results from a
matching-adjusted indirect comparison of efficacy for celticabtagene
autoleucel in CARTITUDE-1 versus idecabtagene vivleuycel in KarMMa for
the treatment of patients with relapsed or refractory multiple myeloma.
Curr Med Res Opin 2023; 39: 81-89. https://doi.org/10.1080/03007995.2022.2139052
- Bar
N, Diels J, van Sanden S, Mendes J, Hernando T, Cost P, Schecter JM,
Lendvai N, Patel N, Ishioda T, et al. Comparative efficacy of
ciltacabtagene autoleucel versus idacabtagene vicleucel in the
treatment of patients with relapsed or refractory multiple myeloma
previously treated with 2-4 prior lines of therapy using a
matching-adjusted indirect comparison. Blood 2023; 142(suppl.1): 2141. https://doi.org/10.1182/blood-2023-182141
- Xu
J, Wang BY, Yu SH, Chen SJ, Yang SS, Liu R, Chen LJ, Hou J, Chen Z,
Zhao WH, et al. Long-term remission and survival in patients with
relapsed or refractory multiple myeloma after treatment with LCAR-B38M
CAR T cells: 5-yeaer follow-up of the LEGAND-2 trial. J Hematol Oncol
2024; 17: 23. https://doi.org/10.1186/s13045-024-01530-z
- Chen
D, Zhu Y, Chen Z, Jiang S, He H, Qiang W, Xiang F, Sun X, Du J. A
5-year follow-up clinical study of the B-cell maturation antigen
chimeric antigen receptor T-cell therapy HDS269B in patients with
relapsed or refractory multiple myeloma. Clin Cancer Res 2024, in
press. https://doi.org/10.1158/1078-0432.c.7429486
- Van
de Donk N, Themeli M, Usmani SZ. Determinants of response and
mechanisms of resistance of CAR T-cell therapy in multiple myeloma.
Blood Cancer Discov 2021; 2: 302-318. https://doi.org/10.1158/2643-3230.BCD-20-0227
- Cohen
AD, Garfall AL, Stadtmauer EA, Melenhorst JJ, Lacey SF, Lancaster E. B
cell antigen maturation antigen-specific CAR T cells are clinically
active in multiple myeloma. J Clin Invest 2019; 129: 2210-2221. https://doi.org/10.1172/JCI126397
- Da
Vià MC, Dietrich O, Truger M, Arampatzi P, Duell J, Heidemeier A, Zhou
X, Danhof S, Kraus S, Chatterje M, et al. Homozygous BCMA gene deletion
in response to anti-BCMA CAR T cells in a patient with multiple
myeloma. Nat Med 2021; 27: 616-619. https://doi.org/10.1038/s41591-021-01245-5
- Samur
MK, Fulciniti MT, Samur AA, Bazarbachi AH, Tai YT, Prabhala R, Alonso
A, Sperling AS, Campbell T, Petrocca F, et al. Biallelic loss of BCMA
as a resistance mechanism to CAR T cell therapy in a patient with
multiple myeloma. Nat Commun 2021 12: 868. https://doi.org/10.1038/s41467-021-21177-5
- Truger
MS, Duell J, Zhou X, Heimeshoff L, Ruckdeschel A, John M, Riedel A,
Huper S, Peter J, Walter W, et al. Single- and double-hit events in
genes encoding immune targets before and after T cell-engaging antibody
therapy in MM. Blood Adv 2021; 5: 3794-3798. https://doi.org/10.1182/bloodadvances.2021004418
- Samur
MK, Samur AA, Corre J, Lannes R, Shah P, Anderson K, Avet-Loiseau H,
Munshi N. Monoallelic deletion of BCMA is a frequent feature in
multiple myeloma. Blood Adv 2023; 7: 6599-6603. https://doi.org/10.1182/bloodadvances.2023010025
- Lee
H, Ahan S, Maity R, Leblay N, Zicheddu B, Truga M, Chojnacka M,
Cirrincione A, Duarante M, Tilmont R, et al. Mechanisms of antigen
escape from BCMA or GPRC5D-targeted immunotherapies in multiple
myeloma. Nat Med 2023; 29: 2295-2306. https://doi.org/10.1038/s41591-023-02491-5
- Samur
MK, Martin N, Thompson E, Fulciniti MT, Samur AA, Kaiser S, Munshi NC.
