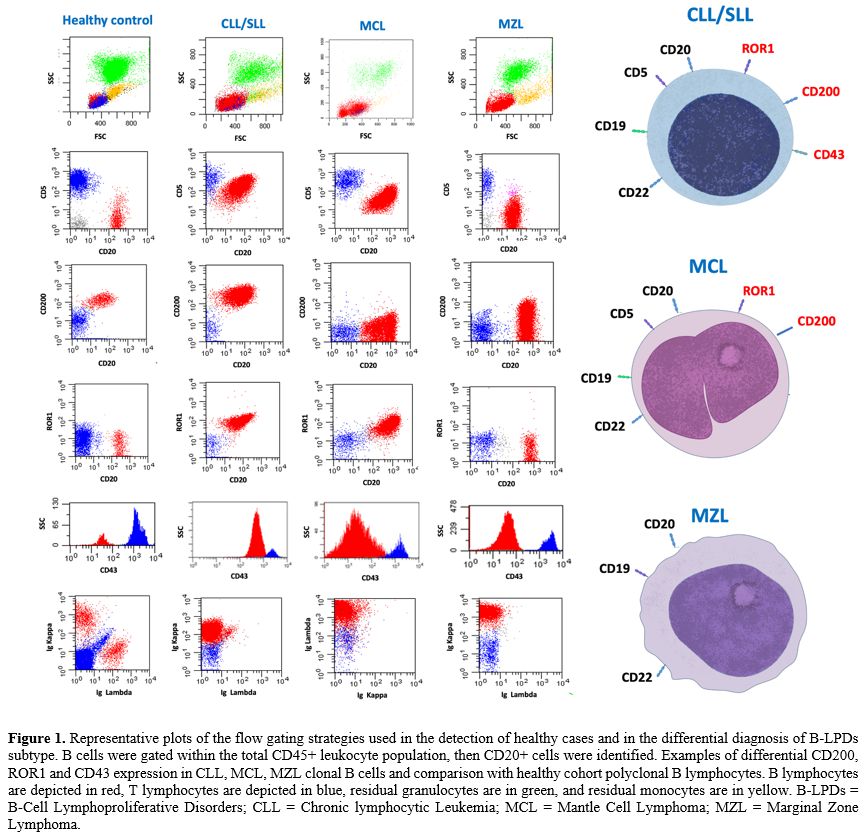
Immunophenotypic characterization employing multiparametric flow cytometry (MFC) is an increasingly important method for either hematological disease diagnosis or prompt exclusion, providing important prognostic disease features.[5-6] Nowadays, MFC is also crucial for B-LPD follow-up monitoring due to the increasingly pivotal role of minimal residual disease (MRD) detection, along with molecular testing.[7-8] Indeed, leukemic cells express peculiar surface and intracytoplasmic antigens, the identification of which allows to define the lineage, the level of differentiation, the maturation stage, and peculiar phenotypic aberrations. MFC has numerous advantages, ranging from the procedure's rapidity, the ability to analyze a broad spectrum of antigens, and the possibility of performing several simultaneous measurements to quantify population frequencies and antigen expression levels.[9-11] Panels for B-LPDs include the pan-B-cell markers (CD19, CD20, CD22 and cyCD79a) and other useful markers exploited for the differential diagnosis and prognostic characterization such as CD10, CD5, CD200, CD23, CD79b, FMC-7, CD103, CD25, CD123, CD43, CD81, CD38, CD49d, surface immunoglobulin (sIg) and Kappa and Lambda light chains.[12-13]
MFC-based algorithms have been developed over time to facilitate differential diagnosis, such as Matutes score for chronic lymphocytic leukemia (CLL).[14] In this respect, some antigens have been pinpointed as "required" for certain B-LPD diagnoses, while others are "recommended." For instance, Rawstron et al. identified CD19, CD5, CD20, CD23, Kappa, and Lambda light chains as “required” antigens for a CLL diagnosis with CD43, CD79b, CD81, CD200, CD10, and ROR1 representing “recommended” markers for differential diagnosis.[15-16] Exploiting these antigens, some authors have tried to increase MFC diagnostic sensitivity for CLL cases, as the study of D’Arena et al. which, exploiting CD200 detection, defined a simplified score, compared with the classical Matutes score, requiring 4 markers instead of 5 (CD5, CD23, CD200, and sIg).[17] Nevertheless, differential diagnosis can still be difficult with classical MFC panels. Thus, novel markers are continuously added to improve diagnostic sensitivity and specificity, including CD200, ROR1, and CD43.
The membrane glycoprotein CD200 (previously known as MRC OX-2) belongs to the immunoglobulin superfamily.[18] It is expressed on different cells, including myeloid, dendritic, endothelial cells, neurons, as well as B and T-lymphocytes.[19] CD200, interacting with its receptor (CD200R), plays a pivotal role in regulating the immune response.[20] CD200 differential expression in B-LPDs was first described in 2009 for being consistently expressed in CLL but often absent in mantle cell lymphoma (MCL), showing its possible diagnostic value.[21] The receptor tyrosine kinase-like orphan receptor 1 (ROR1) is an embryonic transmembrane glycoprotein involved in embryonic development.[22-23] After the embryonic phase, ROR1 is usually largely downregulated in human cells.[24] ROR1 was initially identified through gene expression profiling studies as a CLL-specific marker, proven to be uniformly expressed on CLL cell surfaces. However, it was later described in other B-LPDs.[8,15-16,25-26] Afterward, several studies involving small cohorts of patients highlighted the role of ROR1 expression in the diagnosis and prognosis of B-LPDs, specifically in the discrimination between CLL and CD51 post-germinal center B-cell disorders.[27] Hence, currently, ROR1 represents a valid and solid marker for MFC in B-LPD characterization. CD43 (i.e. leukosialin) is a glycosylated protein expressed in most leukocytes involved in cell adhesion, interaction, activation, and migration while its role in the B cell lineage is less known.[28] CD43 was initially used as an additional marker of CLL, but it has recently gained relevance in the differential diagnosis of B-LPDs, especially in extended panels.[29] CD43 is currently considered an additional target useful in the differential diagnosis of CLL from other neoplasms by WHO,[2] the European Research Initiative on CLL (ERIC), and the European Society for Clinical Cell Analysis (ESCCA).[15-16]
The quantitative analysis of MFC markers expressed as mean fluorescence intensity (MFI) is rarely discussed in the literature,[12] yet its use may be important not only in the differential diagnosis of B-LPDs but also in obtaining prognostic and predictive information.
Therefore, this retrospective study aims to evaluate the expression rate and MFI of CD200, ROR1, and CD43 in various B-LPDs, assess their usefulness in the differential diagnosis of these diseases as part of MFC routine panels, identify an accurate diagnosis, and support histological examination when necessary.
