Chronic inflammation and acute inflammatory events contribute significantly to various complications, such as vaso-occlusive crises and organ damage. Key triggers of the chronic inflammatory state include hemolysis, immune cell activation, and endothelial dysfunction. Research has demonstrated that SCD patients exhibit elevated levels of circulating proinflammatory cytokines, such as interleukin (IL)-1b, IL-6, IL-8, IL-10, and tumor necrosis factor α (TNF- α), during both acute episodes and steady states. These markers contribute to chronic endothelial activation, leukocyte aggregation, and red blood cell adhesion, leading to ischemia and tissue necrosis.[1-3] Pathare et al. identified that SCD patients in a steady state have significant elevations in IL-1β, IL-6, and IFN-gamma compared to normal subjects, noting an increase of type II (humoral immune response) proinflammatory cytokines in steady states and an additional rise of type I (cellular immune response) cytokines during crises.[4]
Blood transfusions, particularly Red Blood Cell Exchange (RBCX), are a vital therapeutic option for SCD, significantly reducing the percentage of circulating sickle red blood cells and enhancing vascular perfusion. This therapeutic and preventive strategy addresses both acute and chronic complications by removing sickle cells, thus diminishing their role in vaso-occlusive and hemolytic events, increasing oxygen transport capacity, and reducing blood viscosity.[5-6] The latest guidelines from the American Society of Hematology recommend automated red cell exchange over simple transfusion or manual red cell exchange for specific groups of SCD patients, namely for stroke prevention, severe recurrent acute chest syndrome, other serious complications such as chronic leg ulcers and priapism, improving quality of life in those with severe symptoms despite optimal medical therapy.[7]
Understanding the inflammatory processes in SCD is essential for developing new therapeutic strategies aimed at modulating inflammation in SCD.[8] Proposed mechanisms for suppressing inflammation include reducing the production of inflammatory cytokines and mitigating the harmful effects of reactive oxygen species.[9] These include TNF-α antagonists, such as Etanercept, which decrease endothelial activation, vaso-occlusion, and pulmonary hypertension in animal models. Ongoing studies are evaluating the effects of different treatments, such as hydroxyurea (HU) and RBCX, on inflammation and oxidative stress.[1,9,10] As inflammation plays a crucial role in SCD pathophysiology, future therapies may increasingly focus on anti-inflammatory approaches, potentially used alongside or as alternatives to HU, especially for patients who cannot undergo hematopoietic stem cell transplant or gene therapy.[8,11,12]
The primary aim of this study was to evaluate the impact of RBCX transfusion on inflammatory markers in pediatric patients with sickle cell anemia who were part of a chronic transfusion program.
Material and Methods
This prospective observational study was conducted on patients with SCD enrolled in a chronic RBCX program at the Pediatric Intensive and Special Care Unit of Hospital Professor Doutor Fernando Fonseca, a district hospital located in Portugal.Study Population. Eligible participants were pediatric patients with SCD who were electively admitted for RBCX at our institution between October 2022 and August 2024.
The inclusion criteria were: (1) a confirmed diagnosis of homozygous HbS SCD through electrophoresis; (2) an age range of two to 20 years; (3) indicating to initiate elective chronic exchange transfusion program as prevention in cerebrovascular disease (primary or secondary prevention), recurrent vaso-occlusive crises (VOCs) or recurrent acute chest syndrome, or stabilization prior to bone marrow transplantation; and (4) consent to participate in the study. Patients with RBCX performed for acute exacerbations were excluded.
Data. Clinical and laboratory data were retrieved from the patient's medical records. Blood samples were collected promptly at time points designated as standard according to established follow-up protocols. This procedure did not involve additional blood collections beyond those that would already be done during the patient's treatment program.
The study received approval from the institutional ethics committee. Prior to enrollment, written informed consent was obtained from all legal representatives and patients aged 15 years or older.
Red Cell Exchange Transfusion. All RBCX procedures were performed using the Spectra Optia® Apheresis System in the pediatric intensive care unit. A hemodilution-depletion protocol was selected for patients with a hematocrit above 24%. A standard depletion protocol is performed in all other situations. A final hematocrit of 28% or 5% above the usual hematocrit is programmed. The number of packed red blood cells is decided according to the target of Hb S at the end of each procedure. In patients with a recent ischemic stroke or the program due to primary prevention, a final HbS under 30% is desired. For patients with a stroke for more than two years, a target of 30-50% is acceptable.
