Importance of Circulating Tumor Plasma Cells (CTPCs). At diagnosis, CTPSs are routinely quantified in peripheral blood by morphology but can be evaluated with much more sensitivity and precision by flow cytometry. Studies carried out using low-sensitivity methods of evaluation of CTPSs have shown a PB involvement by CTPCs of 19-37% in MGUS, 50-75% in Multiple Myeloma (MM), and 100% in plasma cell leukemia (PCL).[2] Using flow cytometry for CTPC detection, 59% of MGUS, 100% of Smoldering MM, and 100% of MM have detectable CTPCs.[2] This study also showed that a higher number of CTPCs in PB was associated with higher levels of bone marrow infiltration and more adverse prognostic features in MM and with shorter time to progression to MM in SMD patients.[2]
The presence and particularly the number of CTPCs was considered a possible negative prognostic factor for MM patients; however, no association was detected between CTPC levels and response to induction treatment.[2] The presence of more than 20% clonal plasma cells by differential count of the leucocytes or by counting more than 2 × 109 per liter in peripheral blood characterizes plasma cell leukemia. However, patients with lower levels of circulating plasma cells have the same adverse prognosis, which challenges the disease definition.[3] However, several recent studies have shown that CTPCs above some levels are associated with reduced PFS in MM patients,[4-11] and to define PCL, the percentage of CTPCs has been lowered. Levels of CTPCs were evaluated by next-generation flow cytometry, and detectable levels were observed in 86.8% of cases; higher levels of CTPCs (>0.1% of all PBMCs) were strongly correlated with an increased BM infiltration by myeloma cells, with ISS-3 stage disease and with the presence of high-risk cytogenetics t(4;14), t(14;16) and del(17p); furthermore, there was a correlation between higher CTPC levels and high serum creatinine levels.[4] Finally, it was observed that there is a trend for inferior PFS in patients with high CTPCs.[5] Low cutoffs, different according to the methods utilized, have been defined as optimal to stratify MM patients.[5-8]
Garcés et al., using the data from GEM2012MENOS65 and GEM2014MAIN clinical trials,[5] and Bertamini et al., using the results from the FORTE trial,[6] have explored the clinical significance of CTPCs and have defined optimal cutoffs, respectively, of 005% and 001%, to stratify MM patients eligible for transplantation. Furthermore, patients with undetectable CTPC levels had very good PFS and MRD status.[5,7] Interestingly, CTPC levels, but not the bone-marrow plasma-cell levels, affected the outcome.[6]
These results were confirmed by Kostopoulos and coworkers, who defined the level of 2x10-4 CTPC, corresponding at about 0.01%, as a reliable cutoff to distinguish high CTPC and low CTPC patients, with the high group exhibiting a significantly shortened progression-free survival (PFS) compared to the low group.[7] In a more recent report updated to 550 MM patients, the same authors have better defined the cutoff of CTPC level at 0.02% and have shown that about 10% of patients with undetectable CTPC levels have a particularly favorable prognosis with a 5-yr PFS and OS of 83% and 97%, respectively and with an achievement of MRD-negativity of 73% after 2-year of treatment.[8]
Jelinek and coworkers have assessed the levels of CTPCs by multiparameter flow cytometry in 395 patients with newly diagnosed MM not eligible for transplantation; patients with CTPCs comprised in the range between 2% and 20% represent about 4% of the whole cohort and displayed shorter PFS compared with patients with <2% CTPCs (3.1 vs 15.6 months, respectively), as well as shorter OS (14.6 vs 33.6 months, respectively).[8] Patients in the 2-20% group had a higher frequency of ISS III stage, elevated LDH levels, and higher frequency of high-risk cytogenetic abnormalities.[9] These observations were also extended to a group of MM patients eligible for transplantation, showing reduced PFS and OS in the group of patients with 2-20% CTPCs compared to those with <2% CTPCs.[9] Finally, patients with 2-20% CTPCs have comparable prognosis with respect to patients with primary PCL.[9] In conclusion, this study showed that a cutoff of CTPCs allowed defining a subgroup of ultrahigh-risk MM patients.[9] All these observations strongly support the inclusion of CTPC evaluation by flow cytometry as a standard part of diagnostic workup of MM patients.[8]
The inclusion of the evaluation of CTPCs at diagnosis by flow cytometry into the standard-risk assessment may improve the identification of high-risk patients, optimizing their treatment as shown by recent studies.[10,11]
All these investigations suggest that plasma cell independence from the bone marrow microenvironment represents a major evolutionary step in disease biology, and accordingly. This characteristic is common in a different way to the two disease entities of PCL and EMD, and both PCL and EMD are included in the group of high-risk and ultrahigh-risk multiple myeloma.[1,12] The revised IMWG definition of PCL requires ≥5% circulating plasma cells (CPCs).[3,12] However, the spectrum of risk exists below this threshold. It has been recognized with sensitive flow cytometric assays, ranging from low risk with no detectable CPCs to HR with many CPCs.[13]
CPCs are also found more frequently detected by flow cytometry in extramedullary plasmacytoma (EMP) patients than in the other forms of myeloma and worsen their prognosis.[13-15]
Extramedullary Multiple Myeloma
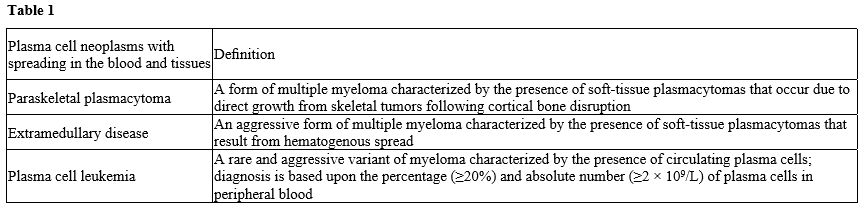
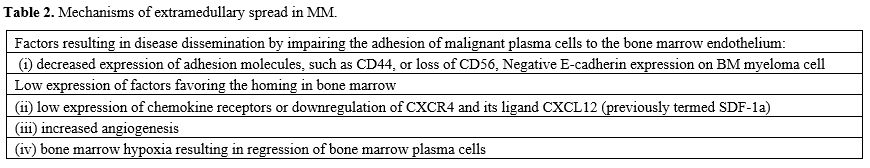
