For this narrative review, we utilized PubMed to search articles about pediatric HL management in LMICs. The search was narrowed to articles between 1990 and 2023, excluding sub-Saharan Africa, which has been the subject of a recent exhaustive report,[8] and far-East LMICs, for which data in the English literature are insufficient. A total of 33 articles were included. We summarized the results reported so far in these papers and discussed the lessons that have been derived from these different real-life experiences.
Epidemiology
The epidemiology of pediatric HL varies greatly among geographic areas with different socio-economic levels. In HICs, HL is the sixth most frequent childhood neoplasm, accounting for 5-6% of childhood cancers.[9] Data regarding the real incidence of pediatric HL in other parts of the world concern only some geographic areas and are often not updated. In developing countries, HL is more frequent, being the fourth most common neoplasm in childhood.[10] In Latin America, the incidence of childhood HL is particularly high, 1-1.5 per 100,000 children.[10]In HICs, HL presents two peaks of age distribution in young adults (20-34 years) and older people (55-74 years).[11] In LMICs, the first peak occurs at a younger age in both children and adolescents, with 20% to 30% of childhood HL cases occurring before the age of 5, compared to about 5% in HICs.[12] Such patterns of occurrence are similar to those of Epstein-Barr virus (EBV) and other infections, suggesting an etiologic role of environmental exposure. In HICs, the incidence of EBV tumor cell positivity for HL ranges from 15% to 25% in adolescents and young adults.[13-15] It is significantly higher - between 70% and 100% - in LMICs where primary EBV infection occurs within a few months to years after birth. EBV-associated HL is more frequent in children below the age of 10, in males, and in the mixed cellularity (MC) subtype.[16,17] In India, EBV has been found in 78% of HL cases at all ages and in up to 98% of childhood HL.[18,19]
MC is the most common subtype of pediatric HL in LMICs (46-60%), and its association with EBV infection is well documented.
In LMICs, there is a male predominance in pediatric HL, particularly under the age of 10, with an M: F ratio between 2.5:1 and 5:1. The M:F ratio reaches 10.5:1 in Indian cases.[20,21] The reasons for this gender distribution are not fully understood. Increased male susceptibility to infection, as well as social-cultural factors leading to reduced attention to female children, have been suggested.[22]
Treatment
