Optimal selection of first-line treatment is currently challenging, as the clinician has to choose among almost equally effective treatment options, taking into account both disease and patient factors and preferences and the unique safety profile of each drug. Patients with CLL in Greece have access to all novel therapies pending approval by the official committee overseeing high-cost drugs.
The scope of this document is to provide recommendations for the treatment of patients with CLL based on the available evidence for both the first-line and the relapsed/refractory setting.
Methodology
The Lymphoma Working Group of the Hellenic Society of Haematology invited a panel of Greek hematology experts to consider the treatment landscape of CLL. The experts performed a systematic review of all available data related to the treatment of CLL over the last two decades, focusing on pivotal randomized phase 3 clinical trials of novel agents. The results of the literature search were presented and discussed.Pretreatment evaluation of clinically meaningful biological factors. Screening for TP53 disruption [(del17p13.1)] and/or TP53 mutation) is mandatory prior to the first and each subsequent line of treatment. Patients with CLL with TP53 mutations may or may not have concomitant del (17p).[5] TP53 abnormalities are associated with poor prognosis, and their evaluation is crucial for making treatment decisions even in the era of targeted therapies.[6-7] Next Generation Sequencing (NGS) allows for the identification of low-burden TP53 mutations (variant allele frequency, VAF, <10%). TP53 pathogenic variants identified by NGS should be considered significant for treatment decisions regardless of the VAF, provided that the laboratory undertaking the analysis is certified for this test by a competent authority (ERIC and/or GenQA) and reports the corresponding limit of detection[8] Immunoglobulin heavy variable (IGHV) gene somatic hypermutation (SHM) status also plays a key role in the prognosis of CLL.[9-10] As this biomarker remains stable over time, assessment of IGHV gene SHM status should be performed only once, ideally prior to first-line treatment. Moreover, the study of B-cell receptor (BCR) immunoglobulins (IGs) stereotypy should be included in pretreatment assessment in CLL since patients in certain stereotyped subsets, such as patients in subset 2 display remarkably consistent clinicobiological profiles and should be treated accordingly.[11]
Consensus:
1. IGHV gene SHM analysis should be performed once during the disease course, ideally before the first-line treatment. Major stereotyped subsets should be defined before treatment initiation
2. Before each line of treatment, FISH for del (17p) and NGS for TP53 mutations are required.
3. G-banding analysis for assessing genomic complexity is not generally recommended in routine care, emphasizing, however, that only the presence of at least five chromosomal aberrations is clinically relevant.[12]
First line therapy
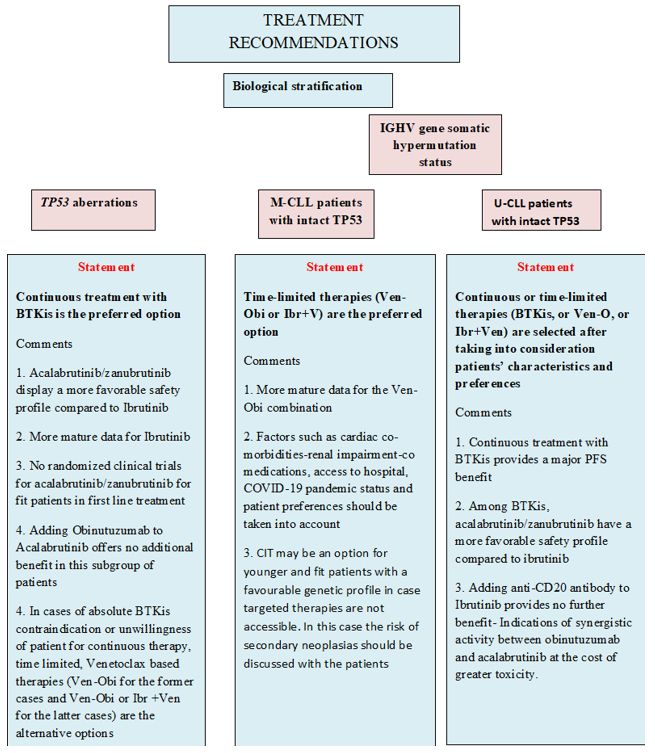
CLL patients with TP53 aberrations (Figure 1). The detection of del(17p) and TP53 mutations in patients with no evidence of active disease is not per se a criterion for starting therapy.[3]In patients meeting the criteria for treatment initiation, the detection of TP53 is an absolute contraindication to the use of chemoimmunotherapy (CIT).[6,13]
Continuous therapy. Continuous therapy with BTKis has shown promising results in the first-line setting. In the National Institutes of Health Clinical Center (NIH) phase 2 trial evaluating only patients with del(17p) or TP53 mutations treated with Ibrutinib, the Progression Free Survival(PFS) and Overall Survival (OS) medians were not reached and the estimated 6-year PFS and OS rates were 60% and 79% respectively.[14-15] In the ALLIANCE trial comparing Ibrutinib and Ibrutinib-Rituximab (IR) to Bendamustine-Rituximab (BR), after a median follow-up of 38 months, the median PFS for patients with del(17p) was not reached for IR versus 7 months for BR.[16] In the ILLUMINATE trial, the estimated 48-month PFS was 74% for patients with del(17p) or TP53 mutations and 77% for those without.[17] Similarly, patients with TP53 aberrations treated with Acalabrutinib with or without Obinutuzumab within the ELEVATE TN trial had a 72-month PFS rate of 56 %. These results suggest that CLL patients with TP53 aberrations could effectively be treated with Acalabrutinib monotherapy without the need for additional Obinutuzumab.[18] The nonrandomized cohort, Arm C, of the phase 3 SEQUOIA trial, which included 109 patients with centrally confirmed del(17p) that received Zanubrutinib showed that after a median follow-up of 18.2 months, the overall response rate was 94.5% with 3.7% of patients achieving complete response with or without incomplete hematologic recovery. The estimated 18-month PFS rate was 88.6%, and the estimated 18-month OS rate was 95.1%. Moreover, in the SEQUOIA trial, there is also a nonrandomized cohort, Arm D, that includes treatment naïve CLL patients with del(17p) treated with the combination of Zanubrutinib and Venetoclax.[19-20]
Time-limited therapies. In the phase III CLL14 trial, 36 and 27 patients displayed TP53 aberrations in the Venetoclax plus Obinutuzumab and in the Chlorambucil-Obinutuzumab arm, respectively.[21] The median PFS for patients with TP53 aberrations was approximately 18 months in patients treated with Chlorambucil-Obinutuzumab versus almost 4 years for Venetoclax plus Obinutuzumab (Ven-Obi).[21] That notwithstanding, the trial results showed that TP53 aberrations remained a relatively poor prognosticator also in the context of Ven-Obi treatment with a hazard ratio (HR) of 3.39 (p=0.03).[21] In the CAPTIVATE phase II trial investigating the effectiveness of the Ibrutinib–Venetoclax (Ibr-Ven) combination in patients aged ≤70 years with previously untreated CLL, 27/159 (17%) pts had TP53 aberrations. Ibr-Ven resulted in high complete response (CR) and undetectable Minimal Residual Disease (uMRD) rates across patient subgroups, including those with TP53 aberrations. Specifically, the best overall response rates by investigator assessment were 96% in patients with del(17p) and/or mutated TP53, while at 4, the 4-year PFS and OS rates were 63% and 96%, respectively.[22]
Recommendations for CLL patients with TP53 aberrations.
