Prediabetes is typically characterized by impaired fasting glucose (IFG) and/or impaired glucose tolerance (IGT).[4] Although diabetes mellitus (DM) in TDT patients has many similarities with Type 2 Diabetes Mellitus (T2DM) and a few with diabetes Type 1, the diabetes of thalassemia is distinct and is defined as thalassemia related diabetes mellitus (Th-RDM); it has a unique pathophysiology related to iron overload of pancreas, involving anomalies in insulin secretion and peripheral insulin resistance, necessitating a specialized management with various outcomes and monitoring.[5] Moreover, the risk for developing Th-RDM is also positively related to age, body mass index, genetic, environmental, and ethnic characteristics. These factors lead to great variability in following the measurement of progression from prediabetes to diabetes, depending on the criteria of the study population.[2,5]
Proactive surveillance is crucial, as the onset of glucose dysregulation (GD) is often insidious and preceded by a prolonged period of progressive decline of insulin secretion. Although determining the optimal method for early identification of TDT patients at risk for deteriorating glucose homeostasis remains challenging, current standards of care guidelines recommend annual 2-hour oral glucose tolerance test (OGTT) screening, starting at the age of 10 years.[6] However, the exact frequency of screening for diabetes in clinical practice is unknown because there are no robust real-world data to rely on for the determination of an optimal screening frequency.
The OGTT should be performed after a minimum 8-hour fasting period, with plasma glucose (PG) samples collected before and 2 hours after ingesting a standardized glucose load (1.75 g/kg, up to a maximum of 75 g) within a 5-minute interval.[6] Despite OGTT being generally considered the “gold standard” in the diagnosis of diabetes, it is associated with several drawbacks (high burden for patients and healthcare teams, reliance on patients fasting for the correct time), and it is influenced by several pre-analytical and analytical factors (duration of fasting prior to OGTT, glucose load, blood specimen used for diagnosis, blood tubes collection and analytical method).[7]
There is ongoing research to improve the consistency and quality of information derived from OGTT. It analyzes whether a 1-h PG value equal to or exceeding 155 mg/dL could be an adjunctive or alternative diagnostic marker for identifying early glucose dysregulation associated with lower insulin sensitivity and impaired β-cell function, even in those with normal glucose tolerance (NGT).[8,9] It is yet unknown, and needs to be prospectively evaluated, whether impairments in β-cell function determine the rate of progression to overt diabetes. Additionally, multiple timepoint OGTT sampling, assessing PG and insulin secretion every 30 minutes over the course of OGTT, may estimate β-cell secretion and insulin sensitivity in the early natural history of GD.[8,10]
In summary, early diagnosis of GD is essential for the timely identification of high-risk TDT patients who may benefit from intensive iron chelation therapy, lifestyle modification, and, in selected cases, pharmacotherapy. It is crucial in view of the difficulty in reversing pancreatic iron load and GD.[5,9]
Studies on adherence rates to recommended OGTTs are rare and limited to cystic fibrosis, overweight/obese patients, and pregnant women.[11-13] Studies specifically focused on adherence rates to OGTTs in individuals with thalassemias are indeed rare. The existing literature primarily addresses the prevalence of abnormal glucose tolerance in transfusion-dependent β-thalassemic patients and factors that may contribute to diabetes in these patients, such as age, effective transfusion regimen, splenectomy, serum ferritin levels, chronic liver disease, associated endocrine complications, overweight/obesity and compliance with iron-chelation therapy. While guidelines for the management of transfusion-dependent thalassemia mention non-adherence to chelation therapy, they do not specifically address OGTT adherence.[14]
The objective of this multicenter ICET-A network study survey was to collect information from expert clinicians caring for patients with TDT on factors influencing the adherence to OGTT screening in subjects followed in specialized, multidisciplinary, pediatric, and adult Thalassemia Units in order to identify factors improving adherence and implications of OGTT for clinical practice.