Cytokine Measurements. Inflammatory markers, including C-reactive protein (CRP), procalcitonin (PCT), ferritin, IL-1, IL-6, IL-8, and TNF-α, were measured according to standard protocols. Blood samples were collected 12 hours before and after RBCX.
Reference values for laboratory markers were IL-1< 13.6 pg/mL; TNF-α 4.6-12.4pg/mL; IL-6< 7 pg/mL; IL-8< 132 pg/mL; Procalcitonin< 0,05 ng/mL; C-Reactive Protein< 0.50 mg/dL; and Ferritin 13-150 ng/mL.
Statistical Analysis. The statistical analysis was performed by using (SPSS 25, Chicago, IL). Parametric tests were used for data analysis. The Student’s t-test was employed to evaluate significant differences in laboratory values pre- and post-RBCX. The efficacy of RBCX was assessed using McNemar’s Test. A p-value of <0.05 was considered statistically significant.
Results
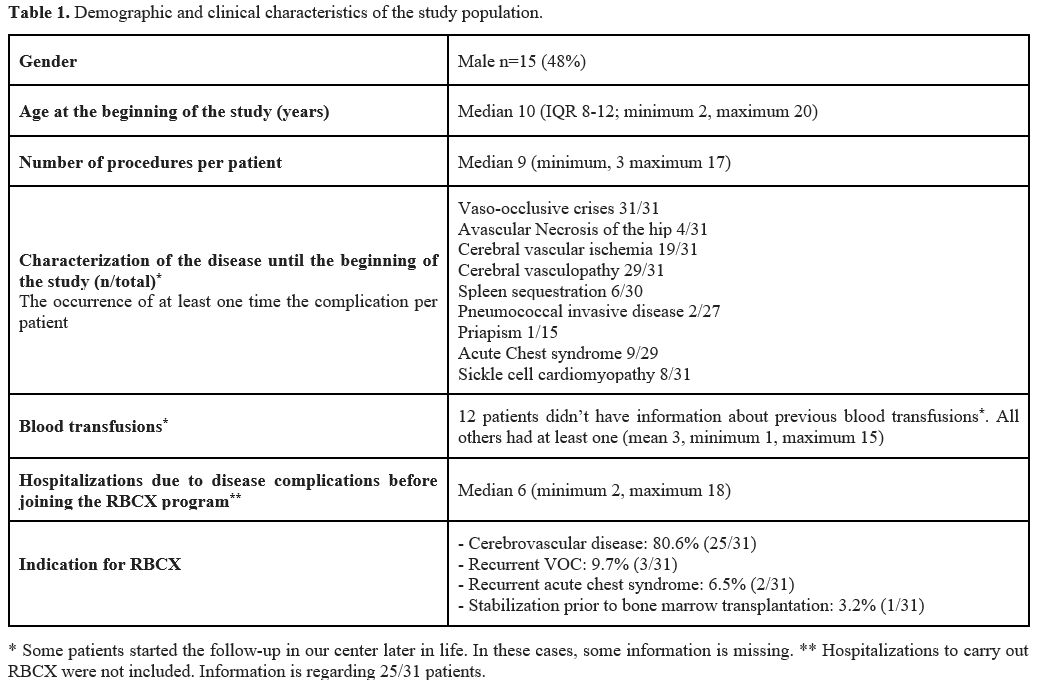
Patient Characteristics. A total of 31 children diagnosed with SCD and undergoing treatment with RBCXs were included in this study. The demographic and clinical characteristics of the study population are outlined in Table 1. Among these patients, 48% were male, with a median age of 10 years (minimum two years; maximum 20 years) at the beginning of the study. Prior to enrollment in the RBCX program, each patient had a median of six hospitalizations, ranging from a minimum of two to a maximum of 18. The primary indication for being under the RBCX program was cerebrovascular disease prevention (both primary and secondary), accounting for 81% of cases. Additionally, only two patients were not under HU treatment during the RBCX program - one patient discontinued HU due to the development of lower limb ulcers, a known complication of this drug; another patient discontinued it to undergo fertility preservation procedures prior to bone marrow transplantation.During the study, eight patients discontinued the RBCX program. Among these, two transitioned to follow-up care at a different hospital, while six successfully achieved their clinical objectives. Of these six, four patients attained transcranial Doppler normalization, thereby meeting the goals of the cerebrovascular disease prevention program and continued treatment solely with HU. One patient discontinued RBCX after two years of treatment without recurrence of acute chest syndrome, maintaining HU therapy. The final patient, enrolled in the program as a bridge to bone marrow transplantation, successfully underwent the transplantation procedure.