Methods
Flow cytometry. B-LPD diagnosis was assessed by MFC using a combination of monoclonal antibodies (mAbs) recommended by the EuroFlow Consortium.[30-31] Peripheral blood (PB) and/or bone marrow (BM) samples were stained within 24 hours of collection before any treatment, including steroids. Total leukocyte cells were incubated with an appropriate volume of mAbs directed against B, T, and NK lymphoid lineage antigens. with a combination of mAbs against CD45, CD5, CD19, CD20, CD22, CD200, ROR1, CD43, CD81, CD27, CD10, CD123, CD103, CD25, CD11c, CD49d, CD38, CD18, CD11a, CD79-b, FMC-7, Ig λ and Ig k, CD3, CD4, CD8, CD16, CD56, CD57, CD158a, CD158b, TCR-αβ, TCR-ϒẟ (Becton et al.; Società Italiana Chimici, SIC, Life Sciences, Rome, Italy, Beckman Coulter, Brea, CA). Data on standardized 8-12 color staining combinations were acquired on FACSCanto II or BD FACS Lyric flow cytometers (Becton Dickinson) by collecting at least 50,000 ungated events and analyzed using the PAINT-A-GATE and FACSDIVA software (Becton Dickinson). Cytometer setup and tracking beads (BD) were used for daily cytometer optimization. Leukemic cells were gated within the total CD45+ leukocyte population, considering that all cases of B-LPDs were positive for the pan-leukocyte antigen. Representative plots of the flow gating strategy are reported in Figure 1. The presence of pathological cells was identified in comparison with the known patterns of antigen expression and MFI profile with the known pattern of normal maturing lymphoid precursors.[32] In all cases, antigen expression was defined by the percentage of pathological lymphocytes that resulted in positive for the different markers in the immunological gate. Furthermore, cell surface antigen expression was estimated by assessing the proportion of positive leukemic cells for each given antigen with a positivity cutoff of more than or equal to 20%. Antigen expression levels were quantified based on MFI values obtained with specific mAbs compared with values given by the internal negative controls, which were represented by a cell population that does not express the antigen of interest and thus remains unlabeled in an antibody-labeled cell suspension but that has been exposed to identical conditions (including exposure to the antibody directed to the antigen of interest) as the cell population under study.Study design. We analyzed data from 3051 MFC routine panels’ results of consecutive patients tested for B-LPDs referred at the Hematology department of “Sapienza” University of Rome between 2009 and 2024. All patients provided informed consent for the investigation of PB and/or BM materials. When the differential diagnosis was not feasible by exploiting MFC alone, by guidelines, the diagnosis was obtained by the integration of clinical data, morphological findings on PB, BM examination, and/or histopathological examination on BM biopsies and/or on lymph nodes, and molecular analysis that were correlated with the final results on MFC. Each diagnosis was made according to the current WHO classification.[2,33-34] In the 2024 WHO classification, the new entity splenic B-cell lymphoma/leukemia with prominent nucleoli (SBLPN) replaced the previous term 'variant villous cell leukemia' (vHCL).[2] Nevertheless, due to specific immunophenotypic disease subset, for clarity, due to novel concerns regarding this reassignment of patients with vHCL,[35] and because the 2022 International Consensus Classification (ICC) of Mature Lymphoid Neoplasms[36] preserved the term vHCL, this will be used throughout the rest of the paper. Moreover, according to the 2024 WHO classification,[2] our cases of monoclonal B-cell lymphocytosis (MBL) were classified in CLL/SLL-type MBL (monoclonal CLL/SLL-phenotype B-cell count ≥0.5 x 109/L and total B-cell count less than 5 x109/L with no other features diagnostic of CLL/SLL) and non-CLL/SLL-type MBL (any monoclonal B-cell expansion with no symptoms or features diagnostic of another mature B-cell neoplasm).
A total of 436 cases were excluded from the final analysis because the exact classification into a specific entity was not provided. For 244 of them, MFC immunophenotyping supported the diagnosis of clonal B-LPDs; however, histological or other complementary exams were not performed. For 192 cases, both MFC and histopathological exams pointed to clonal B-LPD; however, a diagnosis of mature B cell lymphoma non-other specified (NOS) was rendered (Figure 2).
Statistical analysis. Summary statistics, such as mean and standard deviation, median, range, and interquartile range (IQR), were reported by category groups. Differences in the study groups were assessed by the Pearson chi-square test or the Fisher exact test for categorical covariates. Pairwise comparisons using the Wilcoxon rank sum test for independent groups were used for comparisons between categories. All tests were two-sided, accepting p < 0.05 as indicating a statistically significant difference. All analyses were performed using R software [R Core Team (2021). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria].
Results
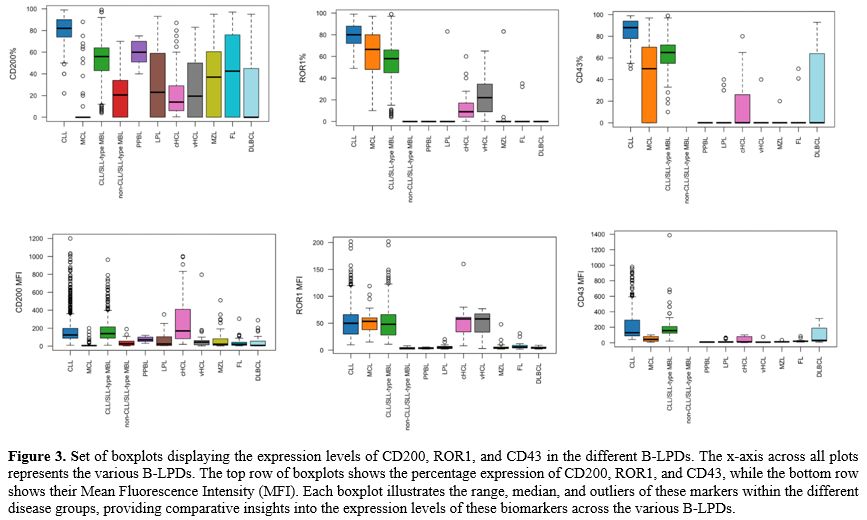
In this study, data from 2615 cases tested by MFC were retrospectively analyzed. Patients’ characteristics are displayed in supplementary table 1. The median age of patients at the MFC test was 66 years (IQR, 53-74), and 1509 (57.7%) were male. The overall median absolute lymphocyte count (ALC) among patients was 8.5 x109/L (IQR, 4.6-16.3), with a median clonal B-cell count of 6.0 x109/L (IQR, 2.3-13.3). Figure 2 presents the distribution of B-LPDs into specific entities. Overall, CLL was the most prevalent diagnosis, accounting for 1.401 cases (53.6%). Additionally, 466 cases (17.8%) were identified as CLL/SLL-type MBL and 32 cases (1.2%) were classified as non-CLL/SLL-type MBL. HCL was diagnosed in 144 cases (5.5%), including 112 cases of classic HCL (cHCL) and 32 cases of variant HCL (vHCL – according to 2022 ICC). Other mature B-cell neoplasia were identified in 294 cases (11.2%). In some of these, MFC phenotype pointed to specific lymphoma subtypes, which were subsequently confirmed by histological examinations (i.e., mantle cell lymphoma, MCL; follicular lymphoma, FL; marginal zone lymphoma, MZL; diffuse large B-cell lymphoma, DLBCL; and lymphoplasmacytic lymphoma, LPL).Figure 3 shows boxplots displaying the expression levels of CD200, ROR1, and CD43 (percentage and MFI) in the major different B-LPDs. Table 1a shows the CD200, ROR1, and CD43 percentage of expression and MFI in the major different B-LPDs, while Table 2a displays the positivity rate of the three antigens.