Material and Methods
Questionnaire survey design. In June 2024, the Coordinator (VDS) of the International Network of Clinicians for Endocrinopathies in Thalassemia and Adolescent Medicine (ICET-A) formulated a questionnaire survey on adherence to OGTT screening based on international recommendations in specialized multidisciplinary pediatric and adult TDT units.[6] The inclusion criteria for the study were patients with TDT above the age of 10 years, regardless of the degree of iron overload and/or associated endocrine complications. The exclusion criteria included (a) the presence of patients with hemoglobinopathies other than TDT, (b) patients who had undergone bone marrow transplantation, and (c) TDT patients with chronic illnesses like chronic renal failure, active HCV, and HIV infection. Physicians were asked to provide contact information and data about their participating centers. Non-adherence to annual OGTT was defined as failure to undergo diagnostic OGTT screening as recommended by current guidelines and/or based on the clinician’s judgment (first step).The questionnaire, revised and approved by the ICET-A Steering Committee (including ATS and PT), consisted of five sections: (a) study population, OGTT screening, and criteria for defining GD and DM, number of patients with GD [isolated- IFG, isolated- IGT, or both, (optional)] and Th-RDM (mandatory); (b) patient barriers to OGTT screening; (c) major difficulties reported by patients during OGTT; (d) suggested actions for improving adherence to OGTT; and (e) alternative tests to OGTT suggested by clinicians for supplementing the screening of GD.
The questionnaire survey comprised 25 questions, including single-choice, multiple-choice, and descriptive answers, organized according to the five sections covering various aspects of glucose homeostasis and OGTT screening in TDT patients. Forty-two items were assessed using simple numeric rating scores, like a 5-point Likert scale. The rating of the assessments was based on the clinical judgment of expert clinicians from 0 to 10: very light interest = 1-2; light interest = 3-4; medium interest = 5-6; relevant interest = 7, and very relevant interest = 8-10. This straightforward approach was selected because it was considered quick, inexpensive, and convenient for capturing clinicians' expertise and perceptions in different fields.[15,16] The questionnaire survey is available from the corresponding author upon reasonable request.
Thirty clinicians working in the specialized multidisciplinary care units for thalassemias were invited to participate via email, regardless of their affiliation with the ICET-A Network. The questionnaire was sent online, followed by two reminders for the centers that had yet to respond to the ICET-A invitation. The deadline for submitting the completed questionnaires was set for the end of August 2024 (second step).
During the survey, the participating centers were asked to verify the accuracy of the information included in the preliminary manuscript draft, which was regularly updated during the initial data analysis (third step). Subsequently, the participating centers were formally requested to review the first version of the manuscript and contribute to the preparation of its final version (last step).
Data presentation. The data are mainly presented as numerical values, percentages, means, medians, and ranges. For the preparation of tables, we arbitrarily used the highest experts' reported scores from 8 to 10. The chi-square (χ2) test was applied to compare the frequencies of qualitative variables across different groups.
Ethics. All procedures were in accordance with the 1964 Helsinki Declaration and its later amendments, October 2013 (www.wma.net). The local Ethics Committee approvals were waived for this study, as no identifiable private information was collected, and an anonymized dataset was analyzed.
Results
a. Survey response rate. The survey received responses from a total of 18 centers from 10 different countries. The response rate of ICET-A Centers was higher (8/10, 80%) compared to invited Centers who were not part of the ICET-A Network (8/20, 40%; χ2 two-tailed: P: 0.038)Details on the distribution of responses in different countries and number of Centers participating in the survey are given in Table 1.
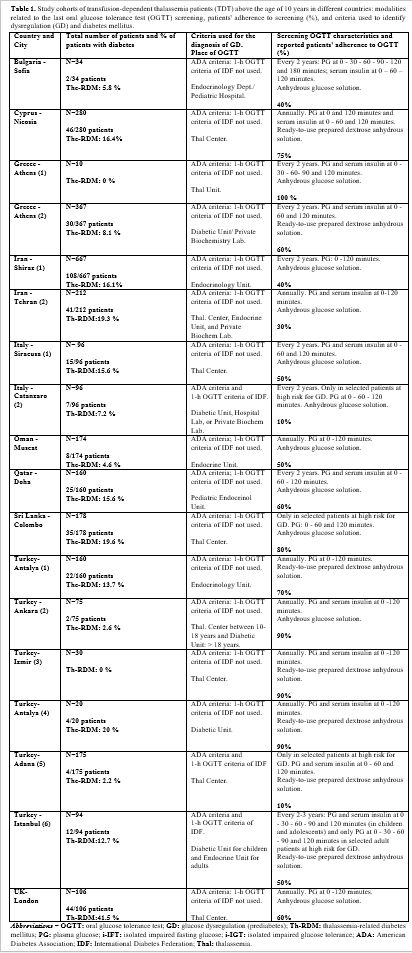
b. Centers’ characteristics. 13 out of 18 centers (72,2%) are affiliated with an academic institution. All centers provide care for children (13%, below 10 years), as well as for adolescents and adults (87%). The total number of TDT patients followed in the participating centers was 3,382. Five out of 18 centers (27.7%) followed less than 100 TDT patients. In 12 out of 18 centers (66.6%), TDT patients were referred to their Endocrine and/or Diabetes clinic or to private biochemistry laboratories for OGTT (Table 1 and Figure 1).
