Introduction
CDC is characterized by organ involvement, mainly in the liver and the spleen.[1] The diagnosis of CDC is very challenging. Diagnostic criteria for proven CDC require obtaining positive cultures for Candida spp. in blood or a sterile tissue specimen.[2] However, not only is the sensitivity of culture tests low, but obtaining tissue through invasive procedures in critically ill patients with suspected CDC is also not always feasible.[1,3-4] For such reasons, there has been a demand for non-invasive diagnostic tests for CDC. The serum β-d-glucan (BDG) assay is a non-invasive test for circulating cell wall components of fungus.[5] The diagnostic performance of BDG varies among different fungal infections and different patient populations.[6-9] Growing evidence supports the utility of BDG for the diagnosis of invasive candidiasis and PCP.[2,10] However, little is known about the diagnostic performance of BDG in patients with CDC. Furthermore, its kinetics during antifungal treatment is rarely investigated. Therefore, in this study, we aimed to investigate the diagnostic sensitivity and kinetics of BDG assay in patients with CDC.Materials and Methods
All eligible adult patients who were diagnosed as CDC in a tertiary hospital in Seoul, South Korea, from January 2017 to December 2019 and underwent BDG assay (Gold Mountain River Tech Development, Beijing, China) were retrospectively reviewed. CDC was defined according to the criteria established by the European Organization for Research and Treatment of Cancer and Mycoses Study Group (EORTC/MSG). Cases were considered to be “proven” if histopathologic, cytopathologic, or direct microscopic examination of a specimen obtained from liver tissue revealed yeast infection with pseudohyphae or true hyphae or if liver biopsy specimens gave positive culture results showing a clinical or radiological abnormality consistent with infection. Cases were classified as “possible” if the patients with relevant host factors such as hematologic malignancies yielded small, target-like abscesses in the liver or spleen (bull’s-eye lesions) or the brain at the time of neutrophil recovery after a prolonged phase of neutropenia. The values of BDG assay (Gold Mountain River Tech Development, Beijing, China) above 