 |
Table 2. A) Antigens positivity rate comparison between B-LPDs of CD200, ROR1, and CD43. B) P-Value Comparisons between selected B-LPDs couples, proving statistically significant differences. |
Chronic Lymphocytic Leukemia and CLL/SLL-type Monoclonal B cell Lymphocytosis. In CLL cases (n=1.401), CD200 was constantly detected (positivity percentage: 100%) and highly expressed [CD200%, mean: 100 (range, 24-100)], with bright intensity [CD200 MFI, median = 125 (range, 10-1200)]. ROR1 expression was similarly positive and highly expressed in all cases [ROR1%, mean: 100 (range, 52-100), positivity rate: 100%] with a median MFI of 50 (range, 10-202). CD43 was positive in all CLL cases (100%), with elevated mean expression percentage [CD43%, mean: 99 (range, 59-100)], and with a bright MFI [CD43 MFI, median: 130 (range, 41-980)]. In the CLL/SLL-type MBL cases (n=466), the expression of CD200, ROR1, and CD43 were as high as in CLL.
Leukemic phase of Mantle Cell Lymphoma. The leukemic phase of MCL was identified in 65 cases (2.5%). In this cohort, CD200 was generally not detected (positivity rate: 17%), thus resulting in a low expression percentage [CD200%, mean: 15 (range, 0-100)], with low MFI [CD200 MFI, median: 6 (range, 1-200)]. ROR1 was expressed in all MCL cases (positivity rate: 100%), with a high mean expression percentage [ROR1%, mean: 88 (range, 43-100)], and with a median MFI of 54 (range, 15-119). CD43 was negative in 74% of the MCL cases tested for such antigen, and its mean expression percentage was quite low [CD43% mean: 32 (range, 0-100)] with a very low MFI [CD43 MFI, median: 44 (range, 7-101)].
Hairy Cell Leukemia (cHCL and vHCL according to 2022 ICC). In cHCL cases (n=112), CD200 was constantly detected (positivity percentage: 100%) and highly expressed [CD200%, mean = 100 (range, 100-100)], with bright intensity [CD200 MFI, median: 170 (range, 20-1000)]. ROR1 was also expressed in the majority of cases (90% of positive cases), with a mean expression percentage of 90% (range, 0-100), whereas its median MFI was 58 (range, 8-160). CD43 was less expressed on cHCL cells surface [CD43%, mean: 43 (range, 0-100) and negative in 57% of cHCL cases], with very low MFI [CD43 MFI, median: 11 (range, 3-101)]. In vHCL cohort (n=32), CD200 [CD200%, mean: 67 (range, 0-100); positivity rate 71%; MFI, median: 45 (range, 2-796)] and ROR1 [ROR1%, mean: 77 (range, 0-100); positivity rate 82%; MFI, median: 58 (range, 3-77)] were less expressed than in cHCL cohort, as well as CD43 that resulted negative in almost all cases [CD43%, mean: 7 (range, 0-100)] with very low MFI [CD43 MFI, median: 8 (range, 3-75)].
Other mature B-cell neoplasms confirmed by histologic examination. FL cohort (n=50) showed intermediate CD200 expression [CD200%, mean: 63 (range, 0-100); positivity rate: 68%] with a low MFI [CD200 MFI, median: 22 (range: 3–305)]. ROR1 resulted negative in almost all cases [ROR1%, mean: 2 (range, 0-47); positivity rate: 5%] and characterized by a low MFI for the rare positive cases [ROR1 MFI, median: 6 (range: 2–31)]. CD43 was usually negative as well [CD43%, mean: 5 (range: 0-66); positivity rate: 8%] and showed a very low MFI [CD43 MFI, median: 19 (range: 8–85)].
MZL cohort (n=83) exhibited a similar pattern to FL, with variable CD200 expression [CD200%, mean: 60 (range: 0-100); positivity rate: 69%; MFI median: 21 (range, 2–511)], usually negative ROR1 [ROR1%, mean: 4 (range, 0-100); positivity rate: 4%] with low MFI in the seldomly positive cases [ROR1 MFI, median: 4 (range, 2–48)], and CD43 negative in almost all cases [CD43%, mean: 1 (range: 0-27); positivity rate: 5%] with a very low MFI [CD43 MFI, median: 12 (range, 8–36)].
DLBCL cases (n=34) were frequently negative for CD200 [CD200%, mean: 34 (range, 0-100); positivity rate: 35%] and with a low MFI [CD200 MFI, median: 8 (range, 2–288)]. ROR1 was universally absent (0%), and CD43 expression was variable [CD43%, mean: 43 (range, 0-100); positivity rate: 43%] with a moderate MFI [CD43 MFI, median: 30 (range, 8–312)].
LPL cohort (n=26) had a variable CD200 expression [CD200%, mean: 59 (range, 0-100); positivity rate: 65%] with a quite low MFI [CD200 MFI, median: 24 (range, 4–353)]. ROR1 resulted almost always negative [(ROR1%, mean: 6 (range, 0-100); positivity rate: 6%] with a low MFI [ROR1 MFI, median: 5 (range, 2–20)] and also CD43 was usually negative [CD43%, mean: 11 (range, 0-51); positivity rate: 24%] with a very low MFI [CD43 MFI, median: 10 (range, 5–65)].
Healthy cohort and Persistent polyclonal B-cell lymphocytosis. Among the 281 cases that resulted not to harbor a clonal B-cell, CD200 was universally present on “normal” B-lymphocytes [CD200%, mean: 100 (range, 100-100); positivity rate: 100%; MFI median: 20 (range 5-52)], while ROR1 and CD43 were absent on their surface. In the polyclonal B-cell lymphocytosis (PPBL)[37] cohort (n=16), the results were similar to the healthy cohort.
Comparative analysis between B-LPDs. We compared the expression percentages and MFI levels of CD200, ROR1, and CD43 across different B-LPDs, assessing whether the observed differences could aid in their differential diagnosis. The comparison between the major B-LPDs revealed important differences in terms of expression percentages and positivity rates, as shown in Table 1b and Table 2b, respectively.