Therapeutic strategies and results in HICs. HL has been one of the most curable cancers since 1950, when the first successful protocols based on chemo and radiotherapy began to be used. In recent decades, the major focus, particularly in pediatric patients, has been to reduce the use of radiotherapy and chemotherapeutic agents such as anthracyclines and procarbazine, which are responsible for various severe long-term toxicities.[23] Nowadays, the survival rate of pediatric HL patients in HICs exceeds 90% with combined chemo-radiotherapy protocols adapted for younger patients.[1-3,5,6] In recent trials, staging, response assessment, and radiotherapy indications have been based on PET evaluations, and the number and intensity of cycles have been adjusted according to the risk group assignment. The 5-year event-free survival (EFS) ranges from 80% to 96% for children with low risk and from 79% to 90% for children with intermediate-high risk disease.[1,3-7,24-27] In the ongoing Children’s Oncology Group (COG) and the European Network Pediatric Hodgkin Lymphoma (EuroNet-PHL) trials, the intensity and duration of chemotherapy, as well as the indication, doses, and fields of radiotherapy, are modulated according to two criteria: the risk category (mainly determined by stage, bulky disease and B symptoms) and the early metabolic response to treatment. Promising immuno-chemotherapy approaches,[28,29] including monoclonal antibodies and checkpoint inhibitors such as brentuximab[30-32] and pembrolizumab,[33] are under evaluation for first-line treatment in pediatric patients, with the aim of further reducing chemotherapy-related toxicity.Reported results in LMICs. There are very little data in the literature on the treatment and outcome of pediatric HL in LMICs prior to the second decade of the 2000s. These studies are generally retrospective and mainly based on single-center experiences. Heterogeneous treatment approaches, including radiotherapy or not, as well as different modalities of data analysis, often censoring abandonment, make these studies difficult to interpret and compare. More recently, prospective cooperative studies have been implemented in Latin America[34] and in the Indian subcontinent.[35-37]
Papers on pediatric HL from LMICs, present in the literature from the 1990s, are summarized in Tables 1-3. These studies include patients up to the age of 14-21. In most studies, the median age is low, around 7 years, with many children less than 5 (from 8.3% up to 37%, median 16%).
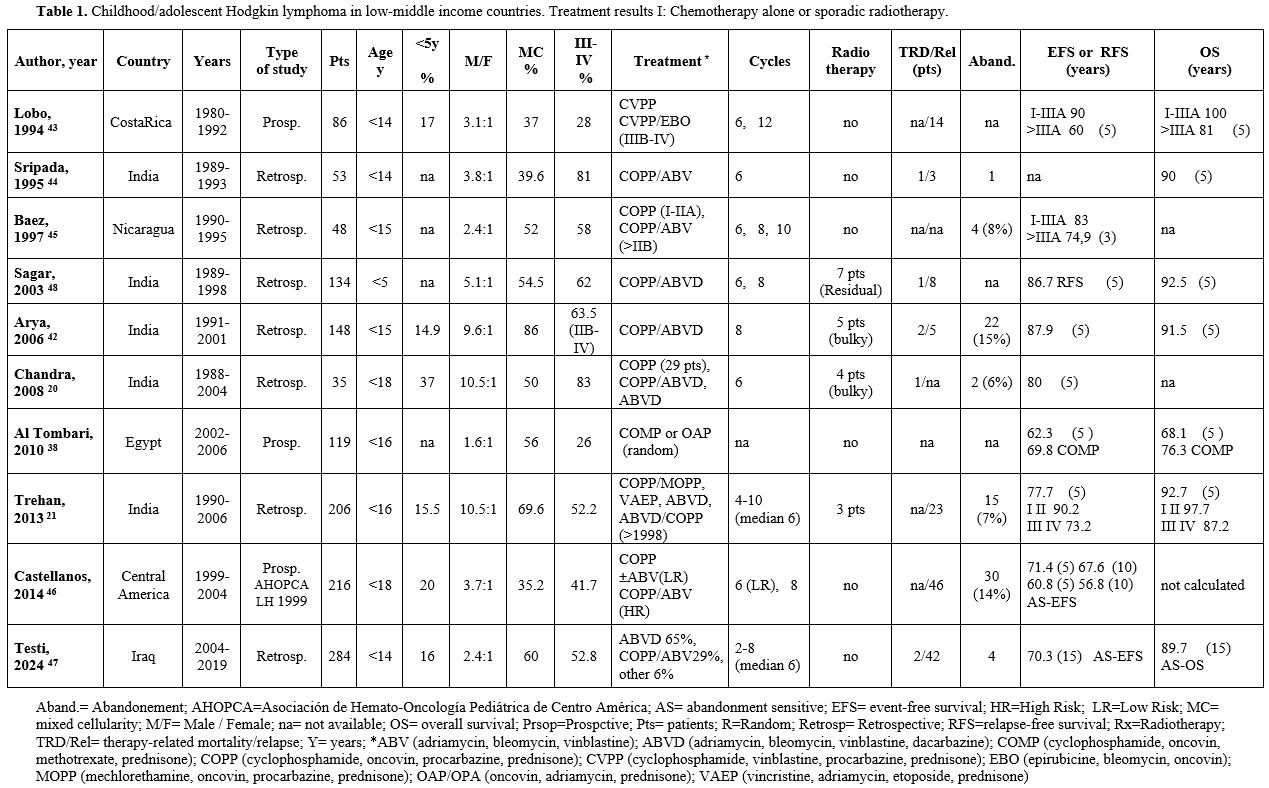 |
Table 1. Childhood/adolescent Hodgkin lymphoma in low-middle income countries. Treatment results I: Chemotherapy alone or sporadic radiotherapy. |
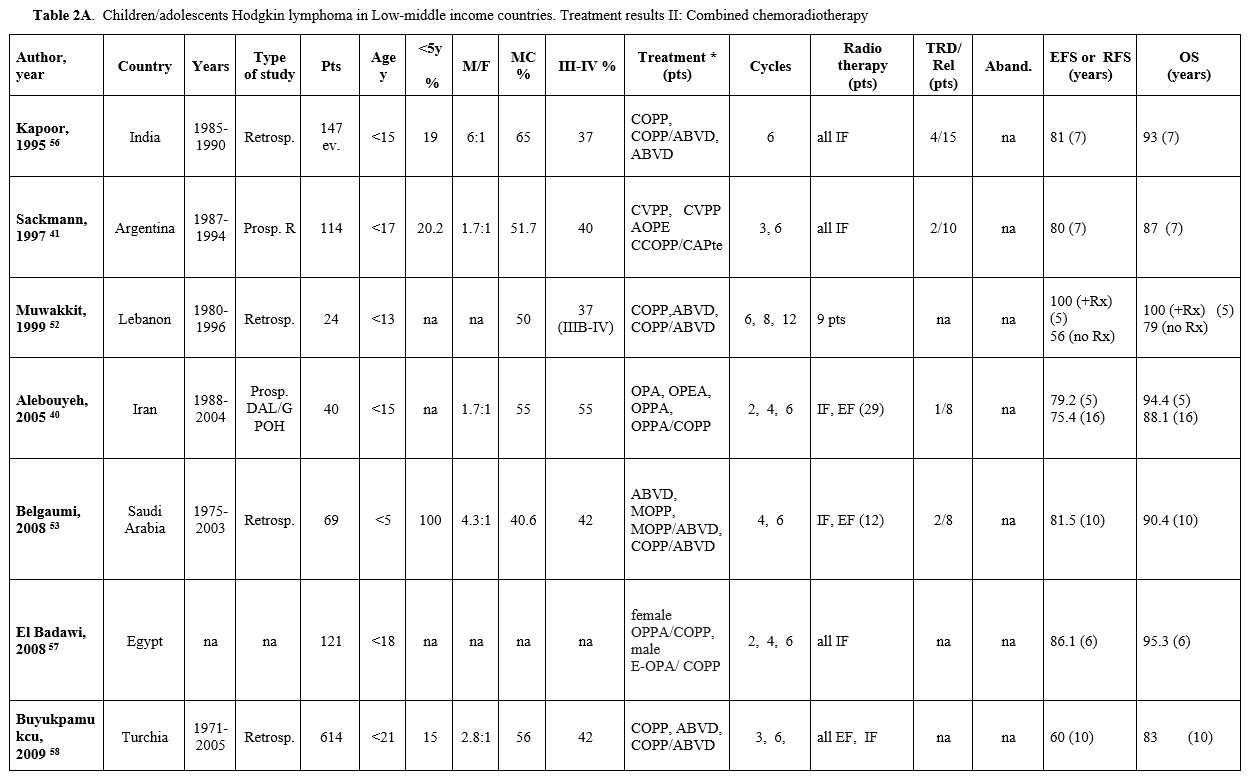 |
Table 2A. Children/adolescents Hodgkin lymphoma in Low-middle income countries. Treatment results II: Combined chemoradiotherapy |
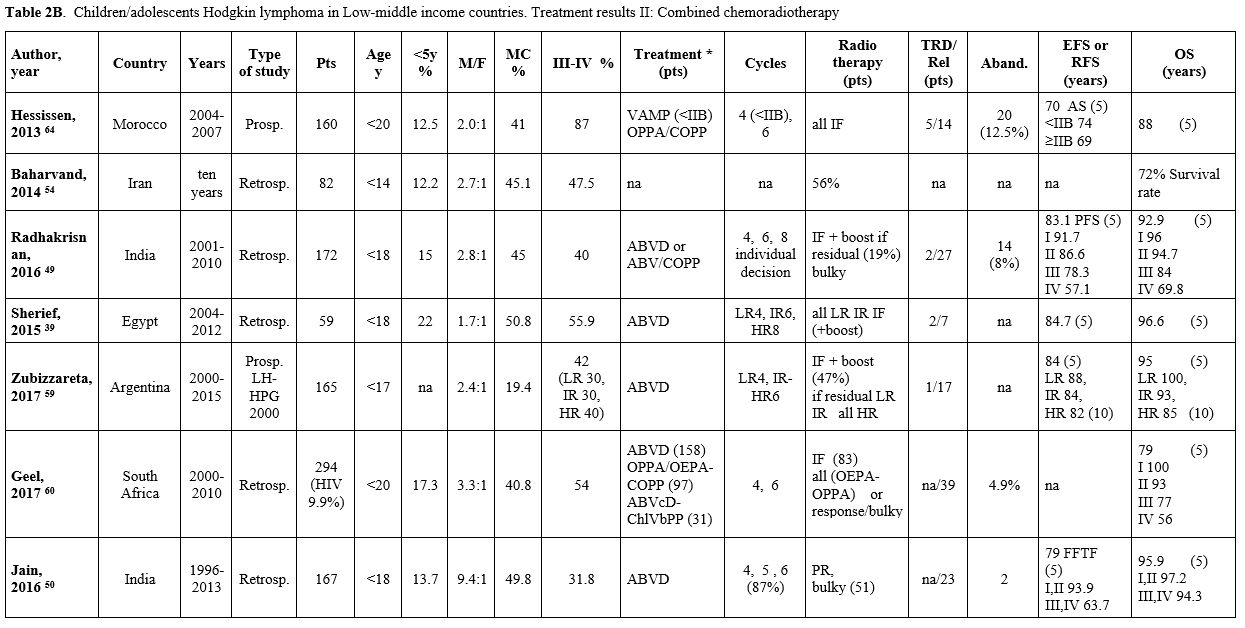 |
Table 2B. Children/adolescents Hodgkin lymphoma in Low-middle income countries. Treatment results II: Combined chemoradiotherapy |
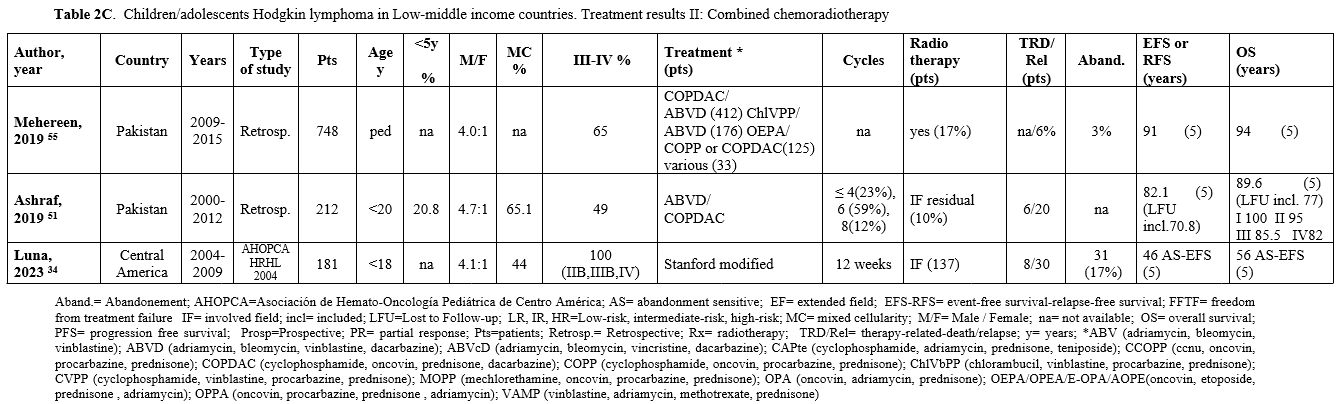 |
Table 2C. Children/adolescents Hodgkin lymphoma in Low-middle income countries. Treatment results II: Combined chemoradiotherapy |
 |
Table 3.