Differences in single cells between BCMA-targeting CAR T-cell therapy
responders and non-responders reveals initial resistance and acquired
resistance are driven by different factors. Blood 2023; 142 (suppl.1):
2106-2107. https://doi.org/10.1182/blood-2022-168903
- Van
Oekelen O, Nath K, Mouhieddine TH, Farzana T, Aleman A, Melkenoff DT,
Ghodkle-Puranik Y, Shah GL, Lesokhin AL, Giralt S, et al. Interventions
and outcomes of patients with multiple myeloma receiving salvage
therapy after BCMA-directed CAR T therapy. Bloos 2023; 141: 756-765. https://doi.org/10.1182/blood.2022017848
- Sun
F, Cheng Y, Wanchai V, Guo W, Mery D, Xu H, Gai D, Siegel E, Bailey C,
Ashby C, et al. Bispecific BCMA/CD24 CAR-T cells control multiple
myeloma growth. Nature Commun 2024; 15: 615. https://doi.org/10.1038/s41467-024-44873-4
- Larson
RC, Kann MC, Graham C, Mount CW, Castano AP, Lee WH, Bouffard AA, Takei
HN, Almazan AJ, Scarfò I, et al. Anti-TACI single and dual targeting
CAR T cells overcome BCMA antigen loss in multiple myeloma. Nat Commun
2023; 14: 7509. https://doi.org/10.1038/s41467-023-43416-7
- Fischer
L, Grieb N, Born P, Weiss R, Seiffert S, Boldt A, Fricke S, Franz P,
Heyn S, Kubasch AS, et al. Cellular dynamics following CAR T cell
therapy are associated with response and toxicity in
relapsed/refractory myeloma. Leukemia 2024; in press. https://doi.org/10.1038/s41375-023-02129-y
- Pont
MJ, Hill T, Cole GO, Abbott JJ, Kelliher J, Salter AI, Hudececk M,
Comstock ML, Rajan A, Patel B, et al. γ-secretase inhibition increases
efficacy of BCMA-specific chimeric antigen receptor T cells in multiple
myeloma. Blood 2019; 134: 1585-1597. https://doi.org/10.1182/blood.2019000050
- Cowan
AJ, Pont MJ, Sather BD, Turtle CJ, Till BG, Libby E, Coffey DG, Tuazon
SA, Wood B, Gooley T, et al. Safety and efficacy of fully human BCMA
CAR T cells in combination with a gamma-secretase inhibitor to increase
BCMA surface expression in patients with relapsed or refractory
multiple myeloma. Blood 2021; 138 (suppl.1): 551-553. https://doi.org/10.1182/blood-2021-154170
- Cowan
AJ, Pont MJ, Sather BD, Turtle CJ, Till BG, Libby E, Coffey DG, Tuazon
SA, Wood B, Gooley T, et al. γ-secretase inhibitor in combination with
BCMA chimeric antigen receptor T-cell immunotherapy for individuals
with relapsed or refractory multiple myeloma: a phase 1, first-in-human
trial. Lancet Oncol 2023; 24: 811-822. https://doi.org/10.1016/S1470-2045(23)00246-2
- Green
DJ, Pont M, Sather BD, Cowan AJ, Turtle CJ, Till BG, Nagengast M, Libby
EN, Becker PS, Coffey DG, et al. Fully human Bcma targeted chimeric
antigen receptor T cells administered in a defined composition
demonstrate potency at low doses in advanced stage high risk multiple
myeloma. Blood 2028; 132 (suppl.1): 1011. https://doi.org/10.1182/blood-2018-99-117729
- Wang
D, Wang J, Hu G, Wang W, Xiao Y, Cai H, Jiang L, Meng L, Yang Y, Zhou
X, et al. A phase I study of a novel fully human BCMA-targeting CAR
(CT103A) in patients with relapsed/refractory multiple myeloma. Blood
2021; 137. 28909-2901. https://doi.org/10.1182/blood.2020008936
- Li
C, Wang D, Yu Q, Li Z, Wang W, Hu G, Mu W, Li C, An N, Long X, et al.