c. Screening frequency and criteria used for GD. Eight out of 18 centers (44.4%) offer annual OGTT screening to patients, while 8 others (44.4%) provide it every 2–3 years. In 2 centers (11.1%), OGTT screening is provided only to selected patients at high risk for GD, namely those with severe iron overload, obesity, family history of diabetes, chronic liver disease, or a previous history of impaired fasting plasma glucose and/or impaired glucose tolerance). In 50% of TDT centers, PG levels were collected only at 0 and 120 minutes, while in the remaining centers, samples were collected at additional intermediate time points (Table 1). For performing OGTT, a ready-to-use prepared dextrose anhydrous solution was used in 6 centers, and in the remaining centers, the solution was prepared with anhydrous glucose in 250–300 ml of cold water.
d. Criteria used for the diagnosis of GD and clinician reported-rates of patients' adhesion to OGTT. Glycemic status after a glucose load was classified by all participating centers according to the contemporary criteria of the American Diabetes Association (ADA).[4] The criteria of the International Diabetes Federation Position Statement for diagnosing hyperglycemia 1-hour post-load were used by 3 out of 18 centers (16.6%).[5]
The mean total percentage of GD reported by 16 out of 18 centers was 32.0% (range:3.3% - 93%; median: 23%), while the mean total percentage of reported Th-RDM was 12.2 ± 9.7 % (range: 0%-41%; median: 13.2 %) (Table1).
Notably, all centers reported a high percentage of poor adherence to OGTT screening (mean 41.3%; range 10-90%) except one (100%), which followed 25 TDT patients (10 of whom were above the age of 10 years; Table 1).
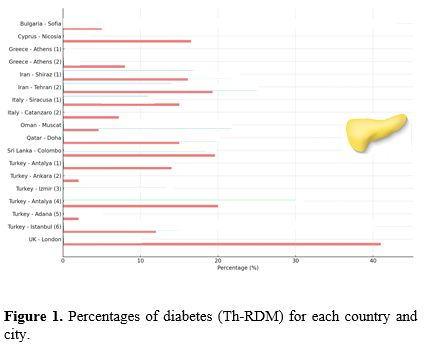
e. Reported barriers to OGTT screening. Figures 2 and 3 summarize the main barriers to OGTT screening and the signs and symptoms reported by the patients or clinicians during OGTT screening. In 3 out of 6 centers using ready-to-use prepared dextrose anhydrous solution, the patients expressed a preference for an alternative diagnostic method to OGTT.
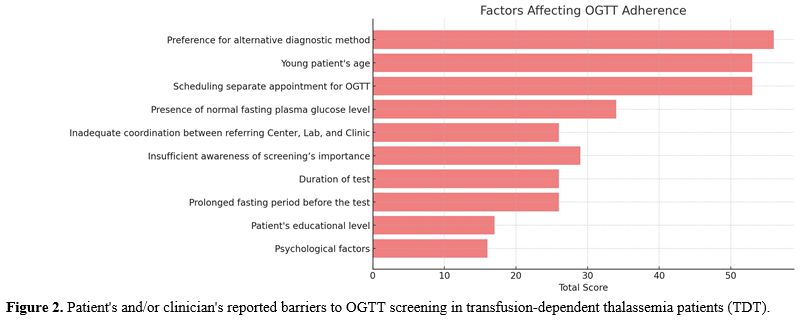 |
Figure 2. Patient's and/or clinician's reported barriers to OGTT screening in transfusion-dependent thalassemia patients (TDT). |
 |
Figure 3. Signs and symptoms observed or reported during OGTT in transfusion-dependent thalassemia patients (TDT). |
f. Actions suggested by clinicians for improving the adherence to OGTT. Hematologists and endocrinologists' most common suggestions for improving adherence to OGTT were flexibility in timing and choice of location, improved collaboration among team members, and the utilization of multiple reminder methods (Figure 4).