- More prolonged disease control achieved with BTKis appears to confer greater benefit to patients with TP53 aberrations compared to other treatments.
- Fixed-duration treatment with the Ven-Obi combination does not appear to overcome the negative prognostic impact of TP53 aberrations.
- CIT is not recommended.
Patients with mutated IGHV genes (M-CLL) without TP53 aberrations (Figure 1). This subgroup displays a favorable risk profile and represents approximately 25-30% of CLL patients at first-line treatment.[2-4] Young and fit M-CLL patients treated with the Fludarabine, Cyclophosphamide, Rituximab combination (FCR) in the CLL-8 trial had a 53.9% PFS at 12.8 years, while similar results have also been reported by the MD Anderson group.[23-25]
Regarding BTKis, subgroup analysis of several studies confirms the high effectiveness of BTKis in M-CLL, mostly in terms of PFS.[16-17,20,26] More mature data was derived from the RESONATE-2 trial for elderly and/or unfit patients in which Ibrutinib was compared to Chlorambucil monotherapy. After a median follow-up of 8 years, PFS at 7 years for M-CLL patients was 68% for Ibrutinib versus 17% for Chlorambucil.[26] The E1912 trial compared the combination of Ibrutinib with Rituximab against FCR for young and fit patients, reporting 5-year PFS rates of 83% for IR vs. 68% for FCR.[27] In the ELEVATE TN trial for elderly and/or unfit patients, the 4-year PFS rates for M-CLL patients were 89%, 81% and 62% for Acalabrutinib plus Obinutuzumab, Acalabrutinib monotherapy and Obinutuzumab plus Chlorambucil respectively; the difference between Acalabrutinib plus Obinutuzumab versus Obinutuzumab plus Chlorambucil was statistically significant (p=0.0012).[18]
In the SEQUOIA trial, Zanubrutinib was also particularly effective in M-CLL patients, inducing high PFS rates (median not reached versus 49.9 months for BR, p<0.00033).[20] Concerning time-limited approaches, in the CLL14 trial, after a follow-up of 72 months, the median PFS for M-CLL patients was not reached for Ven-Obi whereas it was 62.2 months for Chlorambucil-Obinutuzumab; no OS benefit has been shown yet.[21]
Venetoclax-based combinations were also evaluated in the context of the CLL-13/GAIA trial, which reported that the Ven-Obi combination with or without Ibrutinib was superior to CIT (FCR or BR) in terms of PFS, inducing high rates of undetectable MRD in M-CLL, with 3-year PFS rates of 96%, 93.6%, 87% and 89.9% for Ven-Obi-ibrutinib, Ven-Obi, Ven-Rituximab and CIT respectively[28] In the GLOW trial, the combination of Ibr-Ven led to > 90% 2-year PFS rate for M-CLL patients independent of MRD status.[29]
The role of the FCR regimen for fit M-CLL patients without unfavorable cytogenetic characteristics is questionable for the following reasons:
- Inferior results compared to chemo-free regimens in phase III trials.[27-28]
- The use of FCR is associated with severe complications, including myelosuppression, infections, and secondary malignancies.[30-31]
- Not all M-CLL patients are equivalent, as exemplified by those belonging to stereotyped subset #2 who have a particularly adverse prognosis and respond poorly to CIT, including FCR. Information regarding membership in subset #2 must be provided by the laboratory performing IGHV gene analysis.[11]
Recommendations for M-CLL patients.
1. Time-limited treatment options with novel agents are the preferred therapy (Ven-Obi, Ibr-Ven)
2. CIT such as FCR should only be considered for fit and younger patients if targeted therapies are not accessible
Patients with unmutated IGHV (U-CLL) without TP53 aberrations. Patients with U-CLL experience inferior outcomes with shorter survival rates when treated with CIT.[7] Results from pivotal clinical trials in the first-line comparing BTKis versus chemotherapy or CIT highlighted that BTKis with or without anti-CD20 antibodies are clearly superior in U-CLL.[16-18,20,27] In the RESONATE-2 trial, U-CLL patients treated with Ibrutinib had a PFS of 67% versus 6% for Chlorambucil after 5 years of follow-up.[26] In the ALLIANCE trial, after a median follow-up of 33.6 months in patients with U-CLL, the median PFS was not reached for both the Ibrutinib and Ibrutinib-Rituximab arms, whereas it was only 39 months for the BR arm[16] Likewise, in fit patients within the E1912 trial, the combination of Ibrutinib with Rituximab resulted in a significant PFS advantage in U-CLL patients over FCR (5-year PFS 75% for Ibrutinib vs 33% for FCR).[27]
In the ELEVATE-TN trial, after 7 years of follow-up, the median PFS was not reached for U-CLL patients treated with Acalabrutinib plus Obinutuzumab, whereas it was 22.2 months in Obinutuzumab-Chlorambucil arm.[18]
A treatment benefit was also demonstrated for U-CLL patients treated with Zanubrutinib in the SEQUOIA trial.[20]
Concerning time-limited therapies, in the CLL-14 trial, U-CLL patients had significantly superior PFS when treated with the Ven-Obi combination compared to Chlorambucil-Obinutuzumab.[21] In the GLOW trial, PFS at 3.5 years was higher for U-CLL patients on the Ibr-Ven arm compared to the Chlorambucil-Obinutuzumab arm.[29] In conclusion, there is a clear advantage of novel agents over CIT for U-CLL patients. The final decision on the treatment choice concerning targeted therapies should depend on patients’ profiles and preferences as well as the safety profile of each drug. Regarding the latter, Acalabrutinib and Zanubrutinib have fewer cardiovascular adverse events compared to Ibrutinib. The most common cardiac toxicity associated with BTK inhibitors, particularly with Ibrutinib, is atrial fibrillation, while other types of cardiac events include ventricular arrhythmias, heart failure, and hypertension.[32] BTKis should be avoided in patients with severe cardiac failure (ejection fraction<30%), a family history of sudden cardiac arrest, a past medical history of significant ventricular arrhythmia, and in patients with uncontrolled blood pressure.[32] On the other hand, treatment with Venetoclax requires adequate renal function, and patients with severe renal impairment (creatinine clearance >15 and <30ml/min) should only be considered for Venetoclax if the benefit outweighs the risk.[33] Thus, for patients with high tumor burden and/or chronic renal impairment, BTKis are the preferred option.