CD200 expression was ubiquitous in CLL cases, whereas MCL cases showed a much lower percentage of this antigen [CLL, 100% (range 24-100) vs. MCL, 15% (range 0-100)] (p<0.001) and, consequently, CD200 was more frequently positive in CLL [CLL, 100% vs MCL 17%] (p<0.001). Similarly, the MFI for CD200 was markedly higher in CLL, indicating a more intense antigen expression compared to MCL, which presented a usually low CD200 MFI in the seldomly positive cases [CLL 125 (range 10-1200) vs MCL 6 (range 1-200)] (p<0.001). ROR1 expression percentage was high in both CLL and MCL [CLL 100% (range 52-100) vs MCL 88% (range 46-100)], and in both diseases, ROR1 resulted positive in all cases (p>0.9). The MFI for ROR1 was comparable between these two diseases, suggesting no substantial difference for this antigen intensity (p>0.9). In contrast, CD43 was universally expressed in CLL, while it displayed significantly lower positivity levels in MCL [CLL, 100% vs MCL 32%] (p<0.001). The MFI for CD43 was also notably elevated in CLL, reflecting a more intense expression compared to MCL [CLL 130 (range 41-980) vs MCL 44 (7-101)] (p=0.024).
As expected, no difference was detected between CLL and CLL/SLL-type MBL; both displayed uniformly high expression levels of CD200, ROR1, and CD43 (p>0.9) with comparable MFI for each of these antigens (p>0.9).
Confronting CLL and cHCL, both exhibited CD200 positivity in all cases with bright intensity, without any difference in CD200 antigen expression (p>0.9), positivity rate (p>0.9) or in its intensity (p>0.9). In contrast, ROR1 mean expression percentage was statistically higher in CLL [100% (range, 52-100)] compared to cHCL cases [90% (range 0–100%)] (p=0.021), but ROR1 positivity rate and its MFI were similar between these diseases (p>0.9 and p=0.25, respectively). As for CD43, CLL demonstrated a robust antigenic CD43 profile, whereas cHCL exhibits a significantly lower CD43 expression percentage [43% (range, 0–100)] (p<0.001) and CD 43 positivity rate at 43% (p<0.001), with lower MFI [11 (range 3–101)] (p<0.001). In a comparative analysis of CLL and vHCL cases, CLL showed significantly higher expression of CD200 and MFI compared to vHCL [CD200%, mean: 67 (range, 0-100); CD200 MFI, median: 45 (range, 2-796)] (p<0.001). ROR1, mean expression percentage was statistically higher in CLL than in vHCL [100% (range, 52-100)] vs 77% (range 0-100)] (p<0.001), but similar ROR1 positivity rate (p=0.50) and MFI (p>0.9) were evidenced between these two diseases. CD43 was almost completely absent in vHCL [mean expression: 6 (range, 0-100); positivity rate: 6%], while, as mentioned above, CLL demonstrated universal CD43 positivity (p<0.001).
Therefore, in the comparison between cHCL and vHCL, CD200 expression and MFI levels were significantly higher in cHCL compared to vHCL (p<0.001), while no significant differences were observed in either expression or MFI of ROR1. CD43, instead, resulted in an higher mean expression percentage in cHCL compared to vHCL [43% (range, 0-100) vs 7% (range, 0-100)] (p=0.021), with an higher positivity rate (43% vs 6%) (p=0.029), and with an higher median MFI [11 (range, 3-101) vs 8 (range, 3-75)] (p=0.032).
Moreover, in comparative analysis with other mature B cell neoplasms, CLL cases presented significantly higher expression and MFI for all three antigens (CD200, CD43, and ROR1) than FL, MZL, DLBCL, LPL, and non-CLL/SLL-type MBL cases, as displayed in Tables 1b and 2b.
Comparing CLL cases with the healthy cohort, CLL showed the presence of ROR1 and CD43 on the cell surface, which were absent in the healthy lymphocytes (p<0.001). Furthermore, albeit mean expression percentages and positivity rate of CD200 were 100% for both CLL and healthy cases, the CD200 MFI of CLL cells was statistically higher [CLL 125 (range, 10-1200) vs Healthy cohort 20 (range, 5-52)] (p<0.001).
As per MZL cases, clonal cells showed superior expression of CD200 [mean percentage, 60% (range 0-100); positivity rate 69%] and MFI [21 (range 2-511)] compared to MCL (p<0.001), but similar to vHCL (p>0.9). MZL cases were almost always negative for CD43 and ROR1, while ROR1 was universally expressed in MCL (p<0.001) and frequently expressed in vHCL (p<0.001). CD43, instead, resulted in negative results in almost always vHCL and MZL and frequently negative results in MCL.
Comparison between DLBCL and MCL revealed no significant differences in CD200 and CD43 expression percentage (p>0.9) or their respective MFI (p>0.9), resulting mostly negative in these two entities. However, ROR1 was notably absent in DLBCL while being frequently expressed in MCL with significant differences in expression percentages (p<0.001), positivity rate (p<0.001), and MFI (p<0.001).