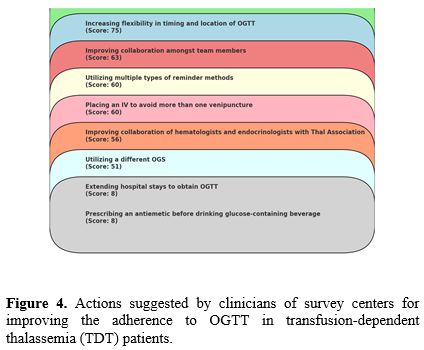 |
|
g. Alternative strategies to OGTT screening suggested by clinicians. Although current guidelines do not recommend alternatives to OGTT testing, numerous approaches to finding a more tolerable option for TDT patients with poor OGTT uptake were reported by hematologists and endocrinologists. The commonest alternatives were: fasting fingerstick glucose self-monitoring, non-fasting (random) fingerstick glucose self-monitoring and continuous glucose monitoring (CGM), although current guidelines do not state specific cut-off values for CGM in patients with TDT (Figure 5).
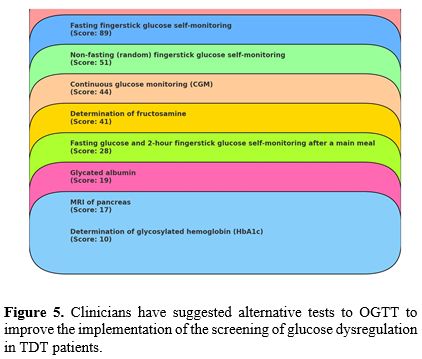 |
|
Discussion
The current gold standard for GD screening is the 2- h OGTT (1.75 g glucose/kg body weight, maximum 75 g) that is recommended annually in all subjects with TDT starting at the age of 10 years. Despite its effectiveness, OGTT has limitations; it is time-consuming, laboratory-dependent, laborious, and poorly tolerated by some patients.[2,9] These and other factors contribute to poor adherence to the annual OGTT among eligible patients, including patient-related barriers and healthcare system issues. Glycated hemoglobin (HbA1c) is commonly used as an alternative test to the fasting plasma glucose and OGTT for the identification of Type 2 Diabetes Mellitus (T2DM) because it is easy to obtain and represents long-term blood sugar levels. The current interpretation of HbA1c values, which corresponds to the calculated estimated glucose level, assumes that the red blood cell (RBC) lifespan is the same for all patients. However, even modest variation in red cell survival could have a significant impact on the HbA1c level. In general, a shorter RBC life span would yield lower levels of HbA1c at a given average whole blood glucose concentration than that of a normal patient.[6]To the best of our knowledge, our survey is the first not only to assess the adherence of individuals with TDT to OGTT screening in specialized multidisciplinary pediatric and adult thalassemia units but also to explore the contributing factors. So far, and despite extensive efforts to communicate the value of regular screening for GD to patients and the parents of children with TDT, adherence to OGTT needs to improve even in developed countries.[17,18]
There are many reasons for poor adherence to annual OGTT among eligible patients, including patient-related barriers, such as: (a) age, (b) social status, (c) insufficient awareness about the importance of screening, (d) prolonged fasting period before OGTT; (e) separate appointment times; (f) poor palatability and tolerance of oral glucose solution (such as intense and unpleasant taste in drinking OGS);(g) difficulties in drinking the oral glucose solution within 5-min; (h) nausea and gastric discomfort during the OGTT test, and (i) additional costs for traveling and testing (in some centers). Moreover, there are also systemic barriers to the provision of optimal healthcare, including (a) separate and long appointment times, (b) inadequate coordination between the referring Centre and laboratory, and (c) limited flexibility in the timing and location of OGTT screening.