Children/adolescents Hodgkin lymphoma in Low-middle income countries.
Treatment results II: ERA based Combined chemoradiotherapy. |
The M:F ratio is high (median 3.8:1), ranging from values similar to Western countries (1.6-1.7:1) in Egypt,[38,39] Iran,[40] and Argentina,[41] and up to 10.5:1 in India[20,21] where very high rates are reported in all studies.
All the studies, except two excluding nodular lymphocyte predominance HL,[61,62] include all HL histologic subtypes. An MC histology is reported in more than 40% of cases, with this histologic subtype being even more represented (up to 86%) in Indian patients.[42] Many patients present with advanced stage (stage III-IV), with a median of 52% (range 26%-87%).
Abandonment, which was differently defined among the studies, was reported in 15 out of the 33 papers, with rates ranging from 1% to 17% (median 7.5%).
The most commonly used regimen was ABVD (adriamycin, bleomycin, vinblastine, dacarbazine), alone or alternated with COPP (cyclophosphamide, oncovin, procarbazine, prednisone), with or without dacarbazine (COPP/ABV(D)). After the 2000s, other schemes derived from the European pediatric protocols, particularly OEPA (oncovin, etoposide, prednisone, adriamycin) and COPDAC (cyclophosphamide, oncovin, prednisone, dacarbazine), have been introduced.
The total number of cycles varied from 3 to 12 (median 6), generally depending on medical decisions according to stage, response to treatment, and availability of radiotherapy. In the absence of radiotherapy, more cycles of chemotherapy were administered (Table 1).
Table 1 describes the studies based on chemotherapy alone. Chemotherapy alone was delivered in six studies due to the unavailability of Radiotherapy[38,43-47]. In four further studies, radiotherapy was sporadically delivered to a very small number of patients based on clinical decisions related to bulky disease at presentation or residual mass after chemotherapy.[20,21,42,48]
Table 2 (A, B, C) reports the studies based on a combined chemo-radiotherapy modality. In some studies, involved field (IF) radiotherapy was planned for patients with bulky or residual disease.[49-55] In other studies, radiotherapy, generally IF, was administered to all patients[34,41,56-58] or to patients selected according to risk.[39,40,59,60] Since 2010, in the most recent prospective protocols, radiotherapy (generally IF) was pre-planned based on ERA and in case of bulky disease[35-37,61-63] (Table 3).
Results according to therapeutic strategies. In the studies based on chemotherapy alone (generally COPP, COPP/ABV(D) or ABVD), the overall 5-year EFS and overall survival (OS) ranged from 69% to 87% (median 77.8%) and from 76.3% to 92.7% (median 91.5%), respectively, with good results (EFS up to 92% and OS up to 100%) in children presenting with localized disease. As previously mentioned, these patients received a high number of chemotherapeutic cycles, up to 10-12. Despite the high chemotherapy burden, the reported therapy-related deaths (TRD) are low (Table 1). However, little information on the long-term side effects is available. The median rate of abandonment is 12%. In the two studies in which abandonment was considered as an event, the 10- and 15-year EFS were 56.8% and 70.3%, respectively.[46,47]
When a chemo-radiotherapy combination was used, the chemotherapy regimens included mainly ABVD, COPP, and COPP/ABVD. Other schemes such as COPDAC and OEPA or OPPA (oncovin, procarbazine, prednisone, adriamycin), derived from pediatric European experiences, have been used in studies from Pakistan and South Africa since 2000. The overall 5-10-year EFS ranged from 60% to 91% (median 81.5%), and the OS ranged from 72% to 96.6% (median 91.6%), with EFS up to 91.7% and OS up to 100% in children with early-stage disease. The median rate of abandonment, reported only in a few papers, was 8%. In the two studies in which abandonment was considered as an event, the 5-year EFS was 46% and 70%, respectively.[34,64]
In the more recent studies, with different combined chemo-radiotherapy programs modulated according to the initial stage and ERA, the 3-5-year EFS and OS were 83.3% and 92.2%, respectively. Patients with localized disease reached a 3-year EFS and OS of 100%.[62,63]
No significant differences were observed among the three treatment strategies (Tables 1-3), with a median EFS of 72.8%, 81.5%, and 83.3% and OS of 91.5%, 91.6%, and 92%, respectively, for chemotherapy alone, chemo-radiotherapy, and ERA-based chemo-radiotherapy groups.
Discussion
Most children in the world live in LMICs, where over 80% of pediatric cancers occur.[65] Unfortunately, the good results of pediatric cancer treatment achieved in HICs are still not attained in the other parts of the world.[66] This is also true for a highly curable disease like HL. In LMICs, the survival rates reported in the published papers are acceptable, particularly for children with early-stage disease, though lower compared to those achieved in HICs and often at the cost of a high therapeutic burden. Moreover, we must consider that data on HL - and all pediatric cancers - are completely lacking for most LMICs. More data are recently emerging from Latin America with the implementation of cooperative studies and from the Indian subcontinent.The data that emerge from this review confirm:
- Lower age and the high incidence of MC in the pediatric HL cohorts.
- The high incidence of MC is probably related to the high incidence of early EBV infection in LMICs.
- The high M: F ratio. Cultural reasons, with a lower interest in female children, are evident, particularly in the reports from India. The M: F ratio appears to be reducing, though remaining high also in the more recent studies.
- The advanced stage of disease presentation is often due to delayed referral and diagnosis for social and economic reasons.