Discussion
Routinely, MFC panels for B-LPDs may be, in certain cases, sufficient to make a simple diagnosis and to prove cell clonality; however, nowadays, the addition of new markers with a qualitative or semiquantitative diagnostic interpretation may help to improve the likelihood of a correct immunophenotypic differential diagnosis. As already mentioned, CD200, ROR1, and CD43 are three antigens expressed in various hematological diseases, the simultaneous use of which in MFC panels may improve the diagnostic yield.In hematological diseases, CD200 is expressed in most CLL, cHCL, and vHCL but is absent in most cases of MCL and variably expressed in other B-LPDs. The addition of CD200 to MFC panels, along with its MFI, improves the sensibility of the B-LPDs diagnostic algorithm, especially for the differential diagnosis of CD5-positive B-LPDs.[38-39] Alapat et al. evaluated 107 B-LPD samples. Bright CD200 expression was observed in all 19 CLL cases, with at least one log shift in MFI compared to the isotype control antibody, while it was uniformly absent in MCL (4 cases).[40] Sanders et al. showed that 45 CLL cases presented CD200 homogeneous and strong expression, with all 14 MCL cases resulting in CD200 negative. Furthermore, 11 atypical CLL cases expressed CD200 but with lower intensity than typical CLL ones.[38] CD200 expression has proven to be a reliable marker for differentiating between CLL and other B-LPDs, not only MCL. In a study of 49 cases, El Desoukey et al. showed that CD200 was highly bright expressed on CD5/CD19-positive clones in all B-CLL patients (100%), with a mean of 94% (SD, 11%), whereas in patients with other B-LPDs, including MCL, FL, and splenic marginal zone lymphoma (SMZL), presented antigen expression below the positivity cutoff (20%), with a mean of 10% (SD, 8%) and with a dim pattern. Conversely, in cHCL CD200 was strongly and brightly expressed as well.[41] Regarding HCLs, on a sample of 180 B-LPDs cases Pillai et al showed that cHCL exhibited very high levels of CD200, with a higher MFI compared to vHCL (just 10% was CD200 positive), MZL, and lymphoplasmacytic lymphoma (LPL).[42] Moreover, Salem et al. focused on MFC analysis of 59 cHCL and 15 vHCL cases. CD200 was evaluated in a small subset of these cases (17/59 cHCL, 7/15 vHCL), resulting expressed in all cHCL specimens with mostly bright expression.[43]
ROR1 is highly and uniformly expressed in CLL patients as well. In the study of Uhrmacher et al., 177 samples of CLL patients were analyzed, including 105 untreated and 72 previously treated patients. ROR1 was uniformly expressed on untreated CLL cell surfaces (mean 97.6%). No significant difference was found between ROR1 expression levels during different time points of treatment, and ROR1 expression levels were uniformly high independently from treatment.[44] Such a finding has been confirmed by our group as well, pinpointing that ROR1 expression and MFI may represent an accurate and reliable marker to evaluate CLL MRD in combination with other molecules such as CD5, CD19, CD43, and CD81.[45-46] Evaluation of CD43 in MFC panels may improve differential diagnosis ability for B-LPDs as well. In a study by Sorigue et al., 643 cases of B-LPDs were subclassified according to the 5-point Moreau system (MS), and the differential expression of CD43 was analyzed in each group. CD43 was positive in 425/443 (96%) of MS4-5 (therefore, diagnostically certain CLL). Thus, CLL diagnosis is very unlikely in the case of CD43-negativity.[29]
To the best of our knowledge, our study includes the highest reported number of B-LPD samples analyzed by MFC for diagnostic purposes. In our study, CLL cases presented a pathognomonic combination of surface antigens as CD5/CD19/CD22/CD23 and low MFI of CD20 and of the clonal sIg (data not shown), as clearly expected. Moreover, CD200, ROR1, and CD43 were universally expressed in CLL cases with high mean expression and bright MFI, whereas CD43 may play an important role in the differential diagnosis of CLL, especially for atypical cases, because CLL diagnosis is very unlikely in the case of CD43 negativity. Furthermore, CD43 MFI can represent an important tool for B-LPD diagnosis. In MCL cases, instead, while ROR1 was universally expressed with an MFI comparable to CLL cases, the expression of CD200 and CD43 resulted in mostly negative. Moreover, all cHCL cases expressed CD200 with bright intensity (as CLL cases), but ROR1 and CD43 expression was variable and meanly lower than in CLL samples. As per vHCL cases, CD200, CD43 and ROR1 expression was variable, with CD43 usually negative and with overall mean expression lower than CLL cases. Furthermore, FL, MZL, DLBCL, LPL, and non-CLL/SLL-type MBL samples presented variable expression of CD200 that was meanly lower than in CLL samples, but CD43 and ROR1 were usually negative. Such evidence is consistent with the one reported in the literature.
Focusing on the reproducibility of our results, our MFC panel may be sufficiently easily applicable as a screening tool for lymphocytosis. In fact, a three-step MFC was used: the first step included an initial screening with hematopoietic lineage antigens such as CD20/CD5/CD3/CD45/CD4/CD8/CD38/CD10; once the positivity of mature B-line antigens (such as CD20) was established, the second panel should include the three antibodies against the antigens investigated in this study (CD200, ROR1 and CD43) added to other antigens useful for the diagnosis of B-LPDs (for example, CD20/ROR1/CD3/CD5/CD43/CD200/CD23/CD45); lastly, the use of a simple panel for clonality assessment such as CD19/CD20/CD5/κ-λ light chains. Other methods have been developed over time for lymphocytosis screening, such as the automatized EuroFlow Lymphoid Screening Tubes (LSTs), which provide reliable and reproducible tools for fast and simplified identification of normal vs pathological lymphocytes.[47] Nevertheless, we reckon that our MFC approach might be easily applicable and not only discriminate pathological from normal lymphocytes but also might improve differential diagnosis sensitivity and specificity in B-LPDs.
Moreover, of note was the detection in a few of the reported cases (1.2%) of a dual pathology (both B and T pathological cells) because the diagnostic workup always included the analysis of T-cell subpopulations. In these cases, further investigations were performed by an extensive T-cell panel and clonality testing, including phenotypic analysis of the V-beta repertoire and/or molecular biology techniques for TCR analysis.
Conclusions
Our study shows that the inclusion of CD200, CD43, and ROR1 and their MFIs in routine MFC analysis improves the diagnostic accuracy of B-LPDs (especially CLL) and helps in the differential diagnosis between CLL and MCL, resulting in indispensable in the diagnostic workup. Routine MFC panels, enriched with these three antigens, allow an exceptionally rapid diagnostic evaluation of patients presenting with lymphocytosis. Such panels may be used along with classical MFC scores such as the Matutes score, but they may be considered diagnostically accurate on their own. Moreover, it is important to consider that while routinely performed MFC analysis can pinpoint a CLL diagnosis, for all other B-LPDs, by guidelines, additional diagnostic exams are required, such as histologic, cytogenetic, and mutational analysis. Nevertheless, these “enriched” MFC panels can suggest and support additional diagnostic exams for B-LPDs. Hence, we believe that, nowadays, CD200, CD43, and ROR1 antigens detection has become a pivotal part of the diagnostic process of B-LPDs.Author Contributions
Alessandro Laganà and Raffaele Maglione: Writing – original draft in equal contribution. Alessandro Costa: Data collection, acquisition, analysis, and interpretation. Maria Laura Bisegna: Contribution to the lab work and review of the manuscript. Maria Laura Milani: Flow cytometry data collection, acquisition, and analysis. Biancamaria Mandelli: Contribution to the lab work and data analysis. Luigi Petrucci: Followed the patients and supervised data collection. Valeria Filipponi, Tania Soriano, Eugenio Santacroce: Contribution to the lab work and data collection. Maria Grazia Nardacci: Contribution to the lab work and complementary exams. Carla Giordano: Provided histological diagnosis and manuscript editing. Maurizio Martelli: Supervision and Manuscript editing. Maria Stefania De Propris: Conceptualization, Investigation, Formal analysis, Writing – original draft.References
- Al-Zubaidi HK, Hughes SF. The use of CD200 in the
differential Diagnosis of B-Cell lymphoproliferative disorders. Br J
Biomed Sci.2023;80:11573. https://doi.org/10.3389/bjbs.2023.11573 PMid:37822353 PMCid:PMC10563807
- WHO
Classification of Tumours Editorial Board. Haematolymphoid tumours.