To overcome the barriers to testing, especially for patients who are resistant to testing, clinicians from different centers have proposed several interventions. These include: (a) the regular use of reminder letters or phone calls, with special emphasis on high-risk patients; (b) providing more detailed explanations about the purpose of OGTT screening; and (c) improving collaboration among team members (hematologists, endocrinologists, diabetologists) and Thalassemia Associations.
It is hoped that such collaboration and peer communication could improve adherence rates and help patients and their families stay informed about the latest advances and knowledge in the field of GD. However, these strategies still need to be validated, and further studies are needed to evaluate the efficacy of these methods for improving patient compliance and to determine whether they could be generalized to other centers.
Our survey has several limitations that should be considered in the design of future studies. First, we developed a non-tested questionnaire survey and adapted some existing items specifically for TDT patients. Second, some of the questions in the survey were guided questions with forced-choice answers. However, free-text comments were collected to help mitigate this bias. Third, our survey presents only a general view of GD in TDT patients, and we did not determine the percentage of adherence to OGTT among specific patients at high risk for GD. Finally, we did not interview patients or parents of adolescents about their OGTT experience, which could have provided additional insights for improving adherence and enhancing patient comfort and overall satisfaction.
The survey has some strong points, including being the first report focused on OGTT adherence in children, adolescents, and adults with transfusion-dependent thalassemia (TDT). It features a large patient sample size and collects data from centers across multiple countries, offering a diverse and comprehensive perspective through examining extremely heterogeneous cohorts, especially in regard to age, severity of phenotypes, and also of treatment with transfusion and chelation. The questionnaire was specifically tailored to TDT patients, ensuring the relevance and specificity of the data collected. Additionally, the survey gathered insights from healthcare professionals, adding expert perspectives on OGTT adherence and barriers. To further enrich the data, the survey included opportunities for free-text comments, which helped mitigate the bias of forced-choice answers and provided valuable qualitative insights.
Future directions for clinical practice should focus on determining the optimal OGTT screening intervals, including whether 2- to 3-year intervals might be beneficial for patients with good adherence to iron chelation therapy and normal OGTT values (PG at 0, 1, and 2 hours).[18] Conversely, shorter screening intervals might be advantageous for patients with test values closer to the ADA cut-off levels. It is also desirable to develop alternative, highly sensitive screening methods that are less cumbersome than the OGTT.
Continuous glucose monitoring (CGM) or postprandial monitoring through finger sticks may offer more acceptable and effective methods for detecting glucose variations and intolerance but could be even more cumbersome than OGTT, and further studies are needed in this area to evaluate if abnormalities detected by CGM and missed by OGTT have prognostic value for clinical outcomes, and if patients benefit from treatment initiated based on CGM results.
Conflicting data have been reported about the diagnostic role of HbA1c and fructosamine in evaluating GD in β-TDT patients. Although the use of HbA1c as a screening test for abnormalities of glucose homeostasis in thalassemia appears to be less accurate,[6,19] a recent study analyzed the role of HbA1c and fructosamine in children with β-TDT and GD. Fructosamine was more specific compared to HbA1c in detecting blood glucose intolerance and more sensitive for diagnosing DM.[20] However, considering the limited evidence on the diagnostic performance of these markers, further research is necessary to define their cut-off values and exact role in the diagnosis and management of TDT patients. Alternative methods of glycaemic control should take into consideration if there is a discrepancy between blood glucose and HbA1c.
In summary, although identifying the optimal method for detecting patients at risk for deterioration of glucose homeostasis remains challenging, the OGTT currently remains the best and simplest method to assess glucose homeostasis, compared to other methods, in patients with TDT.[5]
Conclusions
This survey provides valuable insights into the current practices and challenges associated with OGTT adherence among transfusion-dependent thalassemia (TDT) patients across 18 international TDT centers. Despite the recognized importance of OGTT in screening for glucose dysregulation, adherence remains suboptimal due to a variety of patient-related and systemic barriers. Future research should focus on optimizing screening intervals and exploring novel technologies to ensure early detection and intervention for glucose dysregulation in this vulnerable population.Authors' contributions
VDS conceived and designed the study, distributed the survey to the ICET-A Network and non-ICET-A members, analyzed the data, and drafted the first version of the manuscript. IE supervised the study design, and ATS prepared the original figures. DC, SD, CK, ATS, and PT critically reviewed and edited the manuscript for relevant intellectual content. All participating authors working in Thalassemia centers are the guarantors of the data included in the manuscript and take full responsibility for its content. All authors read and approved the final version of the manuscript.References
- Kattamis A, Kwiatkowski JL, Aydinok Y.