Extramedullary myeloma disease (EMD) is a rare manifestation of multiple myeloma (MM) characterized by the proliferation of malignant plasma cells outside the bone marrow. It is traditionally considered as a group of patients associated with poor prognosis. However, functional whole-body techniques should ideally be used to detect EMD. A consensus statement by the International Myeloma Working Group (IMWG) specifically recommends 18F-FDG PET/CT for this purpose; magnetic resonance (MRI) is the best imaging approach for spinal and central nervous system (CNS) involvement.[15] The condition of EMD can be diagnosed at the time of primary diagnosis and is defined as primary EMD (pEMD) (3-5%) or at the time of disease relapse and is defined as secondary EMD (sEMD) (6-20%).[14,15] Here, we discuss the diagnosis, molecular abnormalities, and prognostic criteria of extramedullary multiple myeloma (EMM), also called myeloma extramedullary disease (EMD), in comparison with plasma cell myeloma (PCL). In one hypothetical model of EMD pathogenesis, metastatic myeloma cells initially exit the bone marrow, translocate into the blood as clonal circulating plasma cells (CPCs), and finally settle in peripheral tissues and form an extramedullary plasmacytoma (EMP).[1,15] The interaction between myeloma cells and the BM microenvironment activates signaling cascades and mediates chemotaxis and adhesion of myeloma cells to BM. The mechanisms of extramedullary spread in MM are not well understood. Some possible mechanisms are: (i) decreased expression of adhesion molecules, such as CD44, or loss of CD56, which could result in disease dissemination by impairing the adhesion of malignant plasma cells to the bone marrow endothelium, (ii) low expression of chemokine receptors or downregulation of CXCR4 and its ligand CXCL12 (previously termed SDF-1a), which is linked to the bone marrow homing of myeloma cells, (iii) increased angiogenesis or (iv) bone marrow hypoxia resulting in the egress of bone marrow plasma cells. Therefore, it is reasonable to say that the overexpression of CD56 on myeloma cells favors their adherence capacity within the BM while its downregulation favors the migration of myeloma cells in the PB Tumor dissemination occurs due to (i) low expression of chemokine receptors and adhesion molecules,1 (ii) under expression of membraneembedded CS81/CD82 tetraspanins and overexpression of tumor promoter heparanase enzyme, (iii) upregulation of CXCR4 by various growth factors and hypoxic conditions in tumor microenvironment and acquisition of EM phenotype regulated by CXCR4.[15-17] Furthermore, the loss of E-cadherin expression and the induction of Ncadherin are known as hallmarks of the epithelial-tomesenchymal transition, an essential initial step in the process of metastasis in solid tumors. Negative Ecadherin expression on BM myeloma cell membranes was significantly associated with the spreading of CMTC and the presence of soft tissue masses arising from bone lesions and breaking through the cortical bone, referred to as extramedullary disease (EMD) (Table 2).[18]Bladé and coworkers have distinguished two types of EMD, one with location of soft tissue masses in extraosseous sites resulting from hematogenous spreading and the other with extension of malignant plasma cells to contiguous soft tissues through disruption of cortical: (i) the first is defined as extramedullary disease (EM-E) and (ii) the second as paraskeletal plasmacytoma (EM-B), about 80% of the EMD are paraskeletal.[15] At diagnosis, EM-E is typically found in skin and soft tissues; at relapse, typical sites are represented by the liver, kidneys, lymph nodes, central nervous system, breast, pleura, and pericardium.[15-17] In a recent review of the literature, Bansal et al. proposed a classification of EMD into three subgroups: (i) boneassociated EMD with MM with soft tissue mass arising from bone lesions and growing contiguously; (ii) boneindependent EMD with MM, with isolated extra-osseous plasma cell tumors not contiguous with bone lesions; (iii) organ-infiltrating EMD with CNS myeloma, diffuse liver involvement or other extra-osseous tissues.[17]
EMD may occur alongside MM diagnosis in the later stages of disease development or in relapse. The EMD is usually observed with concomitant involvement of bone marrow and extramedullary sites, but in some patients, there is involvement of multiple extramedullary sites without bone marrow involvement. EMD, particularly in the form of EM-E, is considered a high-risk factor associated with reduced OS compared to MM without EMD.[1,11,15]
The reported incidence of EMD varies considerably in different studies, and this is due also to the differences in the diagnostic approach; thus, in newly diagnosed MM, the reported incidence varies from 0.5% to 4.8%, and in relapsed MM from 3.4% to 14%.[15] However, at least another 20% of patients develop EMD during their disease course.[15] The paraskeletal forms bone-associated (EM-B) are at diagnosis 2-4 times more frequent than the forms bone independent, Their prognosis tends to be like that of myeloma without extramedullary disease; on the contrary, the subgroup with hematogenous spreading and localizing the soft tissue has a very bad prognosis.[15,17] The presence of Circulating Tumor Plasma Cells worsens the prognosis.[11,13]
Studies Defining Cytogenetic and Molecular Abnormalities in Emm (EMD)
Primary genetic events involved in MM include immunoglobulin heavy chain gene translocations and hyperdiploidy. In general, patients with translocations t(4;14), t(14;16), and t(14;20) are considered high-risk, whereas patients with t(11;14) are considered standard-risk and have a better prognosis. As MM progresses, secondary genetic aberrations develop, including mutations and copy number abnormalities, del(13q), del(17p), del(1p), and gain of 1q.[19] A few studies have defined the cytogenetics[20-28] and molecular[29-34] abnormalities observed in EMD, many of them are high risk, such as t(4;14), del(17p13), del(13) and chromosome 1 aberrations and p53 mutations (Table 3).High-risk cytogenetic abnormalities were generally more frequent in EMD patients than in patients with MM. High risk translocations like t(4;14), t(14;16), or t(14;20) were considered primary genetic events, and C-MYC translocation and also IgH translocations were found more frequently in myeloma patients with EMM. The same was true for some secondary genetic aberrations. Deletion of 17p was found more frequently (>30%) in patients with EMM than in those without EMM,[20,21,22,24,36] and its frequency was higher in EM-E subjects than in EM-B.[23] Similarly, more frequent in EMM was the 13q deletion (Table 3).[20,21,23,25,34]
Gains of 1q and 1p deletion were also more frequent in patients with EMM than without, particularly in relapse.[24,28,34,36] The deleted or mutated P53 gene was more frequent in bone marrow cells of myeloma patients with EMM.[26,29,35,36] Hyperploidity was less frequent in EMM,[34] and no MM patient with t(11;14) developed EMM.[20] The presence of high risk cytogenetic aberration, like t(4;14) could be predictive of the evolution of MM in EMM.