In these countries, the most used therapeutic regimens were the well-known ABVD, COPP, or COPP/ABV regimens, which are less toxic and can be administered as outpatients. Radiotherapy was not always available, and the number of chemotherapy cycles administered was frequently high, with a risk of late side effects, usually not reported in the published papers. In more recent years, treatment has also been modulated according to ERA with a consequent decrease in the chemo/radiotherapy burden. Based on the ERA evaluation, PET-CT should be the optimal choice, but unfortunately, it is not always available in LMICs where contrast-enhanced CT may be a valid option.[67] With the ERA-based strategies, good results were achieved in India with the ABVD protocol.[35,36] More aggressive schemes (OEPA/COPDAC), according to the European protocols, gave good results but increased the TRD due to the absence of appropriate supportive therapy.[37,61,62] In a very interesting study from South Africa, in high-risk patients, the more toxic OEPA scheme was replaced by ABVD[63] according to a EuroNet-CHL-inspired protocol. This was associated with a 2-year OS of 92.6% and very good results also in high-risk patients (OS 91%).
Key Points
Unavailability of radiological facilities. Proper staging and response evaluation with PET is often not possible, limiting therapy modulation and potentially leading to overtreatment.Inconsistent Availability of Chemotherapy Drugs. Treatment cycles are sometimes inadequate due to the omission or substitution of unavailable drugs.
Inadequate supportive treatment. Intensive treatments used in advanced stages in HICs can cause excessive toxicity without proper patient compliance and supportive measures.
Inconsistent Quality of Radiotherapy. Radiotherapy in LMICs, even when available, may not always meet the quality requirements necessary for safe delivery.
Difficulties in solving the Problem of gonadal toxicity. Implementing fertility preservation measures, now widely adopted in HICs, can be difficult or impossible in LMICs.[68,69]
Difficulties in long-term follow-up. Without long-term follow-up, obtaining information on the rate and severity of late treatment effects is particularly challenging in LMICs, making it difficult to modulate HL treatment effectively while maintaining good outcomes.
Which strategy for LMICs? The ARIA Guidelines
The St. Jude Global and the International Society of Pediatric Oncology (SIOP) created the adapted Resource and Implementation Application (ARIA) guidelines to provide pediatric oncologists worldwide with safe and evidence-based guidelines for diagnosing and treating pediatric cancers. The Aria guidelines for childhood HL identify three categories of centers according to resource availability, detailing the minimal requirements for diagnosis, staging, and treatment for each category. Treatment recommendations are tailored according to patient risk groups (low-risk, intermediate-risk, or high-risk) and center resource categories, with more intensive treatments reserved for high-resource centers. Caution is advised in radiotherapy indications, requiring precise quality assessment for safe delivery. Adapted radiotherapy guidelines for LMICs were recently implemented by the Pediatric Oncology in Developing Countries (PODC) committee of SIOP in collaboration with the Pediatric Radiation Oncology Society (PROS).[70]The novel immunotherapeutic drugs could play an important role in LMICs, especially for high-risk and non-responding patients, offering more effective treatments without increasing chemotherapy-related TRD. However, the primary obstacles remain the costs and availability of these approaches in LMICs.
Conclusions
The so-far reported survival data obtained in LMICs for childhood HL may be considered satisfactory, particularly in early-stage patients. However, these results are obtained in most studies at the cost of a high chemotherapy or radiotherapy burden. The lack of long-term follow-up data means insufficient information on the late effects of these extensive treatments. To improve these results, contrarily to what has been described for aggressive non-Hodgkin lymphoma,[66] the main issue is not to deliver aggressive treatment to all patients, but rather a timely hospital referral and the modulation of treatment based on an accurate evaluation of the extension of the disease and, subsequently, on the response to treatment over time. This “modulation strategy” will need to be adapted to the different realities within LMICs. The availability of diagnostic tools such as PET or only CT, the possibility of safely delivering aggressive treatment for high-risk/not-responding patients, and finally, the availability of affordable radiotherapy may be different between countries and between hospitals in the same country. For these reasons, it is important to implement practical guidelines for the treatment of pediatric HL in LMICs that take into account the heterogeneity of the different realities. The ARIA guidelines for pediatric HL give harmonized, evidence-based general indications that will help pediatric oncologists worldwide. However, only local pediatricians, with their profound knowledge and experience of their specific local contexts, can correctly adapt these guidelines to each unique situation.Acknowledgements
The authors received no financial support for the research, authorship, and/or publication of this paper.Author contributions
MLM reviewed the literature and wrote the manuscript; AMT and RF critically reviewed and contributed to the final draft of the paper; SAH and MFAJ, with their extensive working experience at the Children Welfare Teaching Hospital in Baghdad, inspired and contributed to the discussion of the review.All authors revised and approved the final version of the manuscript.
References
- Belsky JA, Hochberg J, Giulino-Roth L. Diagnosis and management of
Hodgkin lymphoma in children, adolescents, and young adults. Best Pract
Res Clin Haematol. 2023;36(1):101445.
https://doi.org/10.1016/j.beha.2023.101445
- Lo AC,
Dieckmann K, Pelz T, Gallop-Evans E, Engenhart-Cabillic R, Vordermark
D, Kelly KM, Schwartz CL, Constine LS, Roberts K, Hodgson D. Pediatric
classical Hodgkin lymphoma. Pediatr Blood Cancer. 2021;68 Suppl
2:e28562. https://doi.org/10.1002/pbc.28562
- Mauz-Korholz
C, Landman-Parker J, Balwierz W, mmann RA, Anderson RA, Attarbaschi A,
Bartelt JM, Beishuizen A, Boudjemaa S, Cepelova M, Claviez A, Daw S,
Dieckmann K, Fernández-Teijeiro A, Fosså A, Gattenlöhner S, Georgi T,
Hjalgrim LL, Hraskova A, Karlén J, Kluge R, Kurch L, Leblanc T, Mann G,
Montravers F, Pears J, Pelz T, Rajić V, Ramsay AD, Stoevesandt D,
Uyttebroeck A, Vordermark D, Körholz D, Hasenclever D, Wallace WH.
Response-adapted omission of radiotherapy and comparison of
consolidation chemotherapy in children and adolescents with
intermediate-stage and advanced-stage classical Hodgkin lymphoma
(EuroNet-PHL-C1): a titration study with an open-label, embedded,
multinational, non-inferiority, randomised controlled trial. Lancet
Oncol. 2022;23(1):125-137. https://doi.org/10.1016/S1470-2045(21)00470-8
- Keller
FG, Castellino SM, Chen L, Pei Q, Voss SD, McCarten KM, Senn SL, Buxton
AB, Bush R, Constine LS, Schwartz CL. Results of the AHOD0431 trial of
response adapted therapy and a salvage strategy for limited stage,
classical Hodgkin lymphoma: a report from the Children's Oncology
Group. Cancer. 2018;124(15):3210-3219. https://doi.org/10.1002/cncr.31519
- Kelly
KM, Cole PD, Pei Q, Bush R, Roberts KB, Hodgson DC, McCarten KM, Cho
SY, Schwartz C. Response-adapted therapy for the treatment of children
with newly diagnosed high risk Hodgkin lymphoma (AHOD0831): a report
from the Children's Oncology Group. Br J Haematol. 2019;187(1):39-48. https://doi.org/10.1111/bjh.16014
- Friedman
DL, Chen L, Wolden S, Buxton A, McCarten K, FitzGerald TJ, Kessel S, De
Alarcon PA, Chen AR, Kobrinsky N, Ehrlich P, Hutchison RE, Constine LS,
Schwartz CL. Dose-intensive response-based chemotherapy and radiation
therapy for children and adolescents with newly diagnosed
intermediate-risk hodgkin lymphoma: a report from the Children's
Oncology Group Study AHOD0031. J Clin Oncol. 2014;32(32):3651-3658. https://doi.org/10.1200/JCO.2013.52.5410
- Metzger
ML, Weinstein HJ, Hudson MM, Billett AL, Larsen EC, Friedmann A, Howard
SC, Donaldson SS, Krasin MJ, Kun LE, Marcus KJ, Yock TI, Tarbell N,
Billups CA, Wu J, Link MP. Association between radiotherapy vs no
radiotherapy based on early response to VAMP chemotherapy and survival
among children with favorable-risk Hodgkin lymphoma. JAMA.