Periodic Red Blood Cell Exchange results. Among the 31 patients included in the study, 14 individuals (45%) were treatment-naïve, having not previously undergone any RBCX prior to study enrollment. In contrast, the remaining 17 patients had prior RBCX experience, with a median of 13 procedures per patient (minimum two; maximum 28 procedures).
A total of 286 RBCX procedures were analyzed. Each patient had a median of nine procedures (minimum three; maximum 16) conducted at intervals of approximately 38 days. On average, 5.6 ± 1 units of packed red blood cells (ranging from 3 to 7) were utilized per procedure for each patient. No major adverse events associated with the RBCX technique were reported, and none of the patients required hospitalization during the study period due to disease-related complications.
The hematological parameters measured before and after transfusion are summarized in Table 2. The mean Hb level increased by approximately 1.5 g/dL following RBCX. The reduction in HbS was significant, decreasing by 69% (from 37.6 ± 12.2% to 11.8 ± 7.4%; p < 0.001). Additionally, leukocyte and platelet counts were significantly reduced by 20% and 45%, respectively (p < 0.001), while hematocrit increased by 14.8% (from 25.4 ± 3.1% to 29.8 ± 1.8%, p < 0.001).
Considering all patients, no significant differences were observed in serum levels of IL-6, IL-8, PCT, ferritin, or TNF-α before and post-RBCX. However, serum IL-1 levels were significantly higher pre-RBCX, declining from 51.6 ± 80.5 pg/mL to 40.6 ± 70.3 pg/mL (p < 0.001). There was also a slight CRP reduction from 0.38 ± 0.68 mg/dL to 0.33 ± 0.64 mg/dL (p = 0.006). A possible correlation between the reduction in HbS and the decrease in IL-1 was evaluated, but no statistical significance was found.
Subgroup analysis of first-time RBCX patients (treatment-naïve) and non-naïve patients is summarized in Tables 3 and 4. Comparing both groups, changes in pre and post-RBCX values were similar between the two groups (naive vs. non-naïve), with the exception of pre-RBCX IL-1 values. The naïve patients showed higher pre-RBCX IL-1 levels (65.2 ± 87.4 pg/mL) compared to those with prior RBCX experience (41.0 ± 73.4 pg/mL; p = 0.03), yielding a difference of 22.6 pg/mL (95% CI [2.1 to 43.2]). After the procedure, the IL-1 difference between the two groups was not significant (49.6 ± 76.6 and 33.7 ± 64.4, respectively, p=0.1). Additionally, in the subgroup of children naïve of RBCX, IL-6 levels increased significantly by 17.3% from pre- to post-RBCX (p < 0.001).
 |
Table 3. Pre- and post-RBCX hematological parameters considering only the 14 patients who didn’t perform any RBCX before enrolling the study (treatment-naïve). |
 |
Table 4. Pre- and post-procedure values of the 17 patients who already performed an RBCX before enrolling in the study. |
When data were categorized into two groups based on laboratory reference values (high or normal), IL-1 emerged as a significant inflammatory marker (Table 5). Pre-RBCX, elevated levels of IL-1 were observed in 50.8% of patients, whereas post-RBCX decreased to 44.2% (p = 0.006). In contrast, IL-8, TNF-α, IL-6, and CRP were within normal ranges for the majority of patients prior to RBCX, with 98%, 87%, 85%, and 82%, respectively. Interestingly, IL-6 levels were significantly elevated in the post-procedure group (p < 0.001).