The mutations characterizing the EMM belong mostly to the RAS family,[30-36] with a prevalence of BRAF genes.[30,34,36] Although the presence of high-risk cytogenetic aberrations is elevated, it does not completely justify the bad prognosis of MM with EM-E, which is itself an independent risk factor that should be included among the risk factors.[10,11] It is noteworthy that although there is no difference in the presence of high risk cytogenetic aberrations, there is a difference in survival between the two forms of EMM, EM-E, and EM-B.[22,23]
Besse et al. explored cytogenetic abnormalities of 31 EM patients by FISH analysis. Of these, 16 patients had soft tissue-related EM, and 15 had bone-related EM. In these patients, 25 samples of bone marrow plasma cells (BMPCs) and 18 samples of tumor plasma cells were examined with FISH for del(13q14), 14q32 disruptions, Del(17)(p13), (4;14)(p16;q32), (14;16)(q32;q23), Gain(1)(q21), Hyperdiploidy; there was no significant difference in the frequency of these abnormalities, apart from a significant major frequency of hyperdiploidy in BMTPCs and a not significant increase of t(14;16) in extramedullary plasma cell; however, in the 12 EMM patients where TPCs were examined in paired BM and EMD samples, also these differences were not significant.
The frequency of genomic events was increased in patients at the time of EMD diagnosis compared to MM samples obtained prior to EMD evolution.[23]
Kriegova et al. have used whole-genome optical mapping to explore the genomic architecture of EMM; this technique shows a significant advantage in detecting small and large structural rearrangements as well as complex rearrangements across the whole genome that are undetectable using traditional methods.[25] Large intrachromosomal rearrangements within chromosome 1 were detected in all EMM samples. These rearrangements predominantly involve deletions without or with inversions, englobe hundreds of genes, and determine copy number alterations encompassing large regions of chromosome 1. Compared to MM, EMM displayed more deletions and fewer intrachromosomal translocations; finally, 2/7 of the EMM analyzed displayed copy number loss in the 17p 13 region.[25]
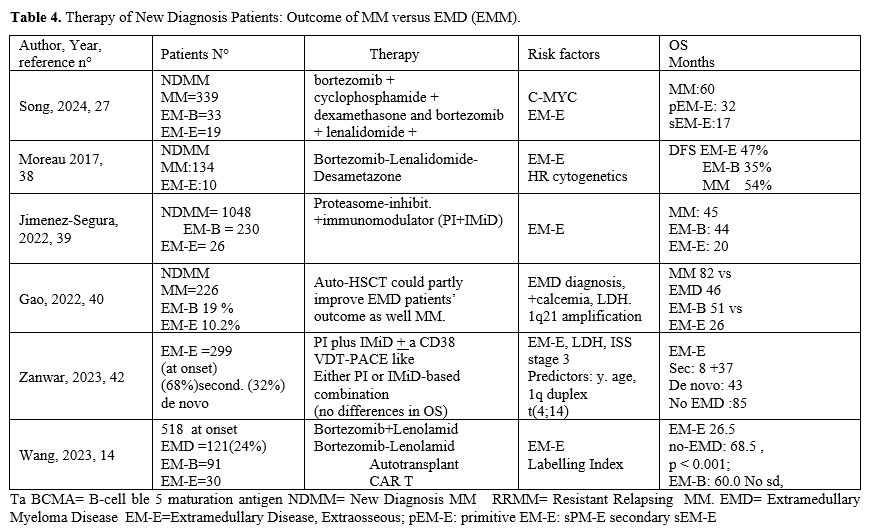
Song et al. have analyzed a total of 439 patients with NDMM, divided into those without EMD (non-EMD, n = 339), those with EMD with primary para-osseous plasmacytoma (pEMD-B, n = 48), those with primary EMD with soft-tissue involvement (pEMD-S, n = 33), and those with secondary EMD (sEMD, n = 19).[27] The incidence of EMD was 18.5% (81/439) at diagnosis and 22.8% (100/439) throughout the disease course. Comparison of FISH results showed a higher proportion of RB1 deletion (n = 20; 60.0% vs. 20.0%, p = .013) and MYC translocation (n = 12; 44.4% vs. 12.5%, p = .041) in the extramedullary tissues than in the paired bone marrow samples. At diagnosis, the percentage of MYC translocations in the sEMD group was notably higher than that in the non-EMD group (55.6% vs. 15.5%, p = .012). The median overall survival (OS) of patients with pEMD-S (32 months) and sEMD (17 months) was significantly shorter (both p = .001) than that of non-EMD patients (60 months).[27]
The mutations of the genes of the RAS family have been described by a few authors.[30-34,37] These mutations seem to be more frequent in the extramedullary tissues and secondary EMM after more relapses[33,34] and can coexist with other mutations, like TP53[33] and with chromosome 1 abnormalities;[33,34] it is noteworthy that the prognosis of patients with RAS family mutations is worse in patients with EMM than in MM, particularly when associated to chromosome 1 abnormalities.[33,34]
Furthermore, Jelinek and coworkers, in the transcriptomic analysis of EMM cells, suggest a higher proliferation and decreased homing to bone marrow (downregulation of CXCR4) compared to MM cells.[34] Importantly, EMM samples displayed reduced expression of immunotherapeutic targets, such as CD38, SLAMF7, and GPRC5D.[34]
Significantly, mice with deletion of the X-linked Utx associated with the activating BrafV600E mutation developed MM-like neoplasms, expanding its clonal plasma cells both in the bone marrow and extramedullary organs.[35]
Circulating tumor DNA (ctDNA) profiling could be adopted as an alternative to MM bone marrow aspirates for the evaluation of genetic abnormalities.[37] The analysis of 8 EMM patients using ctDNA showed that 100% of these patients exhibited RAS pathway mutations: 5/8 KRAS, 2/8 NRAS, and 1/8 BRAF mutations.[36] Interestingly, BRAFV600E mutation, in cooperation with UTX gene inactivation in germline center B cells, promotes the development of MM with extramedullary disease.[37] 7.1%). All these data suggest that RAS pathway mutations play an important role in the development of EMD (Table 3).