Recommendations:
1. Targeted therapies are preferred for patients with U-CLL over CIT.
2. Cardiotoxicity is a class effect of BTKis, and alternative treatment options should be considered for patients at increased cardiac risk.
3. Among BTKis, Acalabrutinib and Zanubrutinib show a favorable safety profile compared to Ibrutinib.
The role of anti-CD20 in the context of continuous treatment
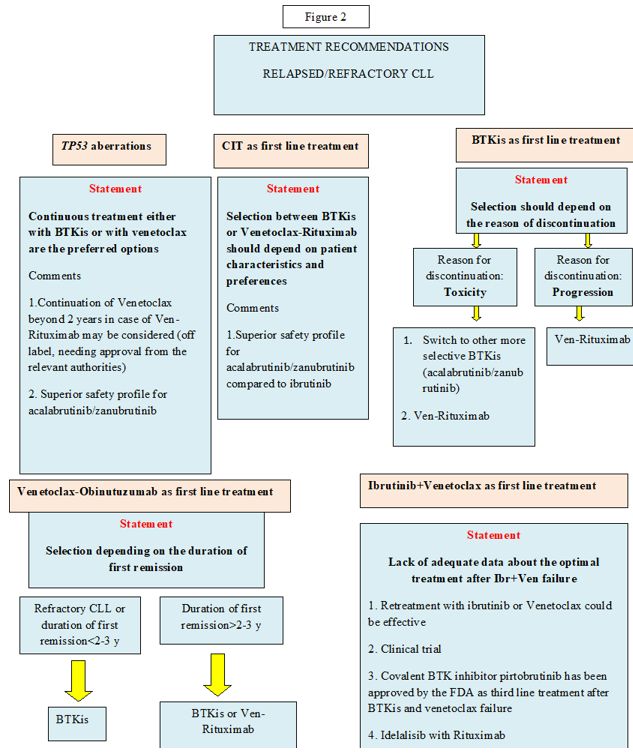
No significant difference was seen in terms of PFS between Ibrutinib monotherapy and Ibrutinib - Rituximab in the ALLIANCE trial.[15] In the ELEVATE TN trial, at 6 years of follow-up, PFS was significantly longer in patients treated with Acalabrutinib plus Obinutuzumab versus Acalabrutinib, while median OS was not reached in any treatment arm and was considerably longer in patients treated with Acalabrutinib-Obinutuzumab versus Obinutuzumab-Chlorambucil combination.[18] However, patients in the Acalabrutinib-Obinutuzumab arm experienced more frequently grade ≥3 adverse events, such as neutropenia and thrombocytopenia.[18] Another important issue concerning the addition of Obinutuzumab to Acalabrutinib concerns the increased vulnerability of patients with CLL receiving anti-CD20 antibodies to severe coronavirus disease 2019 (COVID-19) as well as their impaired immune response to vaccination against COVID-19.[34]Management of relapsed/refractory CLL (Figure 2)
Crucial issues for deciding on treatment of relapsed/refractory(R/R) CLL are the type of first-line treatment and the duration of response after first-line treatment. TP53 aberrations remain the most important prognostic factor also in this setting.There is no role for CIT for patients with R/R CLL as both BTKis and Venetoclax-based regimens proved to be significantly better versus CIT in head-to-head comparisons.[35-39] Regarding continuous treatments, in the RESONATE study, the PFS and OS medians for Ibrutinib were 44 months and 68 months, respectively, compared to 8 and 65 months for Ofatumumab.[35] In the ASCEND trial, 42-month PFS rates were 62% for Acalabrutinib versus 19% for Idelalisib-R and BR, whereas, in the ALPINE study, Zanubrutinib showed a PFS superiority compared to Ibrutinib (12-month PFS rates of 97% vs 93% respectively.[36-38] Regarding time-limited therapies, the phase 3 MURANO trial reported a survival advantage for the combination of Venetoclax plus Rituximab (VR) over BR, with median PFS rates of 53.6 months for VR vs 17 months with BR, and 5-year OS rates of 82% versus 62.2% respectively.[39] Venetoclax monotherapy has also been studied in a phase II study of 158 patients with del(17p), resulting in a median OS of 62 months and a median PFS of 28 months.[40-41] Continuation of Venetoclax beyond 2 years in the case of the VR combination may be considered in patients with TP53 aberrations.[42]
Sequence of treatment. In cases treated with CIT in the first line, the choice of BTKis versus VR critically depends on patient characteristics and preferences. When BTKis are considered, Acalabrutinib or Zanubrutinib are most likely recommended, as they both show similar efficacy and less toxicity compared to ibrutinib.[38,43] In patients exposed to BTKis as first-line, the reason for BTKis discontinuation should be considered.