2012;307(24):2609-2616. https://doi.org/10.1001/jama.2012.5847
- Kabahweza
HM, Spencer A. Childhood Hodgkin Lymphoma in Sub-Saharan Africa: A
Systematic Review on the Effectiveness of the Use of Chemotherapy
Alone. Glob Pediatr Health. 2024;11:2333794X231223266. https://doi.org/10.1177/2333794X231223266
- Macfarlane
GJ, Evstifeeva T, Boyle P, Grufferman F. International patterns in the
occurrence of Hodgkin's disease in children and young adult males. Int
J Cancer. 1995;61(2):165-169. https://doi.org/10.1002/ijc.2910610204
- Stiller CA, Parkin DM. Geographic and ethnic variations in the incidence of childhood cancer. Br Med Bull. 1996;52(4):682-703. https://doi.org/10.1093/oxfordjournals.bmb.a011577
- Grufferman S, Delzell E. Epidemiology of Hodgkin's disease. Epidemiol Rev. 1984;6:76-106. https://doi.org/10.1093/oxfordjournals.epirev.a036276
- Mahajan
A, Bakhshi S, Seth R, Verma N, Mandal P, Singh M, Jain S, Radhakrishnan
V, Kanvinde S, Arora RS, Dinand V, Kalra M, Taluja A, Mallick S, Kumar
R, Chandra J. Hodgkin Lymphoma in children < 5 years. Do they behave
differently? J Pediatr Hematol Oncol. 2022 May 1;44(4):186-190. https://doi.org/10.1097/MPH.0000000000002423
- Claviez
A, Tiemann M, Lüders H, Krams M, Parwaresch R, Schellong G, Dörffel W.
Impact of latent Epstein-Barr virus infection on outcome in children
and adolescents with Hodgkin's lymphoma. J Clin Oncol. 2005;23(18):
4048-4056. https://doi.org/10.1200/JCO.2005.01.701
- Lee
JH, Kim Y, Choi JW, Young-Sik K. Prevalence and prognostic significance
of Epstein-Barr virus infection in classical Hodgkin's lymphoma: a
meta-analysis. Arch Med Res. 2014;45(5):417-431. https://doi.org/10.1016/j.arcmed.2014.06.001
- Jarrett
RF, Stark GL, White J, Angus B, Alexander FE, Krajewski AS, Freeland J,
Taylor GM, Taylor PRA. Impact of tumor Epstein-Barr virus status on
presenting features and outcome in age-defined subgroups of patients
with classic Hodgkin lymphoma: a population-based study. Blood.
2005;106(7):2444-2451. https://doi.org/10.1182/blood-2004-09-3759
- Glaser
SL, Lin RJ, Stewart SL, Ambinder RF, Jarrett RF, Brousset P, Pallesen
G, Gulley ML, Khan G, O'Grady J, Hummel M, Preciado MV, Knecht H, Chan
JK, Claviez A. Epstein-Barr virus associated Hodgkin's disease:
epidemiologic characteristics in international data. Int J Cancer.
1997;7084:375-382. https://doi.org/10.1002/(SICI)1097-0215(19970207)70:4<375::AID-IJC1>3.0.CO;2-T
- Chabay
PA, Barros MH, Hassan R, De Matteo E, Rey G, Carrico MK, Renault IZ,
Preciado MV. Pediatric Hodgkin lymphoma in 2 South American series: a
distinctive epidemiologic pattern and lack of association of
Epstein-Barr virus with clinical outcome. J Pediatr Hematol Oncol.
2008;30(4):285-291. https://doi.org/10.1097/MPH.0b013e3181647bc3
- Naresh
KN, Johnson J, Srinivas V, Soman CS, Saikia T, Advani SH, Badwe RA,
Dinshaw KA, Muckaden M, Magrath I, Bhatia K. Epstein-Barr virus
association in classical Hodgkin's disease provides survival advantage
to patients and correlates with higher expression of proliferation
markers in Reed-Sternberg cells. Ann Oncol. 2000;11(1):91-96. https://doi.org/10.1023/A:1008337100424
- Dinand
V, Dawar R, Arya LS, Unni R, Mohanty B, Singh R. Hodgkin's lymphoma in
Indian children: prevalence and significance of Epstein-Barr virus
detection in Hodgkin's Reed-Stemberg cells. Eur J Cancer.
2007;43(1):161-168. https://doi.org/10.1016/j.ejca.2006.08.036
- Chandra
J, Naithani R, Singh V, Saxena YK, Sharma M, Pemde H. Developing
anticancer chemotherapy services in a developing country: Hodgkin
lymphoma experience. Pediatr Blood Cancer. 2008;51(4):485-488. https://doi.org/10.1002/pbc.21609
- Trehan
A, Singla S, Marwaha RK, Bansal D, Srinivasan R. Hodgkin lymphoma in
children: experience in a tertiary care centre in India. J Pediatr
Hematol Oncol. 2013;35(3):174-179. https://doi.org/10.1097/MPH.0b013e318271f587
- Nandakumar
A, Anantha N, Appaji L, Swamy K, Mukherjee G, Venugopal T, Reddy S,
Dhar M. Descriptive epidemiology of childhood cancers in Bangalore,
India. Cancer Causes Control. 1996;7(4):405-410. https://doi.org/10.1007/BF00052665
- Ehrhardt
MJ, Flerlage JE, Armenian SH, Castellino SM, Hodgson DC, Hudson MM.
Integration of Pediatric Hodgkin Lymphoma Treatment and Late Effects
Guidelines: Seeing the Forest Beyond the Trees. J Natl Compr Canc Netw.
2021;19(6):755-764. https://doi.org/10.6004/jnccn.2021.7042
- Nachman
JB, Sposto R, Herzog P, Gilchrist GS, Wolden SL, Thomson J, Kadin ME,
Pattengale P, Davis PC, Hutchinson RJ, White K. Randomized comparison
of low-dose involved-field radiotherapy and no radiotherapy for
children with Hodgkin's disease who achieve a complete response to
chemotherapy. J Clin Oncol. 2002;20(18):3765-3771. https://doi.org/10.1200/JCO.2002.12.007
- Mauz-Korholz
C, Hasenclever D, Dorffel W, Ruschke K, Pelz T, Voigt A, Stiefel M,
Winkler M, Vilser C, Dieckmann K, Karlén J, Bergsträsser E, Fosså A,
Mann G, Hummel M, Klapper W, Stein H, Vordermark D, Kluge R, Körholz D.