80 pg/mL were classified as positive. Serum galactomannan (GM) antigen (Platelia Aspergillus enzyme immunoassay; Bio-Rad; Redmond, WA, USA) was considered positive at a level ≥0.5. This study was approved by the institutional review board of Uijeongbu Eulji Medical Center (IRB No. 2022-10-004-001). Informed consent was waived by the ethics committee because no intervention was involved, and no patient-identifying information was included.Results
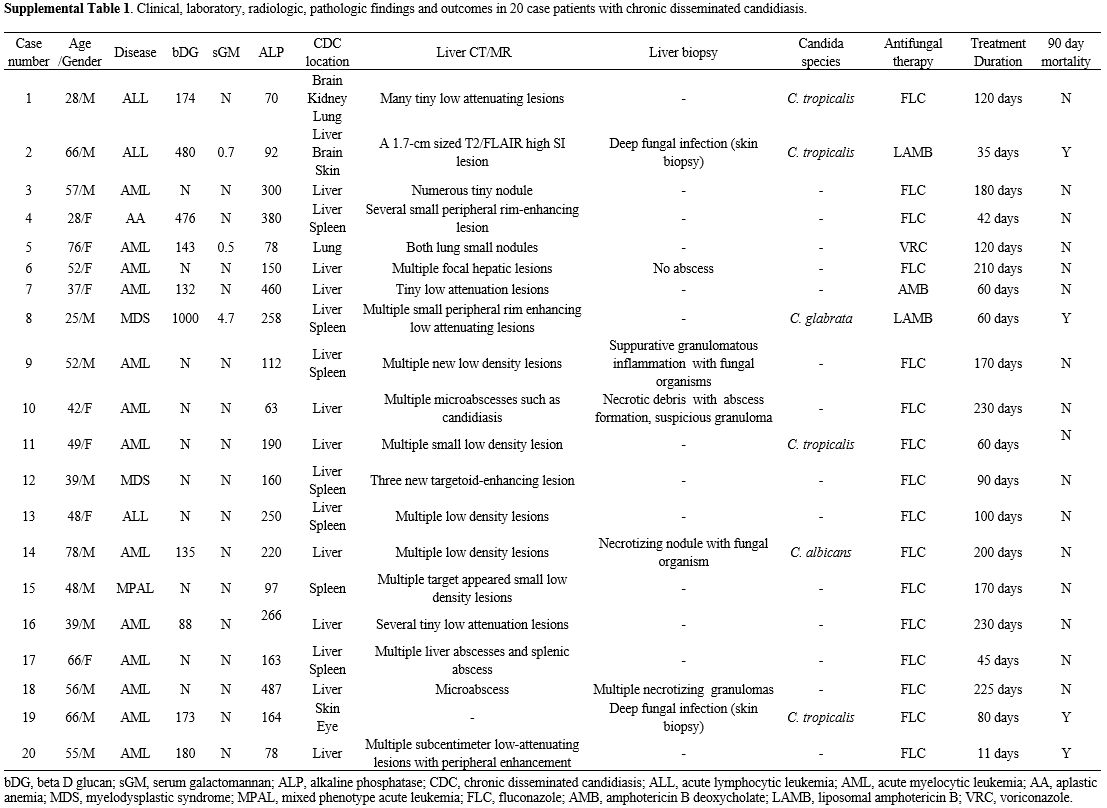
A total of 20 patients with CDC were enrolled. Table 1 shows a comparison of the baseline characteristics and treatment outcomes of BDG-positive and BDG-negative patients with CDC. The median age was 51 years (IQR 39 – 64). Of these, 13 patients had acute myeloid leukemia, three had acute lymphoblastic leukemia, two had myelodysplastic syndrome, and one had aplastic anemia. Two proven CDC cases were confirmed by liver biopsy. Of the six patients with the growth of Candida species in blood or biopsied tissue culture, C. tropicalis was isolated in four patients, and C. glabrata was isolated in one patient. A total of 10 (50%) revealed positive BDG results. The median BDG value was 174 pg/dL (IQR 137–402). Candida organ involvements were most observed in the liver (80%) and spleen (35%), followed by the brain, lung, and skin (10%). The clinical, laboratory, radiologic, and pathologic findings and outcomes of all 20 patients with chronic disseminated candidiasis are presented in Supplementary Table 1.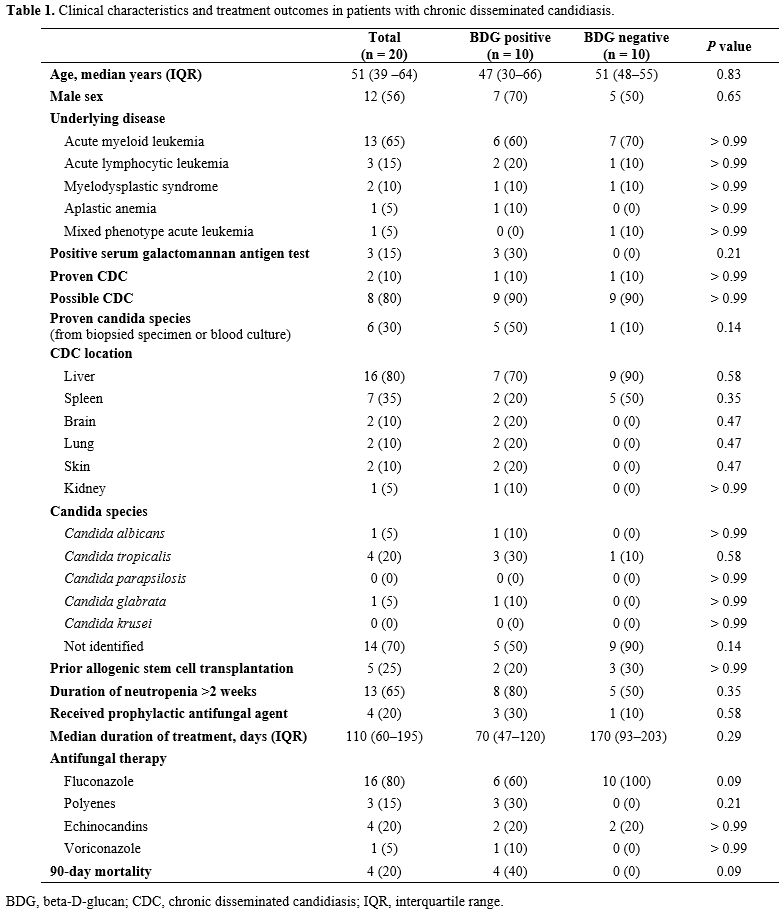 |
|
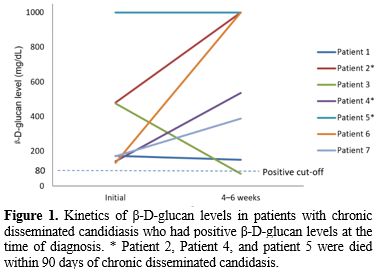
In the seven patients with BDG assay-positive CDC, for whom follow-up BDG results were available, the BDG remained high in six patients (86%) for more than 4 weeks after adequate antifungal therapy. All four patients who died within 90 days of CDC diagnosis had a positive BDG assay, and three of them showed an increasing trend of BDG values during treatment (Figure 1). In the eight patients with BDG assay-negative CDC, BDG levels of seven patients remained negative for 4–6 weeks (Figure 2).
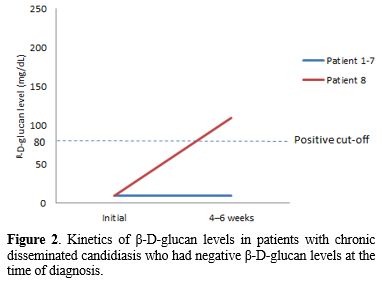 |
Figure 2. Kinetics
of β-D-glucan levels in patients with chronic disseminated candidiasis
who had negative β-D-glucan levels at the time of diagnosis. |