Lyon (France): International Agency for Research on Cancer; 2024. (WHO
classification of tumours series, 5th ed.; vol. 11). https://publications.iarc.who.int/637
- Chan JK, Kwong YL. Common misdiagnoses in lymphomas and avoidance strategies. Lancet Oncol. 2010 Jun;11(6):579-88. https://doi.org/10.1016/S1470-2045(09)70351-1 PMid:20227918
- Deng
J, Zuo X, Yang L, Gao Z, Zhou C, Guo L. Misdiagnosis analysis of 2291
cases of haematolymphoid neoplasms. Front Oncol. 2023 Apr
26;13:1128636. https://doi.org/10.3389/fonc.2023.1128636 PMid:37182167 PMCid:PMC10170766
- Bisegna
ML, Cordone I, Peragine N, Milani ML, Intoppa S, de Fabritiis P,
Martelli M, De Propris MS. Neoplastic bone marrow invasion:rapid
exclusion of hematological disease by flow cytometric routine panels.
Blood Cells Mol Dis. 2023 Mar;99:102721 https://doi.org/10.1016/j.bcmd.2022.102721 PMid:36459839
- De
Propris MS, Musiu P, Intoppa S, Nardacci MG, Pucciarini A, Santi A,
Peragine N, Canichella M, De Luca ML, D'Elia GM, Del Giudice I, Pulsoni
A, Falini B, Guarini A, Martelli M, Tiacci E, Foà R. Hairy cell
leukaemia with low CD103 expression: A rare but important diagnostic
pitfall. Br J Haematol. 2022 Jul;198(2):e28-e31. https://doi.org/10.1111/bjh.18224 PMid:35499213
- Braylan
RC. Impact of flow cytometry on the diagnosis and characterization of
lymphomas, chronic lymphoproliferative disorders and plasma cell
neoplasia. Cytometry. 2004; 58(1):57-61. https://doi.org/10.1002/cyto.a.10101 PMid:14994222
- De
Propris MS, Intoppa S, Milani ML, Mariglia P, Nardacci MG, Peragine N,
Foà R, Guarini A. ROR1 is an accurate and reliable marker of minimal
residual disease in chronic lymphocytic leukaemia. Br J Haematol. 2020
Sep;190(6):e346-e349. https://doi.org/10.1111/bjh.16910 PMid:32579248
- El
Desoukey NA, Afify RA, Amin DG, Mohammed RF. CD200 expression in B-cell
chronic lymphoproliferative disorders. J Investig Med.
2012;60(1):56-61. https://doi.org/10.2310/JIM.0b013e31823908f9 PMid:22064604
- De
Propris MS, Raponi S, Diverio D, Milani ML, Meloni G, Falini B, Foà R,
Guarini A. High CD33 expression levels in acute myeloid leukemia cells
carrying the nucleophosmin (NPM1) mutation. Haematologica. 2011
Oct;96(10):1548-51. doi: 10.3324/haematol.2011.043786. Epub 2011 Jul
26. https://doi.org/10.3324/haematol.2011.043786 PMid:21791474 PMCid:PMC3186318
- Raponi
S, De Propris MS, Intoppa S, Milani ML, Vitale A, Elia L, Perbellini O,
Pizzolo G, Foá R, Guarini A. Flow cytometric study of potential target
antigens (CD19, CD20, CD22, CD33) for antibody-based immunotherapy in
acute lymphoblastic leukemia: analysis of 552 cases. Leuk Lymphoma.
2011 Jun;52(6):1098-107. https://doi.org/10.3109/10428194.2011.559668 PMid:21348573
- Arlindo
EM, Marcondes NA, Fernandes FB, Faulhaber GAM. Quantitative flow
cytometric evaluation of CD200, CD123, CD43 and CD52 as a tool for the
differential diagnosis of mature B-cell neoplasms. Rev Bras Hematol
Hemoter. 2017;39(3):252-258. https://doi.org/10.1016/j.bjhh.2017.05.002 PMid:28830605 PMCid:PMC5567423
- Kroft
SH, Harrington AM. Flow Cytometry of B-Cell Neoplasms. Clin Lab Med.
2017 Dec;37(4):697-723. doi: 10.1016/j.cll.2017.07.001. Epub 2017 Aug
31. https://doi.org/10.1016/j.cll.2017.07.001 PMid:29128065
- Moreau
EJ, Matutes E, A'Hern, RP, Morilla AM, Morilla RM, Owusu-Ankomah KA.,
Seon, BK, Catovsky, D. Improvement of the chronic lymphocytic leukemia
scoring system with the monoclonal antibody SN8 (CD79b). Am J Clinical
Pathol.1997;108(4):378-382. https://doi.org/10.1093/ajcp/108.4.378 PMid:9322589
- Rawstron
AC, Böttcher S, Letestu R, Villamor N, Fazi C, Kartsios H, de Tute RM,
Shingles J, Ritgen M, Moreno C, Lin K, Pettitt AR, Kneba M, Montserrat
E, Cymbalista F, Hallek M, Hillmen P, Ghia P; European Research
Initiative in CLL. Improving efficiency and sensitivity: European
Research Initiative in CLL (ERIC) update on the international
harmonised approach for flow cytometric residual disease monitoring in
CLL. Leukemia. 2013 Jan;27(1):142-9. https://doi.org/10.1038/leu.2012.216 PMid:23041722
- Rawstron
AC, Kreuzer K-A, Soosapilla A, Spacek M, Stehlikova O, Gambell P,
McIverBrown N, Villamor N, Psarra K, Arroz M, Milani R, de la Serna J,
Cedena MT, Jaksic O, Nomdedeu J, Moreno C, Rigolin GM, Cuneo A,
Johansen P, Johnsen HE, Rosenquist R, Utoft Niemann C, Kern W,
Westerman D, Trneny M, Mulligan S, Doubek M, Pospisilova S, Hillmen P,
Oscier D, Hallek M, Ghia P, Montserrat E. Reproducible diagnosis of
chronic lymphocytic leukemia by flow cytometry: An european research
initiative on CLL (ERIC) & European Society for Clinical Cell
Analysis (ESCCA) Harmonisation Project. Cytometry B Clinic Cytom. 2018;
94(1): 121-128. https://doi.org/10.1002/cyto.b.21595 PMid:29024461 PMCid:PMC5817234
- D'Arena
G, Vitale C, Rossi G, Coscia M, Omedè P, D'Auria F, Statuto T, Valvano
L, Ciolli S, Gilestro M, Molica S, Bellesi S, Topini G, Panichi V,
Autore F, Innocenti I, Musto P, Deaglio S, Laurenti L, Del Vecchio L.