Discussion
The findings of this study provide valuable insights into the laboratory outcomes of children with SCD receiving RBCX therapy.The primary indication for long-term RBCX therapy was the prevention of cerebrovascular disease, reported in approximately 81% of the cohort. This approach proved effective, as no neurological events were observed after enrollment in the program. This observation is consistent with established clinical guidelines that recommend RBCX for high-risk individuals to reduce the likelihood of stroke. These results corroborate previous research that underscores the importance of RBCX in the context of stroke prevention in this population.[13] Additionally, the absence of major adverse events and the lack of hospitalizations due to disease complications during the study period further support the safety profile of the RBCX technique.
The analysis of the RBCX procedures evidenced a significant elevation in Hb levels, with an increase of 1.5 g/dL, alongside a reduction in HbS by 69%. These outcomes successfully achieved the target threshold of HbS <30% in most patients (some had a higher target value). Such findings are consistent with prior research that underscores the efficacy of RBCX in enhancing hematological parameters,[5-6] with its immediate benefits, essential for reducing sickling crises and related complications.
Additionally, reducing the white blood cell (WBC) count may offer therapeutic benefits, as WBCs - particularly neutrophils - are known to exacerbate VOCs through their role in vascular adhesion. The substantial decrease in WBC recorded in this study parallels findings from research involving HU, thereby reinforcing the hypothesis that RBCX may play a role in attenuating inflammatory precipitates associated with VOCs.[2]
Furthermore, a significant advantage of apheresis exchange transfusion is its iron neutrality, with the removed HbS containing an equal amount of iron as the administered HbA. Consistent with other studies, our data showed stable iron levels post-RBCX, in contrast to partial exchange programs that often result in iron overload.[14-16] Elevated pre-RBCX ferritin levels in this study likely reflect both chronic transfusion history and disease-related inflammation, complicating its use as a reliable inflammation biomarker in this population.
Previous studies have indicated that inflammation plays a critical role in SCD,[2,13,18] and modulating inflammatory markers through interventions such as RBCX may possess significant therapeutic potential.
In this study, the analysis of inflammatory markers yielded interesting results. While inflammatory markers such as IL-6, IL-8, ferritin, PCT, and TNF-α exhibited no significant alterations following RBCX in the whole population, the observed decrease in IL-1 levels was particularly striking. The significant reduction in IL-1 levels post-treatment (from 51.6 ± 80.5 pg/mL to 40.6 ± 70.3 pg/mL) was noteworthy. Given the established correlation between elevated IL-1 levels and SCD complications, this observed decline presents a possibly relevant finding. Elevated IL-1 levels contribute to the inflammatory environment in SCD by promoting the recruitment of WBC, activation of endothelial cells, and the upregulation of other inflammatory mediators, which further worsen vaso-occlusion and tissue damage.[12,18] Moreover, given that the primary indication for enrolling in our chronic transfusion program was the prevention of cerebrovascular disease, this reduction is especially significant since studies have shown that proinflammatory cytokines, such as IL-1, exacerbate stroke outcomes across all populations (not just in patients with SCD). In fact, IL-1 receptor antagonists are being studied as a potential therapeutic in stroke patients.[19] Therefore, a decline in IL-1 levels induced by RBCX could suggest an amelioration of the inflammatory state.
A slight decline in CRP levels was observed; however, this change should be interpreted with caution. The laboratory cutoff for clinical significance is set at 0.5 mg/mL, and the observed reduction falls well below this threshold. Given the minimal difference, it is unlikely to hold clinical relevance.
Naïve-patients for RBCX had higher pre-RBCX IL-1 levels compared to those with prior RBCX experience, indicating a potential link between prior exposure to RBCX and reduced inflammatory responses. This observation aligns with the concept that repeated RBCX may help in achieving a more stable inflammatory profile.
Contrary to other studies,[2-3] our results showed normal levels of CRP, TNF-α, and IL-8 in most patients with SCD, with no substantial changes post-RBCX. This fact is probably due to our study population being on a regular program of exchange transfusion to prevent disease complications without acute crises during the study.
It is important to consider that 29 of the 31 patients in this study had been on HU therapy for at least four months, which has been shown to reduce the expression of adhesion molecules on red blood cells, leukocytes, and endothelial cells. This therapy also decreases the levels of various inflammatory molecules, such as endothelin-1, TNF-α, IL-1β, and IL-17.[1-3,9,12] This pre-treatment with HU may explain the already lowered levels of inflammatory mediators in our cohort, decreasing the amplitude of changes in mediators reported.