Discussion
CDC, which mainly occurs in patients with hematological malignancy, is difficult to diagnose.[4] Culture-based methods are insensitive, and invasive procedures are not always feasible.[1,3-4] For these reasons, there were expectations for the usefulness of the serum BDG assay, a non-invasive test, in the diagnosis of CDC, but little was known about it.[11-12] In our study, the sensitivity of BDG to CDC diagnosis was not as high as 50%. Although BDG has been reported to be useful for the assessment of deep-seated infections, it has limitations due to the inclusion of very small numbers of CDCs (less than 10 adult patients).[12-13] Based on our study, CDC cannot be ruled out even if BDG is negative if clinically suspected.In previous studies on invasive candidiasis, clinical outcomes were better if BDG decreased after treatment or if BDG was consistently negative from the time of diagnosis.[14] Also, one study reported that a slight decrease in BDG after candidemia was associated with deep-seated candidiasis.[13] For CDC, one of the invasive candidiasis, only one study reported the kinetics of BDG during treatment.[12] Nine CDCs with favorable outcomes showed a tendency for BDG to decrease, and two CDCs with fatal outcomes showed consistently high BDG values.[12] This study is similar to ours. All four patients who died in this study had positive BDG values at the time of diagnosis, and three of them showed a trend of increasing BDG levels until 4-6 weeks after diagnosis. Even in four patients who recovered properly, if BDG was positive at the time of diagnosis, it continued to be positive after 4-6 weeks. All patients with negative BDG levels at diagnosis survived after 90 days, and only one of them had positive BDG. Based on our study, BDG level tends to decrease slowly after CDC treatment, and if it is persistently negative, it may be associated with good clinical outcomes. The effectiveness of beta-D-glucan as a response marker may be affected by the choice of antifungal therapy. In our cohort, the use of fluconazole and amphotericin B, which can exhibit variable efficacy in CDC, may explain the inconsistent results observed. Future studies using more potent antifungals such as echinocandins are warranted.