Procarbazine-free OEPA-COPDAC chemotherapy in boys and standard
OPPA-COPP in girls have comparable effectiveness in pediatric Hodgkin's
lymphoma: the GPOH-HD-2002 study. J Clin Oncol. 2010;28(23):3680-3686. https://doi.org/10.1200/JCO.2009.26.9381
- Dorffel
W, Rühl U, Lüders H, Claviez A, Albrecht M, Bokkerink J, Holte H,
Karlen J, Mann G, Marciniak H, Niggli F, Schmiegelow K, Schwarze E-W,
Pötter R, Wickmann L, Schellong G. Treatment of children and
adolescents with Hodgkin lymphoma without radiotherapy for patients in
complete remission after chemotherapy: final results of the
multinational trial GPOH-HD95. J Clin Oncol. 2013;31(12):1562-1568. https://doi.org/10.1200/JCO.2012.45.3266
- Giulino-Roth
L, Keller FG, Hodgson DC, Kelly KM. Current approaches in the
management of low risk Hodgkin lymphoma in children and adolescents. Br
J Haematol. 2015;169(5):647-660. https://doi.org/10.1111/bjh.13372
- Vassilakopoulos
TP, Liaskas A, Pereyra P, Panayiotidis P, Angelopoulou MK, Gallamini A.
Incorporating Monoclonal Antibodies into the First-Line Treatment of
Classical Hodgkin Lymphoma. Int J Mol Sci. 2023;24(17): 13187. https://doi.org/10.3390/ijms241713187
- Connors
JM, Jurczak W, Straus DJ, Ansell SM, Kim WS, Gallamini A, Younes A,
Alekseev S, Illés Á, Picardi M, Lech-Maranda E, Oki Y, Feldman T,
Smolewski P, Savage KJ, Bartlett NL, Walewski J, Chen R, Ramchandren R,
Zinzani PL, Cunningham D, Rosta A, Josephson NC, Song E, Sachs J, Liu
R, Jolin HA, Huebner D, Radford J; ECHELON-1 Study Group. Brentuximab
Vedotin with Chemotherapy for Stage III or IV Hodgkin's Lymphoma. N
Engl J Med. 2018;378(4):331-344. https://doi.org/10.1056/NEJMoa1708984
- Metzger
ML, Link MP, Billett AL, Flerlage J, Lucas Jr JT, Mandrell BN, Ehrhardt
MJ, Bhakta N, Yock TI, Friedmann AM, de Alarcon P, Luna-Fineman S,
Larsen E, Kaste SC, Shulkin B, Lu Z, Li C, Hiniker SM, Donaldson SS,
Hudson MM, Krasin MJ. Excellent outcome for pediatric patients with
high-risk hodgkin lymphoma treated with brentuximab vedotin and
risk-adapted residual node radiation. J Clin Oncol.
2021;39(20):2276-2283. https://doi.org/10.1200/JCO.20.03286
- Hochberg
J, Basso J, Shi Q, Klejmont L, Flower A, Bortfeld K, Harrison L, van de
Ven C, Moorthy C, Islam H, Gerard P, Voss S, Cairo MS. Risk-adapted
chemoimmunotherapy using brentuximab vedotin and rituximab in children,
adolescents, and young adults with newly diagnosed Hodgkin's lymphoma:
a phase II, non-randomized controlled trial. J Immunother Cancer.
2022;10(5) e004445. https://doi.org/10.1136/jitc-2021-004445
- Castellino
SM, Pei Q, Parsons SK, Hodgson D, McCarten K, Horton T, Cho S, Wu Y,
Punnett A, Dave H, Henderson TO, Hoppe BS, Charpentier A-M, Keller FG,
Kelly KM. Brentuximab Vedotin with Chemotherapy in Pediatric High-Risk
Hodgkin's Lymphoma. New Engl J Med. J Clin Oncol.
2022;387(18):1649-1660. https://doi.org/10.1056/NEJMoa2206660
- Vinti
L, Daw S, Sabado Alvarez C, Mauz-Korholz C, Fagioli F, Beishuizen A,
Michel G, Moleti M, Cepelova M, Thorwarth A, Rigaud C, Plaza Lopez De
Sabando D, Landman-Parker J, Shen J, Pillai P, Marinello P,
Mauz-Koerholz C. Pembrolizumab in children and young adults with
classical hodgkin lymphoma (chl) with slow early response (ser) to
front-line chemotherapy (KEYNOTE-667). Pediatr Blood Cancer. 2023;
Volume 70, Issue S8. Abstract 0053/#1080; 55th Congress of the
International Society of Paediatric Oncology (SIOP) October 11-14,
2023.
- Luna-Fineman S, Castellanos M, Metzger ML, Baez
LF, Peña Hernandez A, Bonilla M, Fuentes-Alabi S, Nieves R, Blanco J,
Rossi E, Devidas M, Chen Y, Arreola M, de Alarcon PA. Treatment of
high-risk Hodgkin lymphoma with a modified Stanford V regimen in the
AHOPCA: Substituting chemotherapy agents and hampered outcomes. Pediatr
Blood Cancer. 2024;71(2):e30792. https://doi.org/10.1002/pbc.30792
- Mahajan
A, Singh M, Bakhshi S, Jain S, Radhakrishnan V, Verma N, Seth R, Arora
RS, Dinand V, Kalra M, Mandal P, Kapoor G, Sajid M, Thulkar S, Arora A,
Taluja A, Chandra J. Treating early-stage Hodgkin lymphoma in
resource-limited settings: InPOG-HL-15-01 experience. Pediatr Blood
Cancer. 2021;68(10):e29219. https://doi.org/10.1002/pbc.29219
- Jain
S, Bakhshi S, Seth R, Verma N, Singh M, Mahajan A, Radhakrishnan V,
Mandal P, Arora R, Dinand V, Kalra M, Sharma A, Taluja A, Thulkar S,
Biswas A, Chandra J. Risk based and response adapted radiation therapy
for children and adolescents with newly diagnosed advanced stage
Hodgkin lymphoma treated with ABVD chemotherapy: a report from the
Indian pediatric oncology group study InPOG-HL-15-01. Leuk Lymphoma.
2022;63(5):1111-1118. https://doi.org/10.1080/10428194.2021.2012659
- Palayullakandi
A, Trehan A, Jain R, Kumar R, Mittal BR, Kapoor R, Srinivasan R, Kakkar
N, Bansal D. Retrospective single-center experience with OEPA/COPDAC
and PET-CT based strategy for pediatric Hodgkin lymphoma in a LMIC
setting. Pediatr Hematol Oncol. 2022 Oct;39(7):587-599. https://doi.org/10.1080/08880018.2022.2044418
- Al-Tonbary
Y, Sarhan MM, El-Ashry RA, Salama E, Sedki M, Fouda A. Comparative
study of two mechlorethamine, vincristine, procarbazine, and prednisone
derived chemotherapeutic protocols for the management of pediatric
Hodgkin lymphoma (HL): single-center 5-year experience. Leuk Lymphoma.