In case of toxicity, dose reduction or treatment with an alternative, more selective BTK could be an option. In case of disease progression, it is absolutely necessary to provide a different treatment approach, such as the Ven-R.[44] On the other hand, if patients had been exposed to Ven-Obi as first-line therapy, the decision should be made on the basis of the reason for discontinuation and the duration of response after Ven-Obi. In case of unmanageable toxicity related to Venetoclax or disease progression on Venetoclax treatment, covalent BTKis represent the next available treatment option.[44] The decision to re-administer Venetoclax after Ven-Obi depends on the duration of the prior response. Retreatment with a Venetoclax-based regimen could be an option in case the duration of remission is greater than 2-3 years. Patients with shorter remissions are not considered suitable for retreatment and should instead proceed to BTKis.[44] Currently, a new group of patients is emerging, including those who have been exposed upfront to both Ibrutinib and Venetoclax. There are no mature data available to support a specific treatment recommendation for patients who progress after this combination. However, a few patients experiencing relapse within the CAPTIVATE trial responded to Ibrutinib retreatment.[20-21] Currently, Pirtobrutinib, a noncovalent BTK inhibitor, has been approved by the FDA (12/2023) for patients after 2 lines of treatment, including BTKis and Venetoclax.[45]
In addition, we should also consider the oral first-in-class phosphatidylinositol 3-kinase delta inhibitor idelalisib in combination with Rituximab, which has shown efficacy in heavily pretreated CLL patients.[46]
Conclusions
The treatment landscape in CLL has radically changed, and the OS of CLL patients has dramatically improved over the last decade due to the advent of novel agents such as BTK and BCL-2 inhibitors. Among almost equally effective treatment options, the clinician, apart from biological dismal prognostic factors such as TP53 abnormalities and unmutated IGHV status, should also take into account several parameters associated with the patient's characteristics as well as with specific side effects of the different regimens. The most important clinical question on the superiority of continuous over time-limited treatment remains, as we will expect the findings from the CLL17 trial of the German CLL Study Group (NCT04608318), which has been conducted in order to address this question. Additionally, concerns about the optimal sequencing of therapies or about the treatment alternatives for double refractory patients need to be further investigated.References
- The Surveillance E, and End Results (SEER) Program
of the National Cancer Institute. Cancer Stat Facts: Leukemia-Chronic
Lymphocytic Leukemia (CLL). https://seer.cancer.gov/statfacts/html/clyl.html; 2021
- Hallek
M. Chronic lymphocytic leukemia: 2015 update on diagnosis, risk
stratification, and treatment. Am J Hematol. 2015;90:446-60. doi:
10.1002/ajh.23979 https://doi.org/10.1002/ajh.23979 PMid:25908509
- Hallek
M, Al-Sawaf O. Chronic lymphocytic leukemia: 2022 update on diagnostic
and therapeutic procedures. Am J Hematol. 2021; 96: 1679-1705. doi:
10.1002/ajh.26367 https://doi.org/10.1002/ajh.26367 PMid:34625994
- Awan
FT, al-Sawaf O, Fischer K, Woyach JA. Current perspectives on therapy
for chronic lymphocytic leukemia. Am Soc Clin Oncol Educ Book. 2020;
40: 320-329. DOI: 10.1200/EDBK_279099 https://doi.org/10.1200/EDBK_279099 PMid:32239979
- Zenz
T, Eichhorst B, Busch R, Denzel T, Häbe S, Winkler D, Bühler A,
Edelmann J, Bergmann M, Hopfinger G, Hensel M, Hallek M, Döhner H,
Stilgenbauer S. TP53 mutation and survival in chronic lymphocytic
leukemia. J Clin Oncol. 2010;28:4473-4479. doi:
10.1200/JCO.2009.27.8762 https://doi.org/10.1200/JCO.2009.27.8762 PMid:20697090
- Campo
E, Cymbalista F, Ghia P, Jäger U, Pospisilova S, Rosenquist R, Schuh A,
Stilgenbauer S. TP53 aberrations in chronic lymphocytic leukemia: an
overview of the clinical implications of improved diagnostics.
Haematologica 2018; 103: 1956-1968. DOI:
10.3324/haematol.2018.187583 https://doi.org/10.3324/haematol.2018.187583 PMid:30442727 PMCid:PMC6269313
- Stilgenbauer
S, Schnaiter A, Paschka P, Zenz T, Rossi M, Dohner K, Bühler A ,
Böttcher S , Ritgen M , Kneba M, Winkler D , Tausch E , Hoth P ,
Edelmann J , Mertens D , Bullinger L, Bergmann M , Kless S , Mack S,
Jäger U , Patten N , Wu L , Wenger MK , Fingerle-Rowson G , Lichter P,
Cazzola M , Wendtner CM , Fink AM , Fischer K , Busch R , Hallek M,
Döhner H. Gene mutations and treatment outcome in chronic lymphocytic
leukemia: results from the CLL8 trial. Blood. 2014;123:3247-3254. doi:
10.1182/blood-2014-01-546150 https://doi.org/10.1182/blood-2014-01-546150 PMid:24652989
- Malcikova
J, Tausch E, Rossi D, Sutton LA, Soussi T, Zenz T, Kater A P, Niemann
CU, Gonzalez D, Davi F, Gonzalez Diaz M, Moreno C, Gaidano G,
Stamatopoulos K, Rosenquist R, Stilgenbauer S, Ghia P, Pospisilova S.
European Research Initiative on Chronic Lymphocytic Leukemia (ERIC) -
TP53 network. ERIC recommendations for TP53 mutation analysis in
chronic lymphocytic leukemia-update on methodological approaches and
results interpretation Leukemia. 2018; 32: 1070-1080. DOI:
10.1038/s41375-017-0007-7 https://doi.org/10.1038/s41375-017-0007-7 PMid:29467486 PMCid:PMC5940638
- Cramer
P, Hallek M. Prognostic factors in chronic lymphocytic leukemia-what do
we need to know? Nat Rev Clin Oncol. 2011;8:38-47. doi:
10.1038/nrclinonc.2010.167. https://doi.org/10.1038/nrclinonc.2010.167 PMid:20956983
- Crombie
J, Davids MS. IGHV mutational status testing in chronic lymphocytic
leukemia. Am J Hematol 2017; 92: 1393-1397 DOI: 10.1002/ajh. 24808 https://doi.org/10.1002/ajh.24808 PMid:28589701 PMCid:PMC5675754
- Gerousi
M, Laidou S, Gemenetzi K, Stamatopoulos K, Chatzidimitriou A.