Prognosis and Therapy of Patients with Emm (EMD)
The prognosis of MM has very much improved with the new drugs and the immunological therapies but not at the same degree in all forms of myeloma, like the EMM.[1,15,17,27] Considering that the old therapies have been abandoned, we will consider the late investigations, which include the new drug Bortezomib and its derivatives and immunotherapies. However, most of the studies are retrospective and report together different trials, various therapies, and patients at onset and at relapse. Furthermore, the classification of EMD follows different criteria, and there is not always a distinction between EM-E and EM-B in EMD. EMM form sometimes corresponds to EMD and other times to EME. Furthermore, the same pathology has been called by different names, and at present, EMD meaning is restricted to the soft tissues without any contact with bone marrow.In any case, all the authors who make a distinction between paraskeletal forms/bone-associated EMD (EMB) and soft/neurological tissues extramedullary forms (EM-E) find a worse prognosis in the EM-E forms either found at the onset of the diseases or at relapse.[11,13,15,17,27,38] Furthermore, the secondary forms have a very poor prognosis, less than 5 months (Table 4).[42]
At the onset of the disease, we have data from a few original articles.[27,38-42] All have found that the absence of EMD at diagnosis was an independent prognostic factor for a longer progression-free survival (PFS and OS). The difference in OS and PFS between patients with and without paraskeletal involvement was abolished by hematopoietic auto-transplant.[39,43]
Jimenez-Segura and coworkers have investigated a group of 1304 patients and observed that 19.6% of these patients at diagnosis had EMM: 17.6% EM-B and 1.9% EM-E. The only factor associated with EMM at relapse was the presence of EMM at diagnosis. In patients undergoing ASCT, only the presence of EM-E was associated with reduced OS compared to EM-B and MM; in patients not eligible for ASCT, the presence of EM-B or EM-E was associated with shorter OS.[39] Gao and coworkers explored 226 MM patients with or without EMD: 19% had EM-B and 10.2% EM-E; the OS for EMM and MM patients was 44 and 82 months, respectively; the PFS 24 and 36 months, respectively.[40]
Goldman-Mazur et al. have explored the predictors of disease progression in 1557 MM patients following primary therapy and observed that short PFS (<2 years) was associated with high-risk cytogenetics, EMD, and plasma cell labeling index.[41]
Zanwar et al. retrospectively analyzed the outcomes of 204 patients with secondary EMD, and 95 with de novo EM-E; the median OS was 0.7 years for secondary EM-E and 3.6 years for de novo EM-E; the median PFS was 2.9 months for secondary EM-E and 12.9 months for de novo EM-E.[42] A multivariate analysis showed that age at diagnosis, 1q duplication, and t(4;14) at diagnosis of MM are independent predictors of the development of secondary EMM.[42] Wang et al. explored 518 MM patients of which 121 presented with EMD; patients with EM-E had the worst PFS and OS; in contrast, EM-B patients displayed PFS and OS comparable to those observed in MM.[14] ASCT significantly improved OS of EMD patients.[14] A prognostic model comprising LDH levels, circulating plasma cells, del(17p), and the type of EMD involvement was developed.[14]
Resistant Relapsing Myeloma Patients with and Without EMD Treated with Immunotherapy
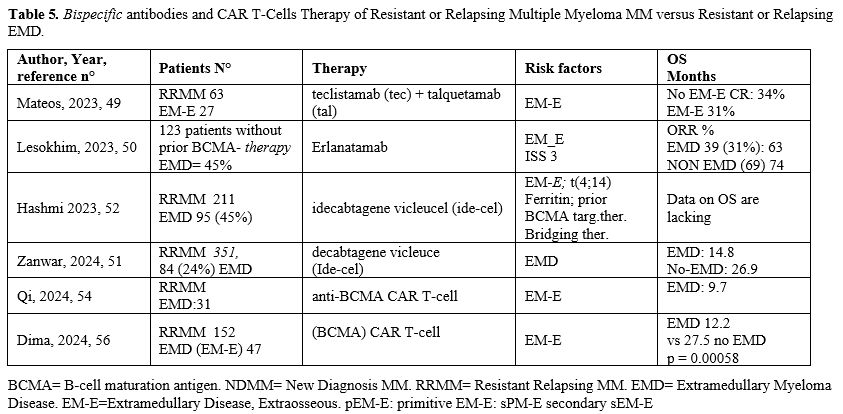
Superiority of CAR T-Cell therapy and or B-specific antibodies vs traditional drugs in resistant relapsing Myeloma (RRMM) is an acquired datum.EMD constitutes 25-45% of RRMM submitted to immune therapy with B-specific Antibodies and/or CAR T-Cells (Tables 4, 5).[15,17,43-58]
 |
|
B-specific Antibodies. In multiple myeloma (MM), bispecific T-cell engagers (BsAb) targeting B-cell maturation antigen (BCMA), G protein-coupled receptor, class C, group 5, member D (GPRC5D), and Fc receptor-like 5 (FcRL5) have already demonstrated remarkable clinical activity in triple-class refractory patients. However, responses to BsAb are not universal, and resistance often emerges while on therapy.[43]
We report the result of trials utilizing the 3 different B-specific antibodies, Taclistamab, directed against CD3 and BCMA (B-cell maturation antigen), Talquetamab against CD3 and GPRC5D (G-protein coupled receptor family C group 5 member D); Erlanatamab is, like Talquetamab directed against CD3 and BCMA, however it is a humanized antibody.
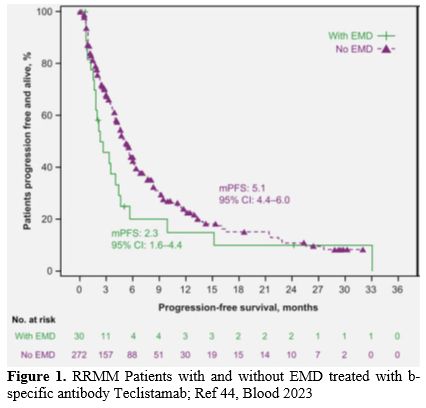
Teclistamab showed remarkable clinical activity in triple-class refractory patients in the multicenter phase I/II tMajesTEC-1 trial, including a total of 165 refractory MM patients; however, the ORR for EMM patients (17%) was 35%; significantly lower than for MM patients without EMD (63%) (Figure 1).[44,45]
 |
|
In a polycentric study of German centers, 123 patients with RRMM, treated with Teclistamab, had an ORR of 52,3% and a PFS of 8,7 months, the 43 patients (36%) with extramedullary disease had a significantly lower ORR (37%) and median PFS (2.1 months).[46]
Similarly, in a pooled analysis of outcomes of R/R patients with EMM, treated with a bispecific antibody Talquetamab, In the MonumenTAL-1 study, the ORR was significantly higher in the overall MM population than in patients with EMD (70-75% vs. 40-45%, respectively);[47] in the context of the LocoMMotion and MoMMent trials, a reduced PFS and OS compared to patients without EMM (PFS: 23 months vs 5.1 months; OS: 7.2 months vs 15.1 months, respectively) was observed.[47,48]
The first results from the RedirecTT-1 study with both the anti-BCMA-directed bispecific antibody Teclistamab (Tec) and the anti-GPRC5D-directed bispecific antibody Talquetamab (Tal) evaluated the safety and efficacy in a group of 63 R/R MM patients, 43% with EMM: ORR was 84% among all evaluable patients and 73% in those with EMD; CR rate was 31% in all evaluable patients and 33% among those with EMD.[49] Importantly, at dose 2 of Tec/Tal, the ORR was 92% in all patients and 83% in those with EMD (Table 4).[49]