Long-term follow-up of fully human BCMA-targeting CAR (CT103A) in
patients with relapsed/refractory multiple myeloma. Blood 2023; 142
(suppl.1): 4854. https://doi.org/10.1182/blood-2023-181394
- Li
C, Wang D, Song Y, Huang H, Li J, Chen B, Liu J, Dong Y, Hu K, Liu P,
et al. CT103A, a novel fully human BCMA-targeting CAR-T cells in
patients with relapsed/refractory multiple myeloma: updated results of
phase 1b/2 study (FUMANBA-1). Hema Sphere 2023; 7(S3): 1627-1628. https://doi.org/10.1097/01.HS9.0000970372.45392.b8
- Harrington
K, Wu R, Hauskins C, Amin R, Long T, Chen A, Rahardjo A, Thayer C,
Navvaro G, Myers M, et al. Development of JCARH125: optimization of a
fully human anti-Bcma CAR for use in the treatment of multiple myeloma.
Blood 2017; 130 (suppl.1): 1813.
- Costa
LJ, Kumar SK, Atrash S, Liedtke M, Kaur G, Derman BA, Bergsagel PL,
Mailankody S, McCarthy PL, Limones J, et al. Results from the first
phase I clinical study of the B-cell maturation antigen (BCMA) nex T
chimeric antigen receptor (CAR) T cell therapy CC-98633/BMS-986354 in
patients (pts) with relapsed/refractory multiple myeloma (RRMM). Blood
2022; 140 (suppl.1): 1360-1362. https://doi.org/10.1182/blood-2022-160038
- Sperling
AS, Nikiforow S, Deman B, Nadeem O, Mo C, Laubach J, Anderson K, Alonso
A; Im SY, Ikgawa S, et al. Phase I study data update of PHE885, a fully
human BCMA-directed CAR-T cell therapy manufactured using the
T-chargeTM platform for patients with relapsed/refractory (R/R)
multiple myeloma (M/M). HemaSphere 2022; 6(S3): P1446. https://doi.org/10.1097/01.HS9.0000848640.53562.8f
- Sperling
AS, Derman BA, Nikiforow S, Im SY, Ikegawa S, Prabhala RH, Rodriguez
DH, Li Y, Quinn DS, Pearson D, et al. Updated phase I study results of
PHE885, a T-charge manufactured BCMA-directed CAR-T cell therapy, for
patients (pts) with r/r multiple myeloma (RRMM). J Clin Oncol 2023;
41(suppl.16): 8004. https://doi.org/10.1200/JCO.2023.41.16_suppl.8004
- Chen
W, Fu C, Fang B, Liang A, Xia Z, He Y, Lu J, Liu H, Hou M, Cai Z, et
al. Phase II study of fully human BCMA-targeting CAR-T cells
(Zevorcabtagene Autoleucel) in patients with relapsed/refractory
multiple myeloma. Blood 2022; 140 (suppl.1) 4964-4965. https://doi.org/10.1182/blood-2022-168610
- Fu
C, Chen W, Cai Z, Yan L, Wang H, Shang J, Wu Y, Yan S, Gao W, Shi X, et
al. Three-year follow-up on efficacy and safety results from phase 1
Lummicar study 1 of Zevorcabtagene Autoleucel in Chinese patients with
relapsed or refractory multiple myeloma. Blood 2023; 142 (suppl.1):
4845. https://doi.org/10.1182/blood-2023-184373
- Metelo
AM, Jozwik A, Luong LA, Dominey-Foy D, Graham C, Attwood C, Inam S,
Dunlop A; Sanchez K, Cuthill K, et al. Allogeneic anti-BCMA CAR T cells
are superior to multiple myeloma-derived CAR T cells in preclinical
studies and may be combined with gamma-secretase inhibitors. Cancer Res
Commun 2022; 2: 158-171. https://doi.org/10.1158/2767-9764.CRC-21-0157
- Lin
RJ, Nager AR, Park S, Sutton J, Lay C, Melton Z, Zhang Y, Boldajipour
B, Van Blarcom TJ, Panowski SH, et al. Design and validation of
inducible TurboCARs with tunable induction and combinatorial cytokine
signaling. Cancer Immunol Res 2022; 10: 1069-1083. https://doi.org/10.1158/2326-6066.CIR-21-0253
- Lin
RJ, Sutton J, Bentley T, Vargas-Inchaustegui DA, Nguyen D, Cheng HY,
Yoon H, Van Blarcom TJ, Sasu BJ, Panowski SH, et al. Constitutive
turbodomains enhance expansion and antitumor activity of allogeneic
BCMA CAR T cells in preclinical models. Sci Adv 2023; 9: eadg8694. https://doi.org/10.1038/s41591-023-02306-7 https://doi.org/10.1126/sciadv.adg8694
- Mailankody