Distinctive Signaling Profiles With Distinct Biological and Clinical
Implications in Aggressive CLL Subsets With Stereotyped B-Cell Receptor
Immunoglobulin Front Oncol. 2021;11:771454DOI: 10.3389/fonc.2021.
771454 https://doi.org/10.3389/fonc.2021.771454 PMid:34804974 PMCid:PMC8595110
- Baliakas
P, Jeromin S, Iskas M, Puiggros A, Plevova K, Nguyen-Khac F, Davis Z ,
Rigolin GM , Visentin A , Xochelli A, Delgado J, Baran-Marszak F,
Stalika E, Abrisqueta P, Durechova K, Papaioannou G, Eclache V, Dimou
M, Iliakis T, Collado R, Doubek M, Calasanz MJ, Ruiz-Xiville N, Moreno
C, Jarosova M, Leeksma AC, Panayiotidis P, Podgornik H, Cymbalista F,
Anagnostopoulos A, Livio Trentin, Stavroyianni N, Davi F, Ghia P, Kater
AP, Cuneo A, Pospisilova S, Espinet B, Athanasiadou A, Oscier D,
Haferlach C, Stamatopoulos K. Cytogenetic complexity in chronic
lymphocytic leukemia: definitions, associations, and clinical impact.
Blood. 2019; 133: 1205-1216. DOI: 10.1182/blood-2018-09-873083. https://doi.org/10.1182/blood-2018-09-873083 PMid:30602617 PMCid:PMC6509568
- Döhner
H, Stilgenbauer S, Benner A, Leupolt E, Kröber A, Bullinger L, Döhner
K, Bentz M, Lichter P. Genomic aberrations and survival in chronic
lymphocytic leukemia. N Engl J Med. 2000;343:1910-1916 DOI:
10.1056/NEJM200012283432602 https://doi.org/10.1056/NEJM200012283432602 PMid:11136261
- Allan
JN, Shanafelt T, Wiestner A, Moreno C, O'Brien SM, Braggio E, Jianling
Li, Krigsfeld G , Dean JP, Ahn IE . Long-term efficacy of first-line
Ibrutinib treatment for chronic lymphocytic leukemia (CLL) with 4 years
of follow-up in patients with TP53 aberrations (del(17p) or TP53
mutation): a pooled analysis from 4 clinical trials. Br J Haematol.
2022 196:947-953. doi: 10.1111/bjh.17984. https://doi.org/10.1111/bjh.17984 PMid:34865212 PMCid:PMC9299890
- Ahn
IE, Tian X, Wiestner A. Ibrutinib for chronic lymphocytic leukemia with
TP53 alterations. N Engl J Med. 2020; 383: 498-500 doi:
10.1056/NEJMc2005943 https://doi.org/10.1056/NEJMc2005943 PMid:32726539 PMCid:PMC7456330
- Woyach
JA, Ruppert AS, Heerema NA, Zhao W, Booth AM, Ding W Bartlett NL ,
Brander DM, Barr PM, Rogers KA, Parikh SA, Coutre S, Hurria A, Brown
JR, Lozanski G, Blachly JS, Ozer HG, Major-Elechi B, Fruth B, Nattam S,
Larson RA, Erba H, Litzow M, Owen C, Kuzma C, Abramson JS, Little RF,
Smith SE, Stone RM, Mandrekar SJ, Byrd JC. Ibrutinib regimens versus
chemoimmunotherapy in older patients with untreated CLL. N Engl J Med.
2018; 379: 2517-2528 doi: 10.1056/NEJMoa1812836 https://doi.org/10.1056/NEJMoa1812836 PMid:30501481 PMCid:PMC6325637
- Moreno
C, Greil R, Demirkan F, Tedeschi A, Anz B, Larratt L, Simkovic M,
Samoilova O, Novak J, Ben-Yehuda D, Strugov V, Gill D, Gribben JG, Hsu
E, Lih CJ, Zhou C, Clow F, James DF, Styles L, Flinn IW . Ibrutinib
plus obinutuzumab versus Chlorambucil plus obinutuzumab in first-line
treatment of chronic lymphocytic leukaemia (iLLUMINATE): a multicentre,
randomised, open-label, phase 3 trial. Lancet Oncol 2019; 20: 43-56.