In spite of the limited efficacy of all available treatments for EMD patients, it is important to note that some new agents in ongoing evaluation in MM patients have shown high rates of response among patients with extramedullary disease. Erlanatamab, a humanized bispecific antibody, after the phase I MagnetisMM-1 study that provided preliminary encouraging safety and efficacy results on R/(RRMM patients, was explored in the registrational phase II MagnetisMM-3 study involving a total of 187 R/R MM patients.[50] 31.7% of the patients have extramedullary disease and the probability of maintaining the response at 15 months in these patients was 63.8% compared to 74.6% in the group of patients without EMD (Table 4).[50]
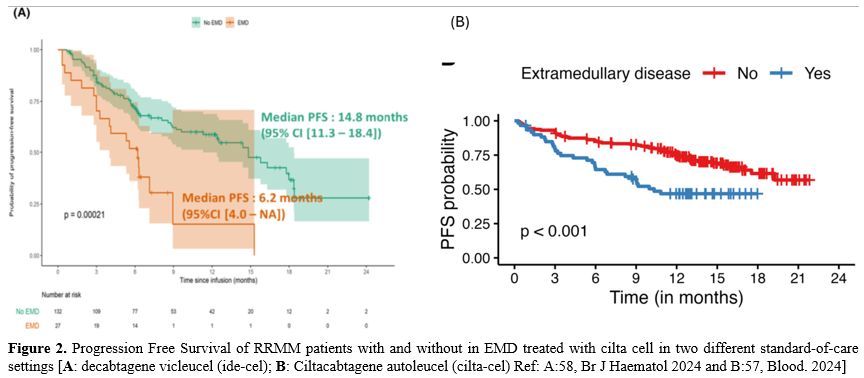
CAR-T. Idecabtagene vicleucel (Ide-Cel), a chimeric antigen receptor T-cell therapy targeting B-cell maturation antigen (BCMA), has demonstrated excellent efficacy and durable responses in patients with relapsed/refractory multiple myeloma (RRMM). However, the outcomes of Ide-Cel in patients with extramedullary disease (EMD) remain incompletely characterized. A multicenter study included patients with RRMM treated with ide-cel between May 2021 and April 2023 across 11 US academic institutions.[51] Visceral or soft tissue lesions non-contiguous from bone were classified as EMD. Time-to-event analyses were performed from the date of ide-cel infusion. Among 351 patients, 84 (24%) had EMD prior to infusion. The median follow-up from ide-cel infusion was 18.2 months (95% CI: 17-19.3). The day 90 overall response rates (ORR) were 52% vs. 82% for the EMD and non-EMD cohorts, respectively (p < 0.001). The median progression-free survival (PFS) was 5.3 months (95% CI: 4.1–6.9) for the EMD cohort vs. 11.1 months (95% CI: 9.2–12.6; p < 0.0001) for the non-EMD cohort. In a multivariable analysis, EMD was an independent predictor of inferior PFS [hazard ratio 1.5 (1.1–2.2), p = 0.02]. The median overall survival was 14.8 months [95% CI: 9-Not reached (NR)] vs. 26.9 months (26.3 vs. NR, p = 0.006) for the EMD and non-EMD cohorts, respectively. In another study,52 211 RRMM patients that received Ide-Cel, 43 (20%) of them had a progressive event ≤3 months of infusion; of 95 EMD (45%), 26 (27%) had a progressive event <3 months from infusion, while of the 116 non-EMD, only 17 (15%) progressed.[52] Therefore, extramedullary disease represents an independent predictor of inferior day 90 ORR and PFS among patients treated with Ide-Cel.
In a Chinese study[53] including 69 RRMM patients treated with combined anti-BCMA and anti-CD19 CAR T-cell, the 15 EMD (24%), with extramedullary disease, had significantly poorer PFS (median of 8.3 months [95% CI, 0.2 to 16.4] v21.4 months [95% CI, 9.2 to 33.5]) and OS (median of 12.3 months v not reached) than patients without extramedullary disease. The same group confirmed these data in a prospective study on thirty-one EMD patients.[54] Discrepancies in treatment response were noted between medullary and extramedullary diseases, with EMD exhibiting suboptimal and delayed response, as well as shortened response duration. The overall response occurred in 90.3% of medullary disease and 64.5% of EMD (p = .031) With a median follow-up of 25.3 months, the median progression-free and overall survival were 5.0 and 9.7 months, respectively. progression within 6 months post-infusion is strongly associated with an increased risk of death (HR = 4.58; p = .029). Compared with non-EMD patients, patients with EMD experienced EMD progression, primarily in the form of BCMA+ progression, with inferior survival outcomes.
Analog results were obtained in 134 patients with RRMM treated with CAR-T cell therapy, utilizing mostly ciltacabtagene autoleucel or idacabtagene vicleucel (Cita.cel); 25.5% of patients were at baseline EMM-E, and the remaining were classified as EMM-B (18.7%) or MM (56%).[55] Compared to MM patients, EMM-B patients had similar PFS and OS, while EMME patients displayed shorter PFS and OS and ORR lower compared to EMM-B or MM.[55]
Very recently in a retrospective analysis involving 152 refractory/relapsed MM patients, have been reported the results obtained with the treatment of a commercial CAR-T cells: the patients with EMM (31%) had an inferior overall survival rate (58% vs. 96%), mPFS (5.1 months vs 12.4 months) and mOS (12.2 vs 27.5 months) compared to 69% non-EMM group.[56] Importantly, patients with paramedullary disease had mPFS and mOS comparable to those observed in patients with bone marrow-only disease.[56] Similar results have been reported in 2 standard-of-care settings, one American recently reported in Blood and presented at ASH Congress 2024.[57] and the other French (Figure 2).[58] It is important to signal the high rate of ORR present also in the high risk, like HR cytogenetics and extramedullary localization. However, these HR forms relapse frequently and have reduced PFS and OS. A tentative to explain the reduced efficacy in patients with EMD has been reported in oral communication of the ASH Congress 2024.[59] This presentation reports the biodistribution of cilta-cel, labelled with Cu-64 SPION, using novel nanoparticle-based tracking technology, combined with immunological correlative studies on blood, bone marrow, and plasmacytoma biopsies, in order to elucidate the biology of resistance in extramedullary myeloma and identify opportunities for novel interventions to improve outcomes. The distribution of radioisotope at 12 hrs was 53% to the liver, 16% to the spleen, and 3% to the bone marrow. Specific uptake at the soft tissue plasmacytomas was not observed over the first 4 days. Analysis of longer-term tracking on MRI by the SPION component of the nanoparticles and correlative immunological studies on blood, marrow, and plasmacytoma biopsies is ongoing.
Similar or better results are possible with anti-GPRC5D CAR T-cell therapy. However, the number of patients treated is low; only 33 11 of them showed extramedullary localization and had an ORR identical (91%) to the patients without EMD, but there are no data about PFS or OS.[60] Thus, the G protein-coupled receptor, class C group 5 member D (GPRC5D), a membrane antigen highly expressed on malignant plasmacytes, is a promising target for MM (Table 5).
