Key clinical indicators for assessing ovarian reserve include sex hormones, antral follicle count, and anti-mullerian hormone (AMH). Sex hormone assessment is limited to the early follicular phase of the menstrual cycle, making it less convenient for patients, especially those with irregular cycles. Antral follicle count measurement requires ultrasound, which can be influenced by operator variability. AMH, produced by granulosa cells of antral and pre-antral follicles, reflects the ovarian reserve.[6] It is unaffected by the hypothalamic-pituitary-ovarian axis and shows minimal fluctuation throughout the menstrual cycle, allowing measurement at any time. This study aims to evaluate serum AMH levels and fertility in women with NTDT.
Materials and Methods
This study included 64 NTDT patients aged 16-40 years treated at the 923rd Hospital of the Joint Logistics Support Force between March 2021 and March 2024. Patients with severe medical conditions, malignant tumors, history of chemotherapy or radiotherapy, autoimmune diseases, pregnancy, or polycystic ovary syndrome were excluded. A control group of 69 healthy women aged 16-40 years was also analyzed. The participants were divided into age subgroups: 16-20, 21-25, 26-30, 31-35, and 36-40 years. Data collected included demographic and laboratory parameters such as age, menstrual history, hemoglobin, serum ferritin (SF), and AMH. This study was approved by the Ethics Committee of the 923rd Hospital.Statistical analysis. Data were analyzed using SPSS Statistics 26.0 (SPSS Inc., Chicago, IL, USA). Continuous variables were presented as mean ± standard deviation or median (range). Between-group differences were assessed using the Student t-test for parametric data and the Mann-Whitney test for non-parametric data. Correlations between AMH and other parameters were analyzed using Pearson's correlation or Spearman's rank test. P-values <0.05 were considered statistically significant.
Results
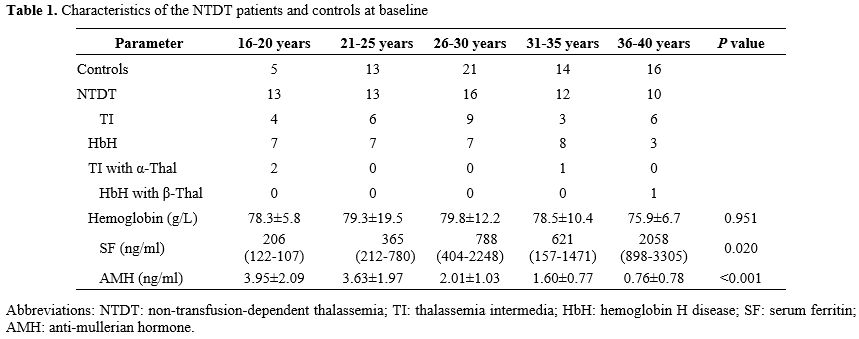
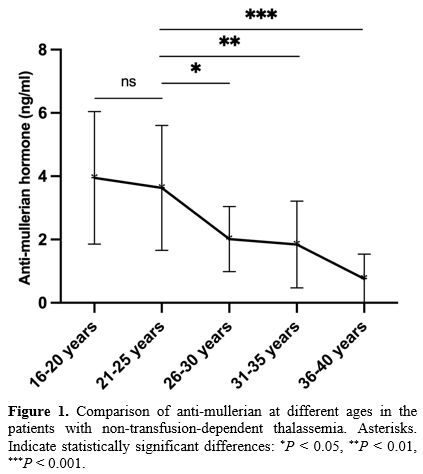
The median age at menarche in NTDT patients was 13 years (range: 13-15). Moreover, 54 had regular menstrual cycles, nine had irregular cycles, and one patient, aged 28, had not yet started menstruating. In total, 84.4% of the patients had regular menstruation, while 14.1% had irregular menstruation. The number of women in each age subgroup is shown in Table 1. The NTDT group comprised 32 HbH patients, 28 with TI, and four with α-thalassemia combined with β-thalassemia. Five patients were diagnosed with subclinical hypothyroidism, and one patient had diabetes mellitus. Hemoglobin levels showed no significant differences across age subgroups in NTDT patients (P > 0.05). Among the 64 patients, 15 had ferritin levels between 800 and 2500 ng/mL, and 11 had ferritin levels greater than 2500 ng/mL, with the majority of these patients being older than 26 years. Of these, four patients did not receive iron chelation therapy, five patients received regular treatment with deferoxamine, deferasirox, or deferiprone, and the remaining 17 received irregular chelation therapy. SF levels increased with age, with the 16-20 and 21-25 age groups showing significantly lower SF