Although IL-6 levels increased post-RBCX in transfusion-naïve patients and showed a higher percentage of elevated values in the normal/high subgroup, this may represent a physiological response to transfusion rather than a pathological inflammatory process. As other studies reported an elevation in IL-6 with a higher duration of VOC episodes, the results found here remain to be elucidated.[7]
It is known that although erythrocyte sickling in response to stressors constitutes the primary underlying defect of SCD, subsequent inflammatory responses to vascular occlusive events contribute to organ damage and further vascular dysfunction.[12] Therapies shown to be beneficial in SCD, such as HU and anti-selectin antibodies, may exert their beneficial effects, in part, via dampening of leukocyte-mediated inflammatory responses. A range of anti-inflammatory drugs, including IL-1 receptor antagonists, like anakinra, and anti-IL-1β, like canakinumab, are under investigation for their potential role in managing SCD.[11,20] Our finding of elevated IL-1 levels reinforces the relevance of these emerging therapies. Future research should aim to clarify the long-term effects of RBCX on inflammatory markers and explore the potential for combined anti-inflammatory therapies in SCD management.
Finally, we emphasize that our study had the limitation that it is a single-centered study of a small cohort of patients. A future study with a control group not included in a chronic transfusion program would be helpful to understand our results better.
Conclusions
In summary, this study highlights the efficacy and safety of RBCX in pediatric patients with SCD, particularly regarding hematological and clinical improvements and the potential for inflammatory modulation. RBCX safely reduces IL-1 levels, a finding that should be further explored in the future. The results of this study may contribute to the comprehension of cytokines in the pathology of SCD. However, the exact rule of these markers still needs to be clarified, with existing literature presenting conflicting findings. Future research should focus on elucidating the implications of these results for long-term patient outcomes and investigating the mechanisms behind the observed changes in inflammatory markers. An improved understanding of these dynamics could contribute to refining patient selection criteria for RBCX and optimizing treatment protocols, thereby enhancing overall patient care in the context of SCD.References
- Dembélé AK, Hermand P, Missud F, Lesprit E, Holvoet
L, Brousse V, Ithier G, Odievre MH, Benkerrou M, Le Van Kim C, Koehl B.
Persistence of chronic inflammation after regular blood transfusion
therapy in sickle cell anemia. Blood Adv. 2023 Feb 14;7(3):309-313. https://doi.org/10.1182/bloodadvances.2022007464
- Zahran
AM, Nafady A, Saad K, Hetta HF, Abdallah AM, Abdel-Aziz SM, Embaby MM,
Abo Elgheet AM, Darwish SF, Abo-Elela MGM, Elhoufey A, Elsayh KI.
Effect of Hydroxyurea Treatment on the Inflammatory Markers Among
Children With Sickle Cell Disease. Clin Appl Thromb Hemost. 2020
Jan-Dec;26:1076029619895111. https://doi.org/10.1177/1076029619895111
- Keikhaei
B, Mohseni AR, Norouzirad R, Alinejadi M, Ghanbari S, Shiravi F, Solgi
G. Altered levels of proinflammatory cytokines in sickle cell disease
patients during vaso-occlusive crises and the steady state condition.
Eur Cytokine Netw. 2013 Mar;24(1):45-52. https://doi.org/10.1684/ecn.2013.0328
- Pathare
A, Al Kindi S, Alnaqdy AA, Daar S, Knox-Macaulay H, Dennison D.