Vegivinti and coworkers have performed a systematic review and meta-analysis of all the studies carried out using bispecific antibodies or CAR-T cells for the treatment of MM patients with either extramedullary disease or with high-risk features and observed that the responses to CAR-T cell therapy were superior for both these types of patients to CAR- T cell therapy than to bispecific antibodies.[61]
However, two very recent studies suggest that the patients resistant or relapsing after CAR T-cell can respond to b-specific antibodies.[62,63] Efficacy and safety of teclistamab were tested in patients with relapsed/refractory multiple myeloma after BCMAtargeting therapies; eligible patients were triple-class exposed, including an immunomodulatory drug, a proteasome inhibitor, and an anti-CD38 monoclonal antibody, and must have also been previously exposed to an anti-BCMA treatment, either CAR-T therapy or an ADC; they had progressive, measurable disease at screening. In forty eligible patients, the ORR was 52.5% (95% CI, 36.168.5). Rates of very good partial response (VGPR) or better and CR or better were 47.5% (95% CI, 31.5-63.9) and 30.0% (95% CI, 16.6-46.5), respectively. Efficacy was similar in patients with different prior treatments. The 12 EMD patients (30%) had a similar ORR (58.3%). The PFR was not reported.[62]
Furthermore, Bispecific antibodies targeting BCMA or GPRC5D were highly effective in relapsed myeloma after CAR T-cell therapy.[63] 139 patients in relapse after (n = 130 ide-cel, n = 9 cilta-cel), received talquetamab (n = 28), teclistamab (n = 37), combinations of immunomodulating drugs (IMiDs), proteasome inhibitors (PIs) or CD38 monoclonal antibodies (n = 43), and others (n = 31). Of them 53% had the extramedullary disease (EMD). Overall response and complete response upon salvage therapies were 79% and 39% for talquetamab, 64% and 32% for teclistamab, 30% and 0% for IMiDs/PIs/CD38, and 26% and 3% for others (P < 0.001). The median OS of all patients was 9 months (95% CI, 4–14 months). Half of the total cohort (53%) showed relapse with EMD, and extramedullary relapse was significantly associated with dismal post-relapse outcome (P= 0.005), with a median OS of 5 months (95% CI, 3–7 months) versus median OS not reached for patients with non-EMD relapse.
Conclusions
At present, true extramedullary myeloma (EMD) has localization in the soft tissues, which results from hematogenic spread; it represents an aggressive form of MM, which can be found at the time of MM diagnosis or at relapse. True EMD is restricted to lasmacytomas that arise from hematogenous spread and have no contact with bony structures. The hematological spread of plasma cells is a very important factor in prognosis; more and more investigations show that the level of circulant plasma tumor cells is a very important risk factor in all forms of MM and should be added to the other factors to determine the ISS. The Paraskeletal plasmacytoma, although localized in soft tissue plasmacytomas, is due to direct growth from skeletal tumors following cortical bone disruption and has a prognosis similar to that of the classical form of MM. The new therapies have improved the prognosis only slightly, and daratumumab, an anti-CD 38 antibody, has limited efficacy in multiple myeloma with extramedullary disease[64] due to decreased CD38 expression on EMM plasma cells.[65]CAR T cells and bispecific antibodies have therapeutic activity in RRMM but less efficacy in EMD. The ORR is not always reduced, but the long-term survival benefits may be limited. The EMD-specific microenvironment potentially impacts treatment. Therefore, the EMD remains classified as ultrahigh-risk myeloma at bad prognosis.
Further efforts are needed to extend EMD remission and improve long-term outcomes.
References
- Rees MJ, D'Agostino M, Leypoldt LB, Kumar S, Weisel
KC, Gay F. Navigating High-Risk and Ultrahigh-Risk Multiple Myeloma:
Challenges and Emerging Strategies. Am Soc Clin Oncol Educ Book. 2024
Jun;44(3):e433520. doi: 10.1200/EDBK_433520. https://doi.org/10.1200/EDBK_433520 PMid:38772002
- Sanoja-Flores
L, Flores-Montero J, Garcés JJ, Paiva B, Garcia-Mateo A, Garcia-Sanchez
A, Corral-Mateos A, Burgos L, Blanco E, Hernandez-Martin J, et al. Next
generation flow cytometry for minimally invasive blood characterization
of MGUS and multiple myeloma at diagnosis based on circulating tumor
plasma cells (CTPC). Blood Cancer J 2018; 8: 117. https://doi.org/10.1038/s41408-018-0153-9 PMid:30455467 PMCid:PMC6242818
- Gundesen MT, Lund T, Moeller HEH. Plasma cell leukemia: definition, presentation and treatment. Curr Oncol Rep 2019; 21: 8. https://doi.org/10.1007/s11912-019-0754-x PMid:30689121 PMCid:PMC6349791
- Terpos
E, Kostopoulos IV, Papanota AM, Papadhimitriou K, Malandrakis P,
Michell P, Ntanasis-Stathopoulos I, Fotiou D, Metousis A, Kanellas N,
et al. Next generation flow cytometry provides a standardized, highly
sensitive and informative method for the analysis of circulating plasma
cells in newly diagnosed multiple myeloma: a single center study of 182
patients. Blood 2019; 134 (suppl.1): 4338. https://doi.org/10.1182/blood-2019-127989
- Garcés
JJ, Cedena MT, Puig N, Burgos L, Perez JJ, Cordon L, Flores-Montero J,
Sanoja- Flores L, Calasanz MJ, Ortiol A, et al. Circulating tumor cells
for the staging of patients with newly diagnosed transplant-eligible
multiple myeloma. J Clin Oncol 2022; 40: 3151-3161. https://doi.org/10.1200/JCO.21.01365 PMid:35666958
- Bertamini
L, Oliva S, Rota-Scalabrini D, Paris L, Moré S, Corradini P, Ledda A,
Gentile M, De Sabbata G, Pietrantuono G, Pascarella A, et al. High
levels of circulating tumor plasma cells as a key hallmark of
aggressive disease in transplant-eligible patients with newly diagnosed
multiple myeloma. J Clin Oncol 2022; 40: 3120-3131. https://doi.org/10.1200/JCO.21.01393 PMid:35666982
- Kostopoulos
IV, Ntanatis-Stathopoulos I, Rousakis P, Eleutherakis-Papaiakovou E,
Panteli C, Malandrakis P, Angelis N, Kanellias N, Orologas-Stavrou N,
Papanota A, et al. Circulating plasma cells in newly diagnosed multiple
myeloma: prognostic and more. J Clin Oncol 2023; 41: 708-710. https://doi.org/10.1200/JCO.22.01606 PMid:36179274
- Kostopoulos
IV, Ntanatis-Stathopoulos I, Rousakis P, Eleutherakis-Papaiakovou E,
Panteli C, Malandrakis P, Angelis N, Kanellias N, Dimitrakopoulou G,
Theodorakakou F, et al. Low levels of circulating tumor cells correlate
with favorable clinical outcome and unique biological features in newly
diagnosed multiple myeloma patients. Blood 2023; 142 (suppl.1): 880. https://doi.org/10.1182/blood-2023-186259
- Jelinek
T, Bezdekova R, Zihala D, Seccikova T, Sithara AA, Pospisilova L,
Sevcikova S, Pothakova P, Stork M, Knecktova Z, et al. More than 2% of
circulating tumor plasma cell leukemia-like multiple myeloma. J Clin
Oncol 2023 41(7):1383-1392. doi: 10.1200/JCO.22.01226 https://doi.org/10.1200/JCO.22.01226 PMid:36315921 PMCid:PMC9995102
- Chu
B, Wang Y, Shi L, Sun K, Bao L. Combination of circulating plasma cells
enhances the efficacy of R2-ISS stage system for risk classification of
newly diagnosed multiple myeloma: a single-center real-world study.