levels than the 26-30 and 36-40 age groups (P < 0.05 for all).The peak AMH levels in NTDT patients were seen in the 16-20 and 21-25 age groups, with a steep decline after the age of 26. AMH levels in the 16-20 and 21-25 age groups were significantly higher than those in the 26-30, 31-35, and 36-40 age groups (P < 0.05 for all) (Figure 1). No significant difference in AMH levels was observed between NTDT and control groups in the 16-20 and 21-25 age groups (P > 0.05 for all). However, in the 26-30, 31-35, and 36-40 age groups, AMH levels were significantly higher in the control group than in the NTDT group (P < 0.05 for all) (Figure 2).
No significant difference in AMH levels was found between HbH and TI patients (Figure 3). However, 3.1% (1/32) of HbH patients had abnormally low AMH, compared to 25% (7/28) of TI patients (P = 0.035), indicating that TI patients were more likely to have reduced AMH levels. No significant correlation was found between AMH levels and hemoglobin or SF levels in NTDT patients (P > 0.05).
 |
|
Discussion
This study utilized AMH levels, a simple and accessible clinical marker, to assess ovarian function in NTDT patients. The findings indicate that AMH levels, a marker of ovarian reserve, were significantly lower in NTDT patients over the age of 25 compared to healthy controls. Ovarian function in NTDT patients appeared to decline 5-10 years earlier than in the general population. Furthermore, patients with TI were at a higher risk of decreased ovarian function compared to those with HbH, suggesting that women with NTDT, especially those with TI, should plan for pregnancy before the age of 25.AMH is a more reliable indicator of ovarian reserve than follicle-stimulating hormone (FSH), as it starts to decline earlier and is more sensitive to changes in ovarian function.[7] AMH levels tend to rise from infancy, peak between ages 15.8 and 25, and begin to decline after 25.[8] In this study, NTDT patients had the highest AMH levels between 16 and 25 years, consistent with the literature. However, while healthy women experience a sharp decline in AMH between 30 and 35 years, NTDT patients showed a rapid decline between 26 and 30 years, about 5 to 10 years earlier than expected.
Hypogonadism is a common endocrine disorder in TDT patients, and AMH levels are inversely related to ferritin levels, with hypogonadism often resulting from iron deposition in hypothalamic-pituitary cells or gonads.[9,10] Although hypogonadism is less common in NTDT compared to TDT, its prevalence remains significant.[11,12] NTDT is characterized by ineffective erythropoiesis, leading to increased intestinal iron absorption and lower hepcidin levels, which results in iron overload and organ damage.[13,14] SF levels in NTDT patients are associated with the risk of hypogonadism.[15] Iron accumulation increases with age, even without transfusion therapy, and ferritin levels were significantly higher in older NTDT patients in this study.[16,17] In this study, SF levels in the 16-20 and 21-25 age groups were lower than that in the 26-30 and 35-40 age groups, which may explain the sharp decline in ovarian function after age 25, as SF gradually accumulates with age in NTDT patients.