Cytokine profile of sickle cell disease in Oman. Am J Hematol. 2004
Dec;77(4):323-8. https://doi.org/10.1002/ajh.20196
- Swerdlow PS. Red cell exchange in sickle cell disease. Hematology Am Soc Hematol Educ Program. 2006:48-53. https://doi.org/10.1182/asheducation-2006.1.48
- Howard J. Sickle cell disease: when and how to transfuse. Hematology Am Soc Hematol Educ Program. 2016 Dec 2;2016(1):625-631. https://doi.org/10.1182/asheducation-2016.1.625
- Chou,
S. T., Alsawas, M., Fasano, R. M., Field, J. J., Hendrickson, J. E.,
Howard, J., Kameka, M., Kwiatkowski, J. L., Pirenne, F., Shi, P. A.,
Stowell, S. R., Thein, S. L., Westhoff, C. M., Wong, T. E., & Akl,
E. A. (2020). American Society of Hematology 2020 guidelines for sickle
cell disease: transfusion support. Blood Adv. 2020;4(2):327-355. https://doi.org/10.1182/bloodadvances.2019001143
- Lynch
K., Mega A., Daves M., Sadiq A., Fogarty H., Piccin A.Liver disease and
sickle cell disease: auto-immune hepatitis more than a coincidence; a
systematic literature review. Mediterr J Hematol Infect Dis 2023,
15(1): e2023060, https://doi.org/10.4084/MJHID.2023.060
- Sarray
S, Saleh LR, Lisa Saldanha F, Al-Habboubi HH, Mahdi N, Almawi WY. Serum
IL-6, IL-10, and TNFα levels in pediatric sickle cell disease patients
during vasoocclusive crisis and steady state condition. Cytokine. 2015
Mar;72(1):43-7. https://doi.org/10.1016/j.cyto.2014.11.030
- Pedrosa
AM, Leal LKAM, Lemes RPG. Effects of hydroxyurea on cytotoxicity,
inflammation and oxidative stress markers in neutrophils of patients
with sickle cell anemia: dose-effect relationship. Hematol Transfus
Cell Ther. 2021 Oct-Dec;43(4):468-475. https://doi.org/10.1016/j.htct.2020.07.011
- Liem
RI, O'Gorman MR, Brown DL. Effect of red cell exchange transfusion on
plasma levels of inflammatory mediators in sickle cell patients with
acute chest syndrome. Am J Hematol. 2004 May;76(1):19-25. https://doi.org/10.1002/ajh.20054
- Venugopal
J, Wang J, Mawri J, Guo C, Eitzman D. Interleukin-1 receptor inhibition
reduces stroke size in a murine model of sickle cell disease.
Haematologica. 2021 Sep 1;106(9):2469-2477. https://doi.org/10.3324/haematol.2020.252395
- Conran N, Belcher JD. Inflammation in sickle cell disease. Clin Hemorheol Microcirc. 2018;68(2-3):263-299. https://doi.org/10.3233/CH-189012
- Tanhehco YC, Shi PA, Schwartz J. Transfusion therapy in sickle cell disease. Ann Blood. 2022;7:9. https://doi.org/10.21037/aob-21-67
- Singer
ST, Quirolo K, Nishi K, Hackney-Stephens E, Evans C, Vichinsky EP.
Erythrocytapheresis for chronically transfused children with sickle
cell disease: an effective method for maintaining a low hemoglobin S
level and reducing iron overload. J Clin Apher. 1999;14(3):122-125. https://doi.org/10.1002/(SICI)1098-1101(1999)14:3<122::AID-JCA3>3.0.CO;2-A
- Hilliard
LM, Williams BF, Lounsbury AE, Howard TH. Erythrocytapheresis limits
iron accumulation in chronically transfused sickle cell patients. Am J
Hematol. 1998;59:28-35. https://doi.org/10.1002/(SICI)1096-8652(199809)59:1<28::AID-AJH6>3.0.CO;2-1
- Swerdlow PS. Red cell exchange in sickle cell disease. Hematology Am Soc Hematol Educ Program. 2006:48-53. https://doi.org/10.1182/asheducation-2006.1.48
- Nader
E, Romana M, Connes P. The Red Blood Cell-Inflammation Vicious Circle
in Sickle Cell Disease. Front Immunol. 2020 Mar 13;11:454. https://doi.org/10.3389/fimmu.2020.00454
- Siransy
LK, Dasse RS, Adou H, Kouacou P, Kouamenan S, Sekongo Y, Yeboah R,
Memel C, Assi-Sahoin A, Moussa SY, Oura D, Seri J. Are IL-1 family
cytokines important in management of sickle cell disease in Sub-Saharan
Africa patients? Front Immunol. 2023 Mar 9;14:954054. https://doi.org/10.3389/fimmu.2023.954054
- Kazmi
S, Salehi-Pourmehr H, Sadigh-Eteghad S, Farhoudi M. The efficacy and
safety of interleukin-1 receptor antagonist in stroke patients: A
systematic review. J Clin Neurosci. 2024 Feb;120:120-128. https://doi.org/10.1016/j.jocn.2024.01.009