DOI: 10.1016/S1470-2045(18)30788-5 https://doi.org/10.1016/S1470-2045(18)30788-5 PMid:30522969
- Sharman
JP, Egyed M, Jurczak W, Skarbnik A, Patel K, Flinn IW, Kamdar M, Munir
T, Walewska R, Hughes M, Fogliatto LM, Herishanu Y, Banerji V, Follows
G, Patricia A. Walker, Karlsson K, Ghia P, Janssens A, Cymbalista F,
Byrd JC, Ferrant E, Ferrajoli A, Wierda WG, Munugalavadla V, Wachira
CW, Wun CC, Woyach JA. Acalabrutinib ± Obinutuzumab Vs Obinutuzumab +
Chlorambucil in Treatment-Naive Chronic Lymphocytic Leukemia: 6-Year
Follow-up of Elevate-TN Blood 2023; 142 (Supplement 1): 636 https://doi.org/10.1182/blood-2023-174750
- Tam
CS, Robak T, Ghia P, Kahl BS, Walker P, Janowski W, Simpson D, Shadman
M, Ganly PS, Laurenti L, Opat S, Tani M, Ciepluch H, Verner E, Šimkovič
M, Österborg A, Trněný M, Tedeschi A, Paik JC, Kuwahara SB, Feng S,
Ramakrishnan V, Cohen A, Huang J, Hillmen P, BrownJR. Zanubrutinib
monotherapy for patients with treatment naive chronic lymphocytic
leukemia and 17p deletion. Haematologica 2021;106:2354-2363. doi:
10.3324/haematol.2020.259432. https://doi.org/10.3324/haematol.2020.259432 PMid:33054121 PMCid:PMC8409041
- Tam
CS, Brown JR, Kahl BS, Ghia P, Giannopoulos K, Jurczak W, Šimkovič M,
Shadman M, Österborg A, Laurenti L, Walker P, Opat S, Chan H, Ciepluch
H, Greil R, Tani M, Trněný M, Danielle M Brander, Flinn IW, Grosicki S,
Verner E, Tedeschi A, Li J, Tian T, Zhou L, Marimpietri C, Paik JC,
Cohen A, Huang J, Robak T, Hillmen P. Zanubrutinib versus bendamustine
and Rituximab in untreated chronic lymphocytic leukaemia and small
lymphocytic lymphoma (SEQUOIA): a randomised, controlled, phase 3
trial. Lancet Oncol. 2022; 23: 1031-1043. DOI:
10.1016/S1470-2045(22)00293-5 https://doi.org/10.1016/S1470-2045(22)00293-5 PMid:35810754
- Al-Sawaf
O, Zhang C, Lu T, Michael Z Liao, Panchal A, Robrecht S, Ching T,
Tandon M, Anna-Maria Fink, Tausch E, Schneider C, Ritgen M, Böttcher S,
Karl-Anton Kreuzer, Chyla B, Miles D, Clemens-Martin Wendtner,
Eichhorst B, Stilgenbauer S, Jiang Y, Hallek M, Fischer K. Minimal
residual disease dynamics after venetoclax-obinutuzumab treatment:
extended off-treatment follow-up from the randomized CLL14 study. J
Clin Oncol. 2021; 39: 4049-4060 DOI: 10.1200/JCO.21.01181 https://doi.org/10.1200/JCO.21.01181 PMid:34709929 PMCid:PMC8678026
- Barr
PM, Allan JN, Siddiqi T, Wierda WG, Lu Tam CS, Moreno CD, Tedeschi A,
Szafer-Glusman E, Zhou C, Abbazio C, Dean JP, Szoke A, Ghia P.
Fixed-duration ibrutinib + venetoclax for first-line treatment of
chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL):
4-y follow-up from the FD cohort of the phase 2 CAPTIVATE study. J Clin
Oncol.Volume 41 Number 16, Suppl 7535 https://doi.org/10.1200/JCO.2023.41.16_suppl.7535
- Fischer
K, Bahlo J, Fink AM, Goede V, Herling CD, Cramer P, Langerbeins P, von
Tresckow J, Engelke A, Maurer C, Kovacs G, Herling M, Tausch E, Kreuzer
KA, Eichhorst B, Böttcher S, John F Seymour JF, Ghia P, Marlton P,
Kneba M, Wendtner CM, Döhner H, Stilgenbauer S, Hallek M. Long-term
remissions after FCR chemoimmunotherapy in previously untreated
patients with CLL: updated results of the CLL8 trial. Blood. 2016; 127:
208-215 DOI: 10.1182/blood-2015-06-651125 https://doi.org/10.1182/blood-2015-06-651125 PMid:26486789
- Thompson
PA, Tam CS, O'Brien SM, Wierda WG, Stingo F, Plunkett W, Smith SC,
Kantarjian HM, Freireich EJ, Keating MJ. Fludarabine, cyclophosphamide,
and rituximab treatment achieves long-term disease-free survival in
IGHV-mutated chronic lymphocytic leukemia. Blood. 2016; 127: 303-309.
DOI: 10.1182/blood-2015-09-667675 https://doi.org/10.1182/blood-2015-09-667675 PMid:26492934 PMCid:PMC4760129
- Thompson
PA, Tam CS, O'Brien SM, Wierda WG, Stingo F, Plunkett W, Smith SC,
Kantarjian HM, Freireich EJ, Keating MJ Fludarabine,
cyclophosphamide,and rituximab treatment achieves long-term
disease-free survival in IGHV-mutated chronic lymphocytic
leukemia.Blood.2016;127:303-309 DOI: 10.1182/blood-2015-09-667675 https://doi.org/10.1182/blood-2015-09-667675 PMid:26492934 PMCid:PMC4760129
- Barr
PM, Owen C, Robak T, Tedeschi A, Bairey O, Burger JA, Hillmen P, Coutre
SE, Dearden C, Grosicki S, McCarthy H, Li JY, Offner F, Moreno C, Zhou
C, Hsu E, Szoke A, Kipps TJ, Ghia P. Up to 8-year follow-up from
RESONATE-2: first-line ibrutinib treatment for patients with chronic
lymphocytic leukemia. Blood Adv. 2022;6:3440-3450 DOI:
10.1182/bloodadvances. 2021006434 https://doi.org/10.1182/bloodadvances.2021006434 PMid:35377947 PMCid:PMC9198904
- Shanafelt
TD, Wang XV, Hanson CA, Paietta EM, O'Brien S, Barrientos J. Jelinek
DF, Braggio E, Leis JF, Zhang CC, Coutre SE, Barr PM, Cashen AF, Mato
AR, Singh AK, Mullane MP, Little RF, Erba H, Stone RM, Litzow M,
Tallman M, Neil E Kay. Long-term outcomes for ibrutinib-Rituximab and
chemoimmunotherapy in CLL: updated results of the E1912 trial. Blood.