The study also found that TI patients were more likely to have lower AMH levels than HbH patients, suggesting a greater risk of diminished ovarian function in TI patients. This may be due to the more severe anemia and iron overload seen in TI patients, who are more prone to iron overload than those with HbH, particularly the patients with deletion HbH.[18,19] Long-term anemia can lead to hypoxia and ischemia in organs and tissues, triggering compensatory hyperplasia. Improving basal hemoglobin levels can help address growth retardation.[1] Additionally, studies have confirmed that serum ferritin levels ≥800 ng/ml serve as a threshold for organ damage in these patients.[20] In our study, some patients had low baseline hemoglobin levels, and most did not receive regular iron removal therapy. We recommend that patients with low AMH levels undergo close monitoring of hemoglobin, ferritin, and sex hormone levels, along with liver and cardiac magnetic resonance imaging to assess iron deposition. Appropriate blood transfusions and iron removal therapy should be considered to prevent further ovarian function decline.
This study had several limitations, including the small sample size, particularly in the age-stratified analysis, which prevented direct comparisons of AMH levels between HbH and TI patients by age. Additionally, due to the limited number of cases, we could not assess whether co-inherited α-thalassemia combined with β-thalassemia affected AMH levels. Larger studies are needed to validate these findings.
Conclusions
Our findings suggest that AMH levels decline 5-10 years earlier in NTDT patients compared to healthy controls, with a particular risk of ovarian insufficiency in patients with TI. Low ovarian reserve could be a contributing factor to subfertility in many women with NTDT. These results require further confirmation in larger, multicenter studies.Acknowledgments
We want to thank all patients for their continuous support and participation in this study.Ethics statement
The study protocol was approved by the Medical Ethics Committee of the 923rd Hospital of the Joint Logistics Support Force of the Peoples Liberation Army.References
- Kattamis A, Kwiatkowski JL, Aydinok Y. Thalassaemia. Lancet. 2022;399:2310-24. https://doi.org/10.1016/S0140-6736(22)00536-0 PMid:35691301
- Viprakasit
V, Ekwattanakit S. Clinical Classification, Screening and Diagnosis for
Thalassemia. Hematol Oncol Clin North Am. 2018;32:193-211. https://doi.org/10.1016/j.hoc.2017.11.006 PMid:29458726
- Castaldi MA, Cobellis L. Thalassemia and infertility. Hum Fertil (Camb). 2016;19:90-6. https://doi.org/10.1080/14647273.2016.1190869 PMid:27335221
- Sinai
Talaulikar V, Chatterjee R, Bajoria R. Reversal of hypogonadotropic
hypogonadism with spontaneous pregnancy in beta-thalassaemia major with
transfusional haemosiderosis. Eur J Obstet Gynecol Reprod Biol.
2017;216:271-2. https://doi.org/10.1016/j.ejogrb.2017.08.012 PMid:28830631
- Chatterjee
R, Bajoria R. Critical appraisal of growth retardation and pubertal
disturbances in thalassemia. Ann N Y Acad Sci. 2010;1202:100-14. https://doi.org/10.1111/j.1749-6632.2010.05589.x PMid:20712780
- La
Marca A, Broekmans FJ, Volpe A, Fauser BC, Macklon NS, Table
ESIGfRE--AR. Anti-Mullerian hormone (AMH): what do we still need to
know? Hum Reprod. 2009;24:2264-75. https://doi.org/10.1093/humrep/dep210 PMid:19520713
- Toner
JP, Seifer DB. Why we may abandon basal follicle-stimulating hormone
testing: a sea change in determining ovarian reserve using
antimullerian hormone. Fertil Steril. 2013;99:1825-30. https://doi.org/10.1016/j.fertnstert.2013.03.001 PMid:23548941
- Lie
Fong S, Visser JA, Welt CK, de Rijke YB, Eijkemans MJ, Broekmans FJ,
Roes EM, Peters WH, Hokken-Koelega AC, Fauser BC, Themmen AP, de Jong
FH, Schipper I, Laven JS. Serum anti-mullerian hormone levels in
healthy females: a nomogram ranging from infancy to adulthood. J Clin
Endocrinol Metab. 2012;97:4650-5. https://doi.org/10.1210/jc.2012-1440 PMid:22993032 PMCid:PMC3683801
- Chang
HH, Chen MJ, Lu MY, Chern JP, Lu CY, Yang YL, Jou ST, Lin DT, Yang YS,
Lin KH. Iron overload is associated with low anti-mullerian hormone in
women with transfusion-dependent beta-thalassaemia. BJOG.