Thalassaemia. Lancet. 2022;399(10343):2310-24. https://doi.
org/10.1016/ S0140-6736(22)00536-0. https://doi.org/10.1016/S0140-6736(22)00536-0 PMid:35691301
- De
Sanctis V, Soliman AT, Daar S, Tzoulis P, Di Maio S, Kattamis C.
Long-term follow-up of β- transfusion-dependent thalassemia (TDT)
normoglycemic patients with reduced insulin secretion to oral glucose
tolerance test (OGTT): A pilot study. Mediterr J Hematol Infect Dis.
2021, 13(1): e2021021. https://doi.org/10.4084/mjhid.2021.021 PMid:33747402 PMCid:PMC7938924
- He
LN, Chen W, Yang Y, Xie YJ, Xiong ZY, Chen DY, Liu NQ, Yang YH, Sun XF.
Elevated Prevalence of Abnormal Glucose Metabolism and Other Endocrine
Disorders in Patients with β-Thalassemia Major: A Meta-Analysis. Biomed
Res Int.2019. 2019:6573497. https://doi.org/10.1155/2019/6573497 PMid:31119181 PMCid:PMC6500678
- El
Sayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins
BS, Gaglia JL, Hilliard ME, Isaacs D, Johnson EL, Kahan S, Khunti K,
Leon J, Lyons SK, Perry ML, Prahalad P, Pratley RE, Seley JJ, Stanton
RC, Gabbay RA, on behalf of the American Diabetes Association . 2.
Classification and diagnosis of diabetes: Standards of Care in
Diabetes-2023. Diabetes Care. 2023; 46 (Suppl.1):S19-S40.
https://doi.org/10.2337/dc23-S002 PMid:36507649 PMCid:PMC9810477
- De
Sanctis V, Soliman A, Tzoulis P, Daar D, Karimi M, Yassin MA, Pozzobon
G, Kattamis C. The clinical characteristics, biochemical parameters and
insulin response to oral glucose tolerance test (OGTT) in 25
transfusion dependent β-thalassemia (TDT) patients recently diagnosed
with diabetes mellitus (DM). Acta Biomed. 2022;92(6):e2021488.
https://doi.org/10.23750/abm.v92i6.12366
- De Sanctis V, Soliman
AT, Elsedfy H, AL Yaarubi S, Skordis N, Khater D, El Kholy M, Stoeva I,
Fiscina B, Angastiniotis M, Daar S, Kattamis C. The ICET-A
recommendations for the diagnosis and management of disturbances of
glucose homeostasis in thalassemia major patients. Mediterr J Hematol
Infect Dis. 2016, 8(1): e2016058.
https://doi.org/10.4084/mjhid.2016.058 PMid:27872738 PMCid:PMC5111521
- De
Sanctis V, Soliman AT, Daar S, Tzoulis P, Di Maio S, Kattamis C. Oral
glucose tolerance test: how to maximize its diagnostic value in
children and adolescents. Acta Biomed. 2022; 93(5): e2022318.
https://doi.org/10.23750/abm.v93i5.13615.
- De Sanctis V, Soliman
A, Tzoulis P, Daar S, Pozzobon G, Kattamis C. A study of isolated
hyperglycemia (blood glucose ≥155 mg/dL) at 1-hour of oral glucose
tolerance test (OGTT) in patients with β-transfusion dependent
thalassemia (β-TDT) followed for 12 years. Acta Biomed. 2021;92(4):
e2021322. https://doi.org/10.23750/abm.v92i4.11105.
- De Sanctis
V, Soliman AT, Daar S, Tzoulis P, Di Maio S, Kattamis C. Glucose
homeostasis and αssessment of β-cell function by 3-hour oral glucose
tolerance (OGTT) in patients with β-thalassemia major with serum
ferritin below 1,000 ng/dl: results from a single ICET-A centre.