2010;51(4):656-663. https://doi.org/10.3109/10428191003624249
- Sherief
LM, Elsafy UR, Abdelkhalek ER, Kamal NM, Elbehedy R, Hassan TH,
Sherbiny HS, Beshir MR, Saleh SH. Hodgkin lymphoma in childhood:
clinicopathological features and therapy outcome at 2 centres from a
developing country. Medicine (Baltimore). 2015;94(15):e670. https://doi.org/10.1097/MD.0000000000000670
- Alebouyeh
M, Moussavi F, Haddad-Deylami H, Vossough P. Successful ambulatory
treatment of Hodgkin's disease in Iranian children based on
German-Austrian DAL-HD 85-90: single institutional results. Ann Oncol.
2005;16(12):1936-1940. https://doi.org/10.1093/annonc/mdi401
- Sackmann-Muriel
F, Zubizarreta P, Gallo G, Scopinaro M, Alderete D, Alfaro E, Casak S,
Chantada G, Felice MS, Quinteros R. Hodgkin disease in children:
results of a prospective randomized trial in a single institution in
Argentina. Med Pediatr Oncol. 1997;29(6):544-552. https://doi.org/10.1002/(SICI)1096-911X(199712)29:6<544::AID-MPO5>3.0.CO;2-K
- Arya
LS, Dinand V, Thavaraj V, Bakhshi S, Dawar R, Rath GK, Singh R, Vats
TS. Hodgkin's disease in Indian children: outcome with chemotherapy
alone. Pediatr Blood Cancer. 2006; 46(1):26-34. https://doi.org/10.1002/pbc.20157
- Lobo-Sanahuja
F, García I, Barrantes JC, Barrantes M, González M, Jiménez R.
Pediatric Hodgkin's disease in Costa Rica: twelve years' experience of
primary treatment by chemotherapy alone, without staging laparotomy.
Med Pediatr Oncol. 1994;22(6):398-403. https://doi.org/10.1002/mpo.2950220609
- Sripada
PV, Tenali SG, Vasudevan M, Viswanadhan S, Sriraman D, Kandasamy R.
Hybrid (COPP/ABV) therapy in childhood Hodgkin's disease: a study of 53
cases during 1989-1993 at the Cancer Institute, Madras. Pediatr Hematol
Oncol. 1995;12(4):333-341. https://doi.org/10.3109/08880019509029583
- Baez
F, Ocampo E, Conter V, Flores A, Gutierrez T, Malta A, Pacheco C,
Palacios R, Biondi A, Riva L, Sala A, Silvestri D, Cavalli F, Sessa C,
Casanova M, Masera G. Treatment of childhood Hodgkin's disease with
COPP or COPP-ABV (hybrid) without radiotherapy in Nicaragua. Ann Oncol.
1977;8(3):247-250. https://doi.org/10.1023/A:1008200210674
- Castellanos
EM, Barrantes JC, Báez LF, Gamboa Y, Peña A, Alabi S, Bonilla M, Wang
H, Metzger ML, de Alarcón PA. A chemotherapy only therapeutic approach
to pediatric Hodgkin lymphoma: AHOPCA LH 1999. Pediatr Blood Cancer.
2014;61(6):997-1002. https://doi.org/10.1002/pbc.24905
- Testi
AM, Al Jadiri MF, Moleti ML, Uccini S, Al-Darraij AF, Al-Saeed RM,
Ghali HH, Sabhan AH, Fadhil SA, Al-Badri SA, Alsaadawi AR, Hameedi AD,
Shanshal MH, Al-Agele YS, Al-Saffar FAR, Yaseen NK, Piciocchi A,
Marsili G, Al-Hadad SA . Hodgkin Lymphoma in children: a 16-year
experience at the Children's Welfare Teaching Hospital of Baghdad,
Iraq. Mediterr J Hematol Infect Dis. 2024;16:e2024o53. https://doi.org/10.4084/MJHID.2024.053
- Sagar
TG, Chandra A, Raman G. Childhood Hodgkin disease treated with COPP/ABV
hybrid chemotherapy: a progress report. Med Pediatr Oncol.
2003;40(1):66-69. https://doi.org/10.1002/mpo.10017
- Radhakrishnan
V, Dhanushkodi M, Ganesan TS, Ganesan P, Sundersingh S, Selvaluxmy G,
Swaminathan R, Rama R, Sagar TG. Pediatric Hodgkin Lymphoma Treated at
Cancer Institute, Chennai, India: Long-Term Outcome. J Glob Oncol.
2016;3(5):545-554. https://doi.org/10.1200/JGO.2016.005314
- Jain
S, Kapoor G, Bajpai R. ABVD-Based Therapy for Hodgkin Lymphoma in
Children and Adolescents: Lessons Learnt in a Tertiary Care Oncology
Center in a Developing Country. Pediatr Blood Cancer.
2016;63(6):1024-1030. https://doi.org/10.1002/pbc.25935
- Ashraf
MS, Naz F, Yakoob MY. Characteristics and Survival Outcomes of Children
With Hodgkin Lymphoma Treated Primarily With Chemotherapy. J Pediatr
Hematol Oncol. 2019:41(6):452-456. https://doi.org/10.1097/MPH.0000000000001496
- Muwakkit
S, Geara F, Nabbout B, Farah RA, Shaab NS, Hajjar T, Khogali M.
Treatment of pediatric Hodgkin's disease with chemotherapy alone or
combined modality therapy. Radiat Oncol Investig. 1999;7(6):365-373. https://doi.org/10.1002/(SICI)1520-6823(1999)7:6<365::AID-ROI7>3.0.CO;2-W
- Belgaumi
A, Al-Kofide AA, Khafaga Y, Joseph N, Jamil-Malik R, Siddiqui KS,
Sabbah RS. Clinical characteristics and outcome of pediatric patients
with stage IV Hodgkin lymphoma. Hematol Oncol Stem Cell Ther.
2009;2(1):278-284. https://doi.org/10.1016/S1658-3876(09)50038-6
- Baharvand
M, Mortazavi H. Characteristics of Hodgkin lymphoma in a defined group
of Iranian pediatric patients. Asian Pac J Cancer Prev.
2014;15(13):5167-5169. https://doi.org/10.7314/APJCP.2014.15.13.5167
- Mehreen
A, Wali RM, Sindhu II, Asad M, Ria S. Retrospective analysis of
clinical features and treatment outcomes of children with Hodgkin's
Lymphoma treated with different chemotherapy protocols at a tertiary
care center in Pakistan. J Pak Med Assoc. 2019;69(9):1266-1272.