2022; 140: 112-120 DOI: 10.1182/blood.2021014960 https://doi.org/10.1182/blood.2021014960 PMid:35427411 PMCid:PMC9283968
- Eichhorst
B, Niemann CU, Kater AP, Fürstenau M, von Tresckow J, Zhang C, Robrecht
S, Gregor M, Juliusson G, Thornton P, Staber PB, Tadmor T, Lindström V,
da Cunha-Bang C, Schneider C, Poulsen CB, Illmer T, Schöttker B,
Nösslinger T, Janssens A, Christiansen I, Baumann M, Frederiksen H, van
der Klift M, Jäger U, Leys MBL, Hoogendoorn M, Lotfi K, Hebart H, Gaska
T, Koene H, Enggaard L, Goede J, Regelink JC, Widmer A, Simon F, De
Silva N, Fink AM, Bahlo J, Fischer K, Wendtner CM, Kreuzer KA, Ritgen
M, Brüggemann M, Tausch E, Levin MD, van Oers M, Geisler C,
Stilgenbauer S, Hallek M; GCLLSG, the HOVON and Nordic CLL Study
Groups, the SAKK, the Israeli CLL Association, and Cancer Trials
Ireland.First Line Venetoclax Combinations in Chronic Lymphocytic
Leukemia.N Engl J Med. 2023;388:1739-1754.
doi:10.1056/NEJMoa2213093. https://doi.org/10.1056/NEJMoa2213093 PMid:37163621
- Moreno
C, Munir T, Owen C, Follows G, Hernandez Rivas JA, Benjamini O,
Janssens A, Levin MD, Robak T, Simkovic M, Voloshin S, Vorobyev VI,
Yagci M, Ysebaert L, Qi Q, Smith E, Srinivasan S, Schuier N, Baeten K,
Caces DB, Niemann CU, Kater AP. First-Line Fixed-Duration Ibrutinib
Plus Venetoclax (Ibr+Ven) Versus Chlorambucil Plus Obinutuzumab
(Clb+O): 55-Month Follow-up from the Glow Study. Blood. 2023; 142
(Supplement 1): 634. https://doi.org/10.1182/blood-2023-177713
- Benjamini
O, Jain P, Trinh L, Qiao W, Strom SS, Lerner S, Wang X, Burger J,
Ferrajoli A, Kantarjian H, O'Brien S, Wierda W, Estrov Z, Keating M.
Second cancers in patients with chronic lymphocytic leukemia who
received frontline fludarabine, cyclophosphamide and Rituximab therapy:
distribution and clinical outcomes. Leuk Lymphoma. 2015; 56: 1643-1650
DOI: 10.3109/10428194.2014.957203 https://doi.org/10.3109/10428194.2014.957203 PMid:25308294 PMCid:PMC4437921
- Kutsch
N, Bahlo J, Robrecht S, Franklin J, Zhang C, Maurer C, De Silva N,
Lange L, Weide R, Kiehl MG, Sökler M, Schlag R, Vehling-Kaiser U,
Köchling G, Plöger C, Gregor M, Plesner T, Herling M, Fischer K, Döhner
H, Kneba M, Wendtner CM, Klapper W, Kreuzer KA, Böttcher S,
Stilgenbauer S, Fink AM, Hallek M, Eichhorst B. Long term follow-up
data and health-related quality of life in frontline therapy of fit
patients treated with FCR versus BR (CLL10 trial of the GCLLSG).
HemaSphere. 2020; 4:e336.DOI: 10.1097/HS9.0000000000000336 https://doi.org/10.1097/HS9.0000000000000336 PMid:32072150 PMCid:PMC7000471
- Awan
FT , Addison D, Alfraih F , Baratta SJ , Campos RN , Cugliari MS, Goh
YT , Ionin VA , Mundnich S, Sverdlov AL , Tam C, Ysebaert L.
International consensus statement on the management of cardiovascular
risk of Bruton's tyrosine kinase inhibitors in CLL Blood Adv. 2022
27:6:5516-5525DOI: 10.1182/bloodadvances.2022007938 https://doi.org/10.1182/bloodadvances.2022007938 PMid:35790105 PMCid:PMC9631706
- Koehler
AB, Leung N, Call TG, Rabe KG, Achenbach SJ, Ding W, Kenderian SS, Leis
JF, Wang Y, Muchtar E, Hayman SR, Hampel PJ, Finnes HD, Schwager SM,
Slager SL, Kay NE, Parikh SA. Incidence and risk of tumor lysis
syndrome in patients with relapsed chronic lymphocytic leukemia (CLL)
treated with venetoclax in routine clinical practice. Leuk Lymphoma.
2020; 61: 2383-2388 doi: 10.1080/10428194.2020 https://doi.org/10.1080/10428194.2020.1768384 PMid:32449401
- Langerbeins
P, Hallek M. COVID-19 in patients with hematologic malignancy.Blood.
2022;140:236-252. DOI: 10.1182/blood.2021012251 https://doi.org/10.1182/blood.2021012251 PMid:35544585 PMCid:PMC9098396
- Munir
T, Brown JR, O'Brien S, Barrientos JC, Barr PM, Reddy NM, Coutre S, Tam
CS, Mulligan SP, Jaeger U, Kipps TJ, Moreno C, Montillo M, Burger JA,
Byrd JC, Hillmen P, Dai S, Szoke A, Dean JP, Woyach JA. Final analysis
from RESONATE: up to six years of follow-up on ibrutinib in patients
with previously treated chronic lymphocytic leukemia or small
lymphocytic lymphoma. Am J Hematol. 2019; 94: 1353-1363. doi:
10.1002/ajh.25638. https://doi.org/10.1002/ajh.25638 PMid:31512258 PMCid:PMC6899718
- Jurczak W, Pluta A, Wach M, Lysak D, Kozak T, Šimkovič M, Kaplan P, Kraychok I, Illes A, de la Serna j, Dolan S, Campbell P, Musuraca G, Jacob A, Avery E, Lee JH, Liang W, Patel P, Quah C, Jurczak W. Three-year follow-up of the ascend trial: acalabrutinib vs Rituximab plus idelalisib or bendamustine in relapsed/refractory chronic lymphocytic leukemia. Blood. 2021; 138:393. DOI: 10.1200/JCO.19.03355 https://doi.org/10.1182/blood-2021-146570 PMID:32459600
- Ghia
P, Pluta A, Wach M, Lysak D, Kozak T, Simkovic M, Kaplan P, Kraychok I,
Illes A, de la Serna J, Dolan S, Campbell P, Musuraca G, Jacob A, Avery
E, Lee JH, Liang W, Patel P, Quah C, Jurczak W W. ASCEND: phase III,
randomized trial of acalabrutinib versus idelalisib plus Rituximab or
bendamustine plus Rituximab in relapsed or refractory chronic
lymphocytic leukemia. J Clin Oncol. 2020; 38: 2849-2861. DOI:
10.1200/JCO.19.03355 https://doi.org/10.1200/JCO.19.03355 PMid:32459600
- Hillmen
P, Eichhorst B, Brown JR, Lamanna N, O'Brien SM, Tam CS, Qiu L,
Kazmierczak M, Zhou K, Šimkovič M, Mayer J, Gillespie-Twardy A, Shadman
M, Ferrajoli A, Ganly PS, Weinkove R, Grosicki S, Mital A, Robak T,
Österborg A, Yimer HA, Salmi T, Ji M, Yecies J, Idoine A, Wu K, Huang
J, Jurczak W. Zanubrutinib versus ibrutinib in relapsed/refractory
chronic lymphocytic leukemia and small lymphocytic lymphoma: interim
analysis of a randomized phase III. J Clin Oncol 2023; 41: 1035-1045.