Mediterr J Hematol Infect Dis 2023;15(1): e2023006.
https://doi.org/10.4084/MJHID.2023.006 PMid:36660350 PMCid:PMC9833310
- De
Sanctis V, Soliman AT, Daar S, Tzoulis P, Kattamis C. Can we predict
incipient diabetes mellitus in patients with transfusion dependent
β-thalassemia (β-TDT) referred with a history of prediabetes? Mediterr
J Hematol Infect Dis. 2024;16(1): e2024005.
https://doi.org/10.4084/MJHID.2024.005 PMid:38223478 PMCid:PMC10786125
- Hicks
R, Ode KL, Vigers T, Chan CL. A provider survey of cystic fibrosis
related diabetes screening and management practices at North American
CF centers. Front Endocrinol.2023;14:1183288.
https://doi.org/10.3389/fendo.2023.1183288 PMid:37274323
PMCid:PMC10232971
- Korner A, Wiegand S, Hungele A, Tuschy S, Otto
KP, l'Allemand-Jander D, Widhalm K, Kiess W, Reinhard H. Longitudinal
multicenter analysis on the course of glucose metabolism in obese
children. Int J Obes (Lond). 2013;37(7):931-6.
https://doi.org/10.1038/ijo.2012.163 PMid:23032406
- Hillier TA,
Pedula KL, Ogasawara KK, Vesco KK, Oshiro CES, Lubarsky SL, Van Marter
J. A Pragmatic, Randomized Clinical Trial of Gestational Diabetes
Screening. N Engl J Med. 2021;11;384 (10):895-904.
https://doi.org/10.1056/NEJMoa2026028 PMid:33704936 PMCid:PMC9041326
- Khamseh
M E, Malek M, Hashemi-Madani N, Ghassemi F, Rahimian N, Ziaee A,
Foroughi-Gilvaee M, Faranoush P, Sadighnia O, Elahinia A, Saeedi V, MR,
Faranoush M. Guideline for the diagnosis and treatment of diabetes
mellitus in patients with transfusion-dependent thalassemia. Iranian J
Blood Cancer. 2023;15 (4):293-303.
https://doi.org/10.61186/ijbc.15.4.293
- Bowling, Ann. Measuring
Disease: A Review of Disease-Specific Quality of Life Measurement
Scales. Philadelphia: Open University Press/Taylor & Francis. 1995;
pp. 1-374. http://hdl.handle.net/10822/1037305. Last Update: December
18, 2023.
- Torrance GW. Measurement of health state
utilities for economic appraisal. J Health Econ.1986;5(1):1-30. https://doi.org/10.1016/0167-6296(86)90020-2 PMid:10311607
- Pepe A,
Pistoia L, Gamberini MR, Cuccia L, Peluso A, Messina G, Spasiano A,
Allò M, Bisconte MG, Putti MC, Casini T, Dello Iacono N, Celli M,
Vitucci A, Giuliano P, Peritore G, Renne S, Righi R, Positano V, De
Sanctis V, Meloni A. The Close Link of Pancreatic Iron With Glucose
Metabolism and With Cardiac Complications in Thalassemia Major: A
Large, Multicenter Observational Study. Diabetes Care 2020; 43
(11):2830-2839. https://doi.org/10.2337/dc20-0908 PMid:32887708
- Noetzli
LJ, Mittelman SD, Watanabe RM, Coates TD, Wood JC. Pancreatic iron and
glucose dysregulation in thalassemia major. Am J Hematol.
2012;87:155-160. https://doi.org/10.1002/ajh.22223 PMid:22120775
- Thewjitcharoen
Y, Elizabeth AE, Butade S, Nakasatien S, Chotwanvirat P, Wanothayaroj
E, Krittiyawong S, Himathongkam T, Himathongkam T. Performance of HbA1c
versus oral glucose tolerance test (OGTT) as a screening tool to
diagnose dysglycemic status in high-risk Thai patients. BMC Endocr Dis.
2019;19:23 https://doi.org/10.1186/s12902-019-0339-6 PMid:30770743
PMCid:PMC6377733
- Mahmoud AA, El-Hawy MA, Allam ET, Salem AH, Hola
AS. HbA1c or fructosamine on evaluating glucose intolerance in children
with beta- thalassemia. Pediatr Res.2024. https://doi.org/10.1038/s41390-024-03146-y.