- Kapoor
G, Advani SH, Dinshaw KA, Muckaden MA, Soman CS, Saikia TK, Gopal R,
Nair CN, Kurkure PA, Pai SK, et al. Treatment results of Hodgkin's
disease in Indian children. Pediatr Hematol Oncol. 1995;12(6):559-569. https://doi.org/10.3109/08880019509030770
- El-Badawy
S, Aboulnaga S, Abou Gabal A, Mokhless A, Zamzam M, Sidhom I, Ebeid E,
Hussein H.. Risk adapted combined modality treatment in children with
Hodgkin's disease: NCI, Cairo. J Egypt Natl Canc Inst.
2008;20(2):99-110.
- Büyükpamukçu M, Varan A, Akyüz C,
Atahan L, Ozyar E, Kale G, Köksal Y, Kutluk T. The treatment of
childhood Hodgkin lymphoma: improved survival in a developing country.
Acta Oncol. 2009;48(1):44-51. https://doi.org/10.1080/02841860802310991
- Zubizarreta
PA, Alfaro E, Guitter M, Sanchez La Rosa C, Galluzzo ML, Millán N,
Fiandrino F, Felice MS. Children and Adolescent Hodgkin Lymphoma in
Argentina: long-term Results After Combined ABVD and restricted
Radiotherapy. J Pediatr Hematol Oncol. 2017;39(8):602-608. https://doi.org/10.1097/MPH.0000000000000943
- Geel
JA, Chirwa TC, Rowe B, Eyal KC, Omar F, Stones DK, et al. Treatment
outcomes of children with Hodgkin lymphoma between 2000 and 2010: First
report by the South African Children's Cancer Study Group. Pediatr
Blood Cancer. 2017;64(10). https://doi.org/10.1002/pbc.26536
- Ghafoor
T. Prognostic factors in pediatric Hodgkin lymphoma: experience from a
developing country. Leuk Lymphoma. 2020;61(2):344-350. https://doi.org/10.1080/10428194.2019.1665666
- Parambil
BC, Narula G, Prasad M, Shah S, Shet T, Shridhar E, Khanna N, Laskar S,
Gujral S, Sankaran H, Banavali S. Clinical profile and outcome of
classical Hodgkin lymphoma treated with a risk-adapted approach in a
tertiary cancer center in India. Pediatr Blood Cancer.
2020;67(2):e28058. https://doi.org/10.1002/pbc.28058
- Geel
J, van Zyl A, Plessis JD, Hendricks M, Goga Y, Carr A, Neethling B,
Hramyka A, Omar F, Mathew R, Louw L, Naidoo T, Ngcana T, Schickerling
T, Netshituni V, Madzhia E, du Plessis L, Kelsey T, Ballot DE, Metzger
ML. Improved survival of children and adolescents with classical
Hodgkin lymphoma treated on a harmonised protocol in South Africa.
Pediatr Blood Cancer. 2024;71(1):e30712. https://doi.org/10.1002/pbc.30712
- Hessissen
L, Khtar R, Madani A, El Kababri M, Kili A, Harif M, Khattab M,
Sahraoui S, Benjaafar N, Ahid S, Howard SC, Benchekroun S. Improving
the prognosis of pediatric Hodgkin lymphoma in developing countries: a
Moroccan Society of Pediatric Hematology and Oncology study. Pediatr
Blood Cancer. 2013;60(9):1464-1469. https://doi.org/10.1002/pbc.24534
- Rodriguez-Galindo
C, Friedrich P, Alcasabas P, Antillon F, Banavali S, Castillo L,
Israels T, Jeha S, Harif M, Sullivan MJ, Quah TC, Patte C, Pui CH, Barr
R, Gross T. Toward the cure of all children with cancer through
collaborative efforts: pediatric oncology as a global challenge. J Clin
Oncol. 2015;33(27):3065-3073. https://doi.org/10.1200/JCO.2014.60.6376
- Moleti
ML, Testi AM, Foà R. Childhood aggressive B-cell non-Hodgkin lymphoma
in low-middle-income countries. Br J Haematol. 2022;196(4):849-863. https://doi.org/10.1111/bjh.17979
- Kalra
M, Bakhshi S, Singh M, Seth R, Verma N, Jain S, Radhakrishnan V, Mandal
P, Mahajan A, Arora RS, Dinand V, Kapoor G, Sajid M, Kumar R, Taluja A,
Mallick S, Chandra J. Response assessment by positron emission
tomography-computed tomography as compared with contrast-enhanced
computed tomography in childhood Hodgkin lymphoma can reduce the need
for radiotherapy in low- and middle-income countries. Pediatr Blood
Cancer. 2023;70(2):e30091. https://doi.org/10.1002/pbc.30091
- Mulder
RL, Font-Gonzalez A, Green DM, Loeffen EAH, Hudson MM, Loonen J, Yu R,
Ginsberg JP, Mitchell RT, Byrne J, Skinner R, Anazodo A, Constine LS,
de Vries A, Jahnukainen K, Lorenzo A, Meissner A, Nahata L,
Dinkelman-Smit M, Tournaye H, Haupt R, van den Heuvel-Eibrink MM, van
Santen HM, van Pelt AMM, Dirksen U, den Hartogh J, van Dulmen-den
Broeder E, Wallace WH, Levine J, Tissing WJE, Kremer LCM, Kenney LB,
van de Wetering MD; PanCareLIFE Consortium. Fertility preservation for
male patients with childhood, adolescent, and young adult cancer:
recommendations from the PanCareLIFE Consortium and the International
Late Effects of Childhood Cancer Guideline harmonization Group. Lancet
Oncol. 2021;22(2):e57-e67. https://doi.org/10.1016/S1470-2045(20)30582-9
- Mulder
RL, Font-Gonzalez A, Hudson MM, van Santen HM, Loeffen EAH, Burns KC,
Quinn GP, van Dulmen-den Broeder E, Byrne J, Haupt R, Wallace WH, van
den Heuvel-Eibrink MM, Anazodo A, Anderson RA, Barnbrock A, Beck JD,
Bos AME, Demeestere I, Denzer C, Di Iorgi N, Hoefgen HR, Kebudi R,
Lambalk C, Langer T, Meacham LR, Rodriguez-Wallberg K, Stern C,
Stutz-Grunder E, van Dorp W, Veening M, Veldkamp S, van der Meulen E,
Constine LS, Kenney LB, van de Wetering MD, Kremer LCM, Levine J,
Tissing WJE; PanCareLIFE Consortium. Fertility preservation for female
patients with childhood, adolescent, and young adult cancer:
recommendations from the PanCareLIFE Consortium and the International
Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet
Oncol. 2021;22(2):e45-e56. https://doi.org/10.1016/S1470-2045(20)30594-5
- Parkers J, Hess C, Burger H, Anacak Y, Ahern V, Howard SC, Elhassan M, Ahmed S, Ghalibafian M, Abbasi AN, Qureshi BM, Zaghloul M, Zubizarreta E, Bey P, Davidson A, Bouffet E, Esiashvili N. Recommendations for the treatment of children with radiotherapy in low- and middle-income countries (LMIC): A position paper from the Pediatric Radiation Oncology Society (PROS-LMIC) and Pediatric Oncology in Developing Countries (PODC) working groups of the International Society of Pediatric Oncology (SIOP). Pediatr Blood Cancer. 2017;64 Suppl 5. https://doi.org/10.1002/pbc.26903