Our study has several limitations. First, as a retrospective study, the BDG assay was performed according to the clinician's judgment, so there was no regular follow-up during the treatment process. Second, only 20 CDC patients were included. However, CDC is a rare disease, and it was a study with the largest number of patients dealing with the relationship between CDC and BDG assay. Although there are limitations, this study will be an invaluable reference for CDC practice in the clinical practice of CDC.
In conclusion, a negative BDG assay appears to be useless for ruling out CDC. The BDG assay decreased slowly during the CDC's adequate treatment.
References
- Rammaert B, Desjardins
A, Lortholary O. New insights into hepatosplenic candidosis, a
manifestation of chronic disseminated candidosis. Mycoses
2012;55:e74-84. https://doi.org/10.1111/j.1439-0507.2012.02182.x
PMid:22360318
- Donnelly
JP, Chen SC, Kauffman CA, Steinbach WJ, Baddley JW, Verweij PE, Clancy
CJ, Wingard JR, Lockhart SR, Groll AH, Sorrell TC, Bassetti M, Akan H,
Alexander BD, Andes D, Azoulay E, Bialek R, Bradsher RW, Bretagne S,
Calandra T, Caliendo AM, Castagnola E, Cruciani M,
Cuenca-Estrella M, Decker CF, Desai SR, Fisher B, Harrison T, Heussel
CP, Jensen HE, Kibbler CC, Kontoyiannis DP, Kullberg BJ, Lagrou K,
Lamoth F, Lehrnbecher T, Loeffler J, Lortholary O, Maertens J,
Marchetti O, Marr KA, Masur H, Meis JF, Morrisey CO, Nucci M,
Ostrosky-Zeichner L, Pagano L, Patterson TF, Perfect JR, Racil Z,
Roilides E, Ruhnke M, Prokop CS, Shoham S, Slavin MA, Stevens DA,
Thompson GR, Vazquez JA, Viscoli C, Walsh TJ, Warris A, Wheat LJ, White
PL, Zaoutis TE, Pappas PG. Revision and Update of the Consensus
Definitions of Invasive Fungal Disease From the European Organization
for Research and Treatment of Cancer and the Mycoses Study Group
Education and Research Consortium. Clin Infect Dis 2020;71:1367-76. https://doi.org/10.1093/cid/ciz1008
PMid:31802125 PMCid:PMC7486838
- Anttila
VJ, Ruutu P, Bondestam S, Jansson SE, Nordling S, Färkkilä M, Sivonen
A, Castren M, Ruutu T. Hepatosplenic yeast infection in patients with
acute leukemia: a diagnostic problem. Clin Infect Dis 1994;18:979-81. https://doi.org/10.1093/clinids/18.6.979
PMid:8086562
- Masood
A, Sallah S. Chronic disseminated candidiasis in patients with acute
leukemia: emphasis on diagnostic definition and treatment. Leuk Res
2005;29:493-501. https://doi.org/10.1016/j.leukres.2004.10.003
PMid:15755501
- Marty
FM, Koo S. Role of (1-->3)-beta-D-glucan in the diagnosis of
invasive aspergillosis. Med Mycol 2009;47 Suppl 1:S233-40. https://doi.org/10.1080/13693780802308454
PMid:18720216
- Karageorgopoulos
DE, Vouloumanou EK, Ntziora F, Michalopoulos A, Rafailidis PI, Falagas
ME. β-D-glucan assay for the diagnosis of invasive fungal infections: a
meta-analysis. Clin Infect Dis 2011;52:750-70. https://doi.org/10.1093/cid/ciq206
PMid:21367728
- Lamoth