CD200 included in a 4-marker modified Matutes score provides optimal
sensitivity and specificity for the diagnosis of chronic lymphocytic
leukaemia. Hematol Oncol. 2018 Mar 30. https://doi.org/10.1002/hon.2510 PMid:29602219
- McCaughan
GW, Clark MJ, Barclay AN. Characterization of the Human Homolog of the
Rat MRC OX-2 Membrane Glycoprotein. Immunogenetics.1987;25(5):329-35. https://doi.org/10.1007/BF00404426 PMid:3032785
- Wright
GJ, Jones M, Puklavec MJ, Brown MH, Barclay AN. The unusual
distribution of the neuronal/lymphoid cell surface Cd200 (Ox2)
glycoprotein is conserved in humans. Immunology. 2001;102(2):173-9. https://doi.org/10.1046/j.1365-2567.2001.01163.x PMid:11260322 PMCid:PMC1783166
- Nathan C, Muller WA. Putting the brakes on innate immunity: A regulatory role for CD200? Nat Immunol. 2001;2(1):17-9. https://doi.org/10.1038/83124 PMid:11135572
- Palumbo
GA, Parrinello N, Fargione G, Cardillo K, Chiarenza A, Berretta S,
Conticello C, Villari L, Di Raimondo F. CD200 expression may help in
differential diagnosis between mantle cell lymphoma and B-cell chronic
lymphocytic leukemia. Leuk Res. 2009 Sep;33(9):1212-6. https://doi.org/10.1016/j.leukres.2009.01.017 PMid:19230971
- Green JL, Kuntz SG, Sternberg PW. Ror receptor tyrosine kinases: orphans no more. Trends Cell Biol 2008;18(11):536-44. https://doi.org/10.1016/j.tcb.2008.08.006 PMid:18848778 PMCid:PMC4672995
- Daneshmanesh
AH, Mikaelsson E, Jeddi-Tehrani M, Bayat AA, Ghods R, Ostadkarampour M,
Akhondi M, Lagercrantz S, Larsson C, Osterborg A, Shokri F, Mellstedt
H, Rabbani H. Ror1, a cell surface receptor tyrosine kinase is
expressed in chronic lymphocytic leukemia and may serve as a putative
target for therapy. Int J Cancer 2008;123(5):1190-5. https://doi.org/10.1002/ijc.23587 PMid:18546292
- Yoda
A, Oishi I, Minami Y. Expression and function of the Ror-family
receptor tyrosine kinases during development: lessons from genetic
analyses of nematodes, mice, and humans. J Recept Signal Transduct Res
2003;23(1):1-15. https://doi.org/10.1081/RRS-120018757 PMid:12680586
- Baskar
S, Kwong KY, Hofer T, Levy JM, Kennedy MG, Lee E, Staudt LM, Wilson WH,
Wiestner A, Rader C. Unique cell surface expression of receptor
tyrosine kinase ROR1 in human B-cell chronic lymphocytic leukemia. Clin
Cancer Res 2008;14(2):396-404. https://doi.org/10.1158/1078-0432.CCR-07-1823 PMid:18223214
- Barna
G, Mihalik R, Timár B, Tömböl J, Csende Z, Sebestyén A, Bödör C,
Csernus B, Reiniger L, Peták I, Matolcsy A. ROR1 expression is not a
unique marker of CLL. Hematol Oncol. 2011;29(1):17-21. https://doi.org/10.1002/hon.948 PMid:20597086
- Rawstron
AC, de Tute RM, Shingles J, Gorman L, Turner K, Evans PAS, Barrans SL,
O'Connor SJM, Burton C, Owen RG, Hillmen P. Improving the Differential
Diagnosis of CD5+ B-Lymphoproliferative Disorders. (Abstract release
date: 05/19/16) EHA Library
- Rosenstein Y, Santana A, Pedraza-Alva G. CD43, a molecule with multiple functions.Immunol Res. 1999;20(3):89-99. https://doi.org/10.1007/BF02786465 PMid:10580634
- Sorigue
M, Juncà J, Sarrate E, Grau J. Expression of CD43 in chronic
lymphoproliferative leukemias. Cytometry B Clin Cytom.
2018;94(1):136-142.0 https://doi.org/10.102/cyto.b.21509 PMid:28073173
- Pedreira
CE, Costa ES, Almeida J, Fernandez C, Quijano S, Flores J, Barrena S,
Lecrevisse Q, Van Dongen JJ, Orfao A; EuroFlow Consortium. A
probabilistic approach for the evaluation of minimal residual disease
by multiparameter flow cytometry in leukemic B-cell chronic
lymphoproliferative disorders. Cytometry A. 2008 Dec;73A(12):1141-50. https://doi.org/10.1002/cyto.a.20638 PMid:18836994
- Van
Dongen JJ, Lhermitte L, Böttcher S, Almeida J, van der Velden VH,
Flores-Montero J, Rawstron A, Asnafi V, Lécrevisse Q, Lucio P,
Mejstrikova E, Szczepański T, Kalina T, de Tute R, Brüggemann M, Sedek
L, Cullen M, Langerak AW, Mendonça A, Macintyre E, Martin-Ayuso M,
Hrusak O, Vidriales MB, Orfao A; EuroFlow Consortium (EU-FP6,
LSHB-CT-2006-018708). EuroFlow antibody panels for standardized
n-dimensional flow cytometric immunophenotyping of normal, reactive and
malignant leukocytes. Leukemia. 2012 Sep;26(9):1908-75. https://doi.org/10.1038/leu.2012.120 PMid:22552007 PMCid:PMC3437410
- Orfao
A, Matarraz S, Pérez-Andrés M, Almeida J, Teodosio C, Berkowska MA, van
Dongen JJM; EuroFlow. Immunophenotypic dissection of normal
hematopoiesis. J Immunol Methods. 2019 Dec;475:112684. https://doi.org/10.1016/j.jim.2019.112684 PMid:31676343
- Campo
E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO
classification of lymphoid neoplasms and beyond: evolving concepts and
practical applications. Blood. 2011 May 12;117(19):5019-32. https://doi.org/10.1182/blood-2011-01-293050 PMid:21300984 PMCid:PMC3109529
- Swerdlow
SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R,
Ghielmini M, Salles GA, Zelenetz AD, Jaffe ES. The 2016 revision of the
World Health Organization classification of lymphoid neoplasms. Blood.
2016 May 19;127(20):2375-90. https://doi.org/10.1182/blood-2016-01-643569 PMid:26980727 PMCid:PMC4874220
- Grever
M, Andritsos L, Anghelina M, Arons E, Banerji V, Barrientos J, Bhat SA,
Blachly J, Broccoli A, Call T, Dearden C, Dietrich S, Else M, Epperla
N, Fagarasanu A, Falini B, Forconi F, Gozzetti A, Hampel P, Hermel DJ,
Iyengar S, Johnston JB, Juliusson G, Kreitman RJ, Lauria F, Lozanski G,
Oakes CC, Parikh SA, Park J, Quest G, Rai K, Ravandi F, Robak T, Rogers
KA, Saven A, Seymour JF, Tadmor T, Tallman MS, Tam CS, Tiacci E,
Troussard X, Wörmann B, Zent CS, Zenz T, Zinzani PL. Hairy cell
leukemia variant and WHO classification correspondence Re: 5th edition
WHO classification haematolymphoid tumors: lymphoid neoplasms.
Leukemia. 2024 Jul;38(7):1642-1644. doi: 10.1038/s41375-024-02280-0.