S, Matous JV, Chhabra S, Liedtke M, Sidana S, Oluwole OO, Malik S, Nath
R, Anwer F, Cruz JC, et al. Allogeneic BCMA-targeting CAR T cells in
relapsed/refractory multiple myeloma: phase 1 UNIVERSAL trial interim
results. Nat Med 2023; 29: 422-429. https://doi.org/10.1038/s41591-022-02182-7
- Dholaria
B, Kocoglu MH, Kin A, Asch AS, Ramakrishnan A, Bachier C, Rodriguez TE,
Shune L, McArthur K McCalgue J, et al. Early results of P-BMCA-ALLO1, a
fully allogeneic chimeric antigen receptor T-cell (CAR-T), in patients
with relapsed/refractory multiple myeloma (RRMM). Blood 2023;
152(suppl.1): 3479. https://doi.org/10.1182/blood-2023-182430
- Smith
EL, Harrington K, Staehr M, Masakayan R, Jones J, Jong TJ, Ng KY,
Ghoddusi M,Purdon TJ, Wang X, et al. GPRC5D is a target for the
immunotherapy of multiple myeloma with rationally designed CAR T cells.
Sci Transl Med 2019; 11: eaau7746. https://doi.org/10.1126/scitranslmed.aau7746
- Xia
J, Li H, Yan Z, Zhou D, Wang Y, Qi Y, Cao J, Li D, Cheng H, Sang W, et
al. Anti-G protein-coupled receptor, class C group 5 member D chimeric
antigen receptor T cells in patients with relapsed or refractory
multiple myeloma: a single-arm, phase II trial. J Clin Oncol 2023; 41:
2583-2593. https://doi.org/10.1200/JCO.22.01824
- Zhang
M, Wei G, Zhou L, Zhou J, Chen S, Zhang W, Wang D, Luo X, Cui J, Huang
S, et al. GPRC5D CAR T cells (OriCAR-017) in patients with relapsed or
refractory multiple myeloma (POLARIS): a first-in-human, single-centre,
single-arm, phase 1 trial. Lancet Hematol 2023; 10: e107-e116. https://doi.org/10.1016/S2352-3026(22)00372-6
- Mailankody
S, Devlin SM, Landa J, Nath K, Diamonte C, Carstens EJ, Russo D,
Auclair R, Fitzgerald R, Cadzin B, et al. GPRC5D-targeted CAR T cells
for myeloma. N Engl J Med 2022; 387: 1196-1206. https://doi.org/10.1056/NEJMoa2209900
- Bal
S, Kocoglu MH, Nadeem O, et al. Clinical activity of BMS-986393
(CC-95266), a G protein-coupled receptor class C group 5 member D
(GPRC5D)-targeted chimeric antigen receptor (CAR) T cell therapy, in
patients with relapsed and/or refractory (R/R) multiple myeloma (MM):
first results from a phase 1, multicenter, open-label study. Blood
2022; 140 (suppl.1): 883-885. https://doi.org/10.1182/blood-2022-162395
- Bal
S, Htut M, Nadeem O, Anderson LD, Kocoglu H, Gregory T, Rossi AC,
Martin T, Egan DN, Costa L, et al. BMS-986393 (CC-95266), a G protein
-coupled receptor class C group 5 member D (GPRC5D)-targetd chimeric
antigen receptor (CAR) T-cell therapy for relapsed/refractory multiple
myeloma (RRMM): updated results from a phase 1 study. Blood 2023; 142
(suppl.1): 219. https://doi.org/10.1182/blood-2023-181857
- Zhou
D, Sun Q, Xia J, Gu W, Qian J, Zhuang W, Yan Z, Cheng H, Zhu F, Qi K,
et al. Anti-BCMA/GPRC5D bispecific CAR T cells in patients with
relapsed or refractory multiple myeloma: a single-arm, single-centre,
phase 1 trial. Lancet Hematol 2024; in press. https://doi.org/10.1016/S2352-3026(24)00176-5
- Garfall
AL, Stadtmauer EA, Hwang WT, Lacey FV, Melenhorst JJ, Krevvata M,
Carroll MP, Matsui WH, Wang Q, Dhodapkar MV, et al. Anti-CD19 CAR T
cells with high-dose melphalan and autologous stem cell transplantation
for refractory multiple myeloma. ICI Insight 2018; 3: e120505. https://doi.org/10.1172/jci.insight.120505
- Yan
Z, Cao J, Cheng H, Qiao J, Zhang H, Wang Y, Shi M, Lan J, Fei X, Jin L,
et al. A combination of humanized anti-CD19 and anti-BCMA CAR T cells
in patients with relapsed or refractory multiple myeloma: a single-arm,
phase 2 trial. Lancet Hematol 2019; 6: e521-e529. https://doi.org/10.1016/S2352-3026(19)30115-2
- Wang
Y, Cao J, Gu W, Shi M, Lan J, Yan Z, Jin L, Xia J, Ma S, Liu Y, et al.