F, Akan H, Andes D, Cruciani M, Marchetti O, Ostrosky-Zeichner L, Racil
Z, Clancy CJ. Assessment of the Role of 1,3-β-d-Glucan Testing for the
Diagnosis of Invasive Fungal Infections in Adults. Clin Infect Dis
2021;72:S102-s8. https://doi.org/10.1093/cid/ciaa1943
PMid:33709130
- Onishi
A, Sugiyama D, Kogata Y, Saegusa J, Sugimoto T, Kawano S, Morinobu A,
Nishimura K, Kumagai S. Diagnostic accuracy of serum 1,3-β-D-glucan for
pneumocystis jiroveci pneumonia, invasive candidiasis, and invasive
aspergillosis: systematic review and meta-analysis. J Clin Microbiol
2012;50:7-15. https://doi.org/10.1128/JCM.05267-11
PMid:22075593 PMCid:PMC3256688
- Son
HJ, Sung H, Park SY, Kim T, Lee HJ, Kim SM, Chong YP, Lee SO, Choi SH,
Kim YS, Woo JH, Kim SH. Diagnostic performance of the (1-3)-β-D-glucan
assay in patients with Pneumocystis jirovecii compared with those with
candidiasis, aspergillosis, mucormycosis, and tuberculosis, and healthy
volunteers. PLoS One 2017;12:e0188860. https://doi.org/10.1371/journal.pone.0188860
PMid:29190812 PMCid:PMC5708637
- Lagrou
K, Chen S, Masur H, Viscoli C, Decker CF, Pagano L, Groll AH.
Pneumocystis jirovecii Disease: Basis for the Revised EORTC/MSGERC
Invasive Fungal Disease Definitions in Individuals Without Human
Immunodeficiency Virus. Clin Infect Dis 2021;72:S114-s20. https://doi.org/10.1093/cid/ciaa1805
PMid:33709126 PMCid:PMC8243279
- Della
Pepa R, Cerchione C, Pugliese N, Colicchio R, Salvatore P, Sirignano C,
Soscia E, Pagano L, Sanguinetti M, Pane F, Picardi M. Diagnostic-driven
antifungal approach in neutropenic patients at high risk for chronic
disseminated candidiasis: preliminary observations on the role of
1,3-β-D-glucan antigenemia and multiphasic contrast-enhanced computed
tomography. Support Care Cancer 2018;26:1691-4. https://doi.org/10.1007/s00520-018-4138-9
PMid:29523967
- Guitard J, Isnard F, Tabone MD, Antignac M, Brissot E, Senghor Y, Petit A, Leverger G, Hennequin C. Usefulness of ß-D-glucan for diagnosis and follow-up of invasive candidiasis in onco-haematological patients. J Infect 2018;76:483-8. https://doi.org/10.1016/j.jinf.2018.01.011 PMid:29432826
- Angebault
C, Lanternier F, Dalle F, Schrimpf C, Roupie AL, Dupuis A, Agathine A,
Scemla A, Paubelle E, Caillot D, Neven B, Frange P, Suarez F, d'Enfert
C, Lortholary O, Bougnoux ME. Prospective Evaluation of Serum β-Glucan
Testing in Patients With Probable or Proven Fungal Diseases. Open Forum
Infect Dis 2016;3:ofw128. https://doi.org/10.1093/ofid/ofw128
PMid:27419189 PMCid:PMC4942764
- Agnelli C, Bouza E, Del Carmen Martínez-Jiménez M, Navarro R, Valerio M, Machado M, Guinea J, Sánchez-Carrillo C, Alonso R, Muñoz P. Clinical Relevance and Prognostic Value of Persistently Negative (1,3)-β-D-Glucan in Adults With Candidemia: A 5-year Experience in a Tertiary Hospital. Clin Infect Dis 2020;70:1925-32. https://doi.org/10.1093/cid/ciz555 PMid:31680136 ì
Supplementary Files
 |
|