Blood 2023; 142 (suppl.1): 4740. https://doi.org/10.1182/blood-2023-181006
- Ikeda
D, Tearo T, Oura M, Uehara A, Tabata R, Narita K, Takeuchi M, Machida
Y, Matsue K, et al. A prognostic model integrating circulating clonal
plasma cells into R-ISS and PET-CT based tumor burden in
transplant-ineligible multiple myeloma. Blood 2023; 142 (suppl.1): 1972. https://doi.org/10.1182/blood-2023-189137
- Fernandez
de Larrea C, Kyle R, Rosiñol L, et al: Primary plasma cell leukemia:
Consensus definition by the International Myeloma Working Group
according to peripheral blood plasma cell percentage. Blood Cancer J
11:192, 2021 https://doi.org/10.1038/s41408-021-00587-0 PMid:34857730 PMCid:PMC8640034
- Geng
S, Wang J, Zhang X, Zhang JJ, Wu F, Pang Y, Zhong Y, Wang J, Wang W,
Lyu X, Huang Y, Jing H. Single-cell RNA sequencing reveals chemokine
self-feeding of myeloma cells promotes extramedullary metastasis. FEBS
Lett. 2020 Feb;594(3):452-465. doi: 10.1002/1873-3468.13623 https://doi.org/10.1002/1873-3468.13623 PMid:31561267
- Wang
J, Shen N, Shen X, Zhang R, Jin Y, Li J, Chen L. Survival trends and
prognostic factors of patients with newly diagnosed multiple myeloma
accompanied with extramedullary disease. Ann Med. 2023;55(2):2281657.
doi: 10.1080/07853890.2023.2281657 https://doi.org/10.1080/07853890.2023.2281657 PMid:38086395 PMCid:PMC10880573
- Bladé
J, Beksoc M, Caers J, Jurcryszyn A, von Lilienfold-Toal M, Moreau P,
Rasche L, Rosinol L, Umani SZ, Zamagni E, Richardson P. Extramedullary
disease in multiple myeloma: a systematic literature review. Blood
Cancer J 2022; 12: 45. https://doi.org/10.1038/s41408-022-00643-3 PMid:35314675 PMCid:PMC8938478
- Bladé
J, Fernández de Larrea C, Rosiñol L. Extramedullary disease in multiple
myeloma in the era of novel agents. Br J Haematol. 2015
Jun;169(6):763-5. doi: 10.1111/bjh.13384. https://doi.org/10.1111/bjh.13384 PMid:25825255
- Bansal R, Rakshit S, Kumar S. Extramedullary disease in multiple myeloma. Blood Cancer J 2021; 11: 161 https://doi.org/10.1038/s41408-021-00527-y PMid:34588423 PMCid:PMC8481260
- Hirao
M, Yamazaki K, Watanabe K, Mukai K, Hirose S, Osada M, Tsukada Y,
Kunieda H, Denda R, Kikuchi T, Sugimori H, Okamoto S, Hattori Y.
Negative E-cadherin expression on bone marrow myeloma cell membranes is
associated with extramedullary disease. F1000Res. 2022 Feb 28;11:245.
doi: 10.12688/f1000research.109551.2. https://doi.org/10.12688/f1000research.109551.2 PMid:35646332 PMCid:PMC9117942
- Cerchione
C, Usmani SZ, Stewart AK, Kaiser M, Rasche L, Kortüm M, Mateos MV,
Spencer A, Sonneveld P, Anderson KC. Gene Expression Profiling in
Multiple Myeloma: Redefining the Paradigm of Risk-Adapted Treatment.
Front Oncol. 2022 Feb 8;12:820768. doi: 10.3389/fonc.2022.820768. https://doi.org/10.3389/fonc.2022.820768 PMid:35211412 PMCid:PMC8861274
- Varga
C, Xie W, Laubach J, Ghobrial IM, O'Donnell EK, Weinstock M, Paba-Prada
C, Warren D, Maglio ME, Schlossman R, Munshi NC, Raje N, Weller E,
Anderson KC, Mitsiades CS, Richardson PG. Development of extramedullary
myeloma in the era of novel agents: no evidence of increased risk with
lenalidomide-bortezomib combinations. Br J Haematol. 2015
Jun;169(6):843-50. doi: 10.1111/bjh.13382. https://doi.org/10.1111/bjh.13382 PMid:26032514
- Rasche
L, Bernard C, Topp MS, Kapp M, Duell J, Wesemeier C, Haralambieva E,
Maeder U, Einsele H, Kno S. Features of extramedullary myeloma relapse:
high proliferation, minimal marrow involvement, adverse cytogenetics: a
retrospective single-center study of 24 cases. Ann Hematol 2012; 91:
1031-1037 https://doi.org/10.1007/s00277-012-1414-5 PMid:22286070
- Billecke
L, Murga Penas EM, May AM, Engelhardt M, Nagler A, Leiba M, Schiby G,
Kröger N, Zustin J, Marx A, Matschke J, Tiemann M, Goekkurt E,
Heidtmann HH, Vettorazzi E, Dierlamm J, Bokemeyer C, Schilling G.