Epub 2024 Jun 8. https://doi.org/10.1038/s41375-024-02280-0 PMid:38851853 PMCid:PMC11216987
- Campo
E, Jaffe ES, Cook JR, Quintanilla-Martinez L, Swerdlow SH, Anderson KC,
Brousset P, Cerroni L, de Leval L, Dirnhofer S, Dogan A, Feldman AL,
Fend F, Friedberg JW, Gaulard P, Ghia P, Horwitz SM, King RL, Salles G,
San-Miguel J, Seymour JF, Treon SP, Vose JM, Zucca E, Advani R, Ansell
S, Au WY, Barrionuevo C, Bergsagel L, Chan WC, Cohen JI, d'Amore F,
Davies A, Falini B, Ghobrial IM, Goodlad JR, Gribben JG, Hsi ED, Kahl
BS, Kim WS, Kumar S, LaCasce AS, Laurent C, Lenz G, Leonard JP, Link
MP, Lopez-Guillermo A, Mateos MV, Macintyre E, Melnick AM, Morschhauser
F, Nakamura S, Narbaitz M, Pavlovsky A, Pileri SA, Piris M, Pro B,
Rajkumar V, Rosen ST, Sander B, Sehn L, Shipp MA, Smith SM, Staudt LM,
Thieblemont C, Tousseyn T, Wilson WH, Yoshino T, Zinzani PL, Dreyling
M, Scott DW, Winter JN, Zelenetz AD. The International Consensus
Classification of Mature Lymphoid Neoplasms: a report from the Clinical
Advisory Committee. Blood. 2022 Sep 15;140(11):1229-1253. doi:
10.1182/blood.2022015851. Erratum in: Blood. 2023 Jan 26;141(4):437. https://doi.org/10.1182/blood.2022015851 PMid:35653592 PMCid:PMC9479027
- Del
Giudice I, Pileri SA, Rossi M, Sabattini E, Campidelli C, Starza ID, De
Propris MS, Mancini F, Perrone MP, Gesuiti P, Armiento D, Quattrocchi
L, Tafuri A, Amendola A, Mauro FR, Guarini A, Foà R. Histopathological
and molecular features of persistent polyclonal B-cell lymphocytosis
(PPBL) with progressive splenomegaly. Br J Haematol. 2009
Mar;144(5):726-31. https://doi.org/10.1111/j.1365-2141.2008.07551.x PMid:19133977
- Sandes
AF, Chauffaille M de L, M.C. Oliveira CR, Maekawa Y. Tamashiro N, Takao
TT, Ritter EC, Rizzatti EG. CD200 Has an important role in the
differential diagnosis of mature b-cell Neoplasms by multiparameter
flow cytometry. Cytometry B Clin Cytom. 2014; 86B: 98-105. https://doi.org/10.1002/cyto.b.21128 PMid:24243815
- Palumbo
GA, Parrinello N, Fargione G, Cardillo K, Chiarenza A, Berretta S,
Conticello C, Villari L, Di Raimondo F. CD200 expression may help in
differential diagnosis between mantle cell lymphoma and B-cell chronic
lymphocytic leukemia. Leuk Res. 2009; 33(9):1212-6. Epub 2009 Feb 20. https://doi.org/10.1016/j.leukres.2009.01.017 PMid:19230971
- Alapat
D, Coviello-Malle J, Owens R, Qu P, Barlogie B, Shaughnessy JD,
Lorsbach RB. Diagnostic Usefulness and Prognostic Impact of CD200
Expression in Lymphoid Malignancies and Plasma Cell Myeloma. Am J Clin
Pathol. 2012; 137(1): 93-100. https://doi.org/10.1309/AJCP59UORCYZEVQO PMid:22180482 PMCid:PMC3632327
- El
Desoukey NA, Dalia Gamil Amin, Aleem Afify RA, Mohammed RA. CD200
expression in B-cell chronic lymphoproliferative disorders. J Investig
Med. 2012;60(1):56-61. https://doi.org/10.2310/JIM.0b013e31823908f9 PMid:22064604
- Pillai
V, Pozdnyakova O, Charest K, Li B, Shahsafaei A, Dorfman DM. CD200 flow
cytometric assessment and semiquantitative immunohistochemical staining
distinguishes hairy cell leukemia from hairy cell leukemia-variant and
other B-cell lymphoproliferative disorders. Am J Clin Pathol.
2013;140(4), 536-543. https://doi.org/10.1309/AJCPEBK31VQQNDDR PMid:24045551
- Salem
DA, Scott D, McCoy CS, Liewehr DJ, Venzon DJ, Arons E, Kreitman RJ,
Stetler-Stevenson M, Yuan CM. Differential expression of CD43, CD81,
and CD200 in classic versus variant hairy cell leukemia. Cytometry B
Clin Cytom. 2019; 96B: 275-282. Epub 2019 May 11. https://doi.org/10.1002/cyto.b.21785 PMid:31077558 PMCid:PMC8191384
- Uhrmacher
S, Schmidt C, Erdfelder F, Poll-Wolbeck SJ, Gehrke I, Hallek, M,
Kreuzer, K. A. Use of the receptor tyrosine kinase-like orphan receptor
1 (ROR1) as a diagnostic tool in chronic lymphocytic leukemia (CLL).
Leuk Res. 2011;35(10):1360-6. Epub 2011 Apr 30. https://doi.org/10.1016/j.leukres.2011.04.006 PMid:21531460
- De
Propris MS, Intoppa S, Milani ML, Mariglia P, Nardacci MG, Peragine N,
Foà R, Guarini A. ROR1 is an accurate and reliable marker of minimal
residual disease in chronic lymphocytic leukaemia. Br J Haematol.
2020;190(6):e346-e349. Epub 2020 Jun 24. https://doi.org/10.1111/bjh.16910 PMid:32579248
- Rawstron
AC, Villamor N, Ritgen M, Böttcher S, Ghia P, Zehnder JL, Lozanski G,
Colomer D, Moreno C, Geuna M, Evans PA, Natkunam Y, Coutre SE, Avery
ED, Rassenti LZ, Kipps TJ, Caligaris-Cappio F, Kneba M, Byrd JC, Hallek
MJ, Montserrat E, Hillmen P. International standardized approach for
flow cytometric residual disease monitoring in chronic lymphocytic
leukaemia. Leukemia. 2007 May;21(5):956-64. doi:
10.1038/sj.leu.2404584. Epub 2007 Mar 15. https://doi.org/10.1038/sj.leu.2404584 PMid:17361231
- Flores-Montero J, Grigore G, Fluxá R, Hernández J, Fernandez P, Almeida J, Muñoz N, Böttcher S, Sedek L, van der Velden V, Barrena S, Hernández A, Paiva B, Lecrevisse Q, Lima M, Santos AH, van Dongen JJM, Orfao A. EuroFlow Lymphoid Screening Tube (LST) data base for automated identification of blood lymphocyte subsets. J Immunol Methods. 2019 Dec;475:112662. https://doi.org/10.1016/j.jim.2019.112662 PMid:31454495
Supplementary Files
 |
|