Long-term follow-up of combination of B-cell maturation antigen and
CD19 chimeric antigen receptor T cells in multiple myeloma. J Clin
Oncol 2022; 40: 2246-2256. https://doi.org/10.1200/JCO.21.01676
- Du
J, Fu W, Jiang H, Dong B, Gao L, Liu L, Ge J, He A, Li L, Lu J, et al.
Updated results of a phase I, open-label study of BCMA/CD19
daul-targeting Fastcar-T GC012F for patients with relapsed/refractory
multiple myeloma (RRMM). HemaSphere 2023; 7(S3): e84060bf. https://doi.org/10.1097/01.HS9.0000970380.84060.bf
- Du
J, Qiang W, Lu J, Jia Y, He H, Liu J, Guo P, Yang Y, Feng Z, Jin L, et
al. Updated results of a phase I open-label single-arm study of dual
targeting BCMA and CD19 Fastcar-T cells (GDC012F) as first-line therapy
for transplant-eligible newly diagnosed high-risk multiple myeloma.
Blood 2023; 142 (suppl. 1): 1022. https://doi.org/10.1182/blood-2023-174841
- Shi
M, Wang J, Huang H, Liu D, Cheng H, Wang X, Chen W, Yan Z, Sang W, Qi
K, Li D, et al. Bispecific CAR T cell therapy targeting BCMA and CD19
in relapsed/refractory multiple myeloma: a phase I/II trial. Nat Commun
2024; 15: 3371. https://doi.org/10.1038/s41467-024-47801-8
- Garfall
AL, Cohen AD, Susanibar-Adaniya S, Hwang WT, Vogl DT, Waxman AJ, Lacey
SF, Gonzalez VE, Fraietta JA, Gupta M, et al. Anti-BCMA/CD19 CAR T
cells with early immunomodulatory maintenance for multiple myeloma
responding to initial or later-line therapy. Blood Cancer Discov 2023;
4: 118-133. https://doi.org/10.1158/2643-3230.BCD-22-0074
- Li
C, Xu J, Luo W, Liao D, Xie W, Wei Q, Zhang Y, Wang X, Wu Z, Kang Y, et
al. Bispecific CS1-BCMA CAR-T cells are clinically active in relapsed
or refractory multiple myeloma. Leukemia 2024; 38: 149-159. https://doi.org/10.1038/s41375-023-02065-x
- Korst
C, O'Neill C, Bruins W, Cosovic M, Twickler I, Verkley C, LeCierre D,
Themeli M, Chion-Sotinel I, et al. Preclinical activity of allogeneic
SLAMF7-specific CAR T-cells (UCARTCS1) in multiple myeloma. J
Immunother Cancer 2024; 12: e008769. https://doi.org/10.1136/jitc-2023-008769
- Gagelmann
N, Garderet L, Iacobelli S, Koster L, Carotti A, Schonland S, Galeni P,
Daskalakis M, Roeloffzen W, Hunter H, et al. Salvage transplant versus
CAR-T cell therapy for relapsed multiple myeloma. Blood 2023; 142
(suppl.1): 3592. https://doi.org/10.1182/blood-2023-184945
- Shi
X, Yan L, Shang J, Kang L, Yan Z, Jin S, Zhu M, Chang H, Gong F, Zhou
J, et al. Anti-CD19 and anti-BCMA CAR T cell therapy followed by
lenalidomide maintenance after autologous stem-cell transplantation for
high-risk newly diagnosed multiple myeloma. Am J Hematol 2022; 97:
537-547. https://doi.org/10.1002/ajh.26486