2011;118:825-31. https://doi.org/10.1111/j.1471-0528.2011.02927.x PMid:21401854
- Uysal
A, Alkan G, Kurtoglu A, Erol O, Kurtoglu E. Diminished ovarian reserve
in women with transfusion-dependent beta-thalassemia major: Is iron
gonadotoxic? Eur J Obstet Gynecol Reprod Biol. 2017;216:69-73. https://doi.org/10.1016/j.ejogrb.2017.06.038 PMid:28732253
- Musallam KM, Rivella S, Vichinsky E, Rachmilewitz EA. Non-transfusion-dependent thalassemias. Haematologica. 2013;98:833-44. https://doi.org/10.3324/haematol.2012.066845 PMid:23729725 PMCid:PMC3669437
- Taher
AT, Musallam KM, Karimi M, El-Beshlawy A, Belhoul K, Daar S, Saned MS,
El-Chafic AH, Fasulo MR, Cappellini MD. Overview on practices in
thalassemia intermedia management aiming for lowering complication
rates across a region of endemicity: the OPTIMAL CARE study. Blood.
2010;115:1886-92. https://doi.org/10.1182/blood-2009-09-243154 PMid:20032507
- Ginzburg
Y, Rivella S. beta-thalassemia: a model for elucidating the dynamic
regulation of ineffective erythropoiesis and iron metabolism. Blood.
2011;118:4321-30. https://doi.org/10.1182/blood-2011-03-283614 PMid:21768301 PMCid:PMC3204905
- Cazzola M. Ineffective erythropoiesis and its treatment. Blood. 2022;139:2460-70. https://doi.org/10.1182/blood.2021011045 PMid:34932791
- Chirico
V, Rigoli L, Lacquaniti A, Salpietro V, Piraino B, Amorini M, Salpietro
C, Arrigo T. Endocrinopathies, metabolic disorders, and iron overload
in major and intermedia thalassemia: serum ferritin as diagnostic and
predictive marker associated with liver and cardiac T2* MRI assessment.
Eur J Haematol. 2015;94:404-12. https://doi.org/10.1111/ejh.12444 PMid:25200112
- Taher
AT, Musallam KM, El-Beshlawy A, Karimi M, Daar S, Belhoul K, Saned MS,
Graziadei G, Cappellini MD. Age-related complications in
treatment-naive patients with thalassaemia intermedia. Br J Haematol.
2010;150:486-9. https://doi.org/10.1111/j.1365-2141.2010.08220.x PMid:20456362
- Taher
A, El Rassi F, Isma'eel H, Koussa S, Inati A, Cappellini MD.
Correlation of liver iron concentration determined by R2 magnetic
resonance imaging with serum ferritin in patients with thalassemia
intermedia. Haematologica. 2008;93:1584-6. https://doi.org/10.3324/haematol.13098 PMid:18728025
- Zeinali
S, Fallah MS, Bagherian H. Heterogeneity of hemoglobin h disease in
childhood. N Engl J Med. 2011;364:2070-1; author reply 1.
- Chui DH, Fucharoen S, Chan V. Hemoglobin H disease: not necessarily a benign disorder. Blood. 2003;101:791-800. https://doi.org/10.1182/blood-2002-07-1975 PMid:12393486
- Huang
Y, Yang G, Wang M, Wei X, Pan L, Liu J, Lei Y, Peng, Long L, Lai Y, Liu
R. Iron overload status in patients with non-transfusion-dependent
thalassemia in China. Ther Adv Hematol. 2022;13:20406207221084639. https://doi.org/10.1177/20406207221084639 PMid:35321211 PMCid:PMC8935562