DOI: 10.1200/JCO.22.00510 https://doi.org/10.1200/JCO.22.00510 PMid:36395435 PMCid:PMC9928683
- Seymour
JF, Kipps TJ, Eichhorst B, Hillmen P, D'Rozario J, Assouline S, Owen C,
Gerecitano J, Robak T, De la Serna J, Jaeger U, Cartron G, Montillo M,
Humerickhouse R, Punnoose EA, Li Y, Boyer M, Humphrey K, Mobasher M,
Kater AP. Venetoclax-Rituximab in Relapsed or Refractory Chronic
Lymphocytic Leukemia. N Engl J Med. 2018;378:1107-20. DOI:
10.1056/NEJMoa1713976 https://doi.org/10.1056/NEJMoa1713976 PMid:29562156
- Stilgenbauer
S, Eichhorst B, Schetelig J, Hillmen P, Seymour JF, Coutre S, Jurczak W
, Mulligan SP, Schuh A, Assouline S, Wendtner CM, Roberts AW, Davids
MS, Bloehdorn J, Munir T, Böttcher S, Zhou L, Salem AH, Desai M, Chyla
B, Arzt J , Kim SY, Verdugo M, Gordon G, Hallek M , Wierda WG .
Venetoclax for patients with chronic lymphocytic leukemia with 17p
deletion: results from the full population of a phase II pivotal trial.
J Clin Oncol. 2018; 36: 1973-1980. DOI: 10.1200/JCO.2017.76.6840 https://doi.org/10.1200/JCO.2017.76.6840 PMid:29715056
- Stilgenbauer
S, Tausch E, Roberts AW, Davids MS, Eichhorst B, Hallek M, Hillmen P ,
Schneider C , Schetelig J , Böttcher S , Kater AP , Jiang Y , Boyer M ,
Popovic R, Ghanim MT , Moran M , Sinai WJ , Wang X , Mukherjee N ,
Chyla B , Wierda WG , Seymour JF. Six-year follow-up and subgroup
analyses of a phase 2 trial of venetoclax for del(17p) chronic
lymphocytic leukemia Blood Adv.2024;8:1992-2004 doi:
10.1182/bloodadvances.2023011741.https://doi.org/10.1182/bloodadvances.2023011741 PMid:38290108 PMCid:PMC11024923
- Hampel
PJ, Parikh SA. Chronic lymphocytic leukemia treatment algorithm Blood
Cancer J. 2022.; 12:161. DOI: 10.1038/s41408-022-00756-9 https://doi.org/10.1038/s41408-022-00756-9 PMid:36446777 PMCid:PMC9708674
- Byrd
JC, Hillmen P, Ghia P, Kater AP , Chanan-Khan A , Furman RR , O'Brien S
, Yenerel MN , Illés A , Garcia-Marco NK , Mato A, Pinilla-Ibarz J,
Seymour JF , Lepretre S , Stilgenbauer S , Robak T , Rothbaum W , Izumi
R , Hamdy A , Patel P, Higgins K, Sohoni S, Jurczak W . Acalabrutinib
versus ibrutinib in previously treated chronic lymphocytic leukemia:
results of the first randomized phase III trial. J Clin Oncol. 2021;
39: 3441-3452. DOI: 10.1200/JCO.21.01210 https://doi.org/10.1200/JCO.21.01210 PMid:34310172 PMCid:PMC8547923
- Eichhorst
B , Ghia P , Niemann CU , Kater AP , M Gregor M , Hallek M, Jerkeman M,
C Buske C. ESMO Clinical Practice Guideline interim update on new
targeted therapies in the first line and at relapse of chronic
lymphocytic leukaemia .Ann Oncol.2024;35:762-768. doi:
10.1016/j.annonc.2024.06.016. https://doi.org/10.1016/j.annonc.2024.06.016 PMid:38969011
- Mato
AR, Shah NN, Jurczak W, Cheah CY, Pagel JM, Woyach JA, Fakhri B, Eyre
TA, Lamanna N, Patel MR, Alencar A, Lech-Maranda E, Wierda WG, Coombs
CC, Gerson JN, Ghia P, Le Gouill S, Lewis DJ, Sundaram S, Cohen JB,
Flinn IW, Tam CS, Barve MA, Kuss B, Taylor J, Abdel-Wahab O, Schuster
SJ, Palomba ML, Lewis KL, Roeker LE, Davids MS, Tan XN, Fenske TS,
Wallin J, Tsai DE, Ku NC, Zhu E, Chen J, Yin M, Nair B, Ebata K,
Marella N, Brown JR, Wang M. Pirtobrutinib in relapsed or refractory
B-cell malignancies (BRUIN): a phase 1/2 study. Lancet.
2021;397:892-901. DOI: 10.1016/S0140-6736(21)00224-5 https://doi.org/10.1016/S0140-6736(21)00224-5 PMid:33676628
- Furman RR, Sharman JP , Coutre SE , Cheson BD, Pagel JM, Hillmen P , Barrientos JC , Zelenetz AD , Kipps TJ , Flinn I , Ghia P , Eradat H , Ervin T , Lamanna N , Coiffier B , Pettitt AR, Ma S , Stilgenbauer S , Cramer P , Aiello M , Johnson DM , Miller LL , Li D , Jahn TM , Dansey RD , Hallek M , O'Brien SM. Idelalisib and Rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014 13;370:997-1007 DOI: 10.1056/NEJMoa1315226 https://doi.org/10.1056/NEJMoa1315226 PMid:24450857 PMCid:PMC4161365