Cytogenetics of extramedullary manifestations in multiple myeloma. Br J
Haematol. 2013 Apr;161(1):87-94. doi: 10.1111/bjh.12223. https://doi.org/10.1111/bjh.12223 PMid:23368088
- Besse
L, Sedlarikova L, Greslikova H, Kupska R, Almasi M, Penka M, Jelinek T,
Pour L, Pou L, Adam Z, et al. Cytogenetics in multiple myeloma patients
progressing into extramedullary disease. Eur J Haematol 2016; 97:
93-100. https://doi.org/10.1111/ejh.12688 PMid:26432667
- Qu
X, Chen L, Qiu H, Lu H, Wu H, Qiu H, Liu P, Guo R, Li J. Extramedullary
manifestation in multiple myeloma bears high incidence of poor
cytogenetic aberration and novel agents' resistance. Biomed Res Int
2015; 2015: 787809. https://doi.org/10.1155/2015/787809 PMid:25984534 PMCid:PMC4423005
- Kriegova
E, Fillerova R, Minarik J, Savara J, Manakova J, Petrackova A, Dihel M,
Balcarkova J, Krhovska P, Pika T, et al. Wole-genome optical mapping of
bone-marrow myeloma cells reveals association of extramedullary
multiple myeloma with chromosome 1 abnormalities. Sci Rep 2021; 11:
14671. https://doi.org/10.1038/s41598-021-93835-z PMid:34282158 PMCid:PMC8289962
- Xia
Y, Shi Y, Zhang J, Zhu Y, Guo R, Zhang R, Shi Q, Li J, Chen L.
Characteristics, and prognostic value of extramedullary chromosomal
abnormalities in extramedullary myeloma. Chinese Med J 2022; 135:
2500-2502. https://doi.org/10.1097/CM9.0000000000002351 PMid:36583869 PMCid:PMC9945484
- Song
Y, Du J, Jin X, Li H, Jia C, Liu Y, Li K, Zhou, Zhuang J. MYC
translocation is a valuable marker for the development and relapse of
extramedullary disease in multiple myeloma. Eur J Haematol 2024;
113(6):824-832. doi: 10.1111/ejh.14296 https://doi.org/10.1111/ejh.14296 PMid:39191670
- Gao
S, Dong F, Yang P, Chen Y, Wang Y, Wang J, Shi Y, Jing H. 1q21+ is
associated with poor prognosis in newly diagnosed multiple myeloma
patients with extramedullary disease: a retrospective study. Ann
Hematol. 2024;103(6):1979-1987. https://doi.org/10.1007/s00277-023-05588-6 PMid:38206369
- Deng
S, Xu Y, An G, Sui W, Zou D, Zhao Y, Qio J, Li F, Hao M, Qiu L.
Features of extramedullary disease of multiple myeloma: high frequency
of p53 deletion and poor survival: a retrospective single-center of 834
cases. Clin Lymphoma Myeloma Leuk 2015; 15: 286-291 https://doi.org/10.1016/j.clml.2014.12.013 PMid:25640025
- Andrulis
M, Lehners N, Capper D, Penzer R, Heining C, Huellein J, Zenz T, von
Deimiling A, Schirmacher P, Ho AD, et al. Targeting the BRAF V600E
mutation in multiple myeloma. Cancer Discov 2013; 3: 862-869. https://doi.org/10.1158/2159-8290.CD-13-0014 PMid:23612012
- De
Haart SJ, Willems SM, Mutis T, Koudijs MJ, van Blokland MT, Lokhorst
HM, de Weger RA, Minnema MC. Comparison of intramedullary myeloma and
corresponding extramedullary soft tissue plasmacytomas using genetic
mutational panel analyses. Blood Cancer J 2016; 6: e426. https://doi.org/10.1038/bcj.2016.35 PMid:27206246 PMCid:PMC4916304
- Liu
Y, Jelloul F, Zhang Y, Bhavsar T, Ho C, Rac M, Lewis NE, Cimera R, Baik
J, Sigler A, et al. Genetic basis of extramedullary plasmablastic
transformation of multiple myeloma. Am J Surg Pathol 2020; 44: 838-848. https://doi.org/10.1097/PAS.0000000000001459 PMid:32118627 PMCid:PMC7225029
- Nakamoto-Matsubara
R, Nardi V, Panaroni C, Fulzele K, Mori T, Verma R, Yee AJ, Branagan
AR, O'Donnell E, Raje NS, et al. Molecular features and clinical
outcomes of extramedullary plasmacytomas. Blood 2021; 138(suppl.1):
398-399 https://doi.org/10.1182/blood-2021-152784
- Saladarriaga
MM, Jayabalan D, Sowa A, Monge J, Rosenblaum C, Pearse R, Nievisky R,
Patel S, Bustoros M. Genomic landscape of multiple myeloma with
extramedullary disease: results from a large patient database. J Clin
Oncol 2023; 41(suppl. 16): 8058 https://doi.org/10.1200/JCO.2023.41.16_suppl.8058
- Jelinek
T, Zihala D, Sevcikova T, Anilkumar Sithara A, Kapustova V,
Sahinbegovic H, Venglar O, Muronova L, Broskevicova L, Nenarokov S,
Bilek D, Popkova T, Plonkova H, Vrana J, Zidlik V, Hurnik P, Havel M,
Hrdinka M, Chyra Z, Stracquadanio G, Simicek M, Hajek R. Beyond the
marrow: insights from comprehensive next-generation sequencing of
extramedullary multiple myeloma tumors. Leukemia. 2024
Jun;38(6):1323-1333. https://doi.org/10.1038/s41375-024-02206-w PMid:38493239 PMCid:PMC11147761
- Rizq
O, Mimura N, Oshima M, Momose S, Takayama N, Itokawa N, Koide S,
Shibamiya A, Miyamoto-Nagal Y, Rizk M, et al. UTX inactivation in
germinal center B cells promotes the development of multiple myeloma
with extramedullary disease. Leukemia 2023; 37: 1895-1907 https://doi.org/10.1038/s41375-023-01928-7 PMid:37198323 PMCid:PMC10457198
- Long
X, Xu Q, Lou Y, Li C, Gu J, Cai H, Wang D, Xu J, Li T, Xhou X, Xiao M,
et al. The utility of non-invasive liquid biopsy for mutational
analysis and minimal residual disease assessment in extramedullary
multiple myeloma. Br J Haematol 2020; 189: e45-e48. https://doi.org/10.1111/bjh.16440 PMid:32191818
- Moreau
P, Göker H, Demiroğlu H, Aksu S, Sayınalp N, Haznedaroğlu IC, et al.
Prospective evaluation of magnetic resonance imaging and
[18F]fluorodeox- yglucose positron emission tomography-computed
tomography at diagnosis and before maintenance therapy in symptomatic
patients with multiple myeloma included in the IFM/DFCI 2009 trial:
results of the IMAJEM study. J Clin Oncol. 2017;35:2911-8. https://doi.org/10.1200/JCO.2017.72.2975 PMid:28686535 PMCid:PMC5578392
- Jimenez-Segura
R, Rosinol L, Cibeira MT, Fernandez de Larrea C, Tovar N, Rodriguez-
Lobato LG, Bladé E, Moreno DF, Oliver-Caldés A, Bladé J. Paraskeletal
and extramedullary plasmacytomas in multiple myeloma at diagnosis and
at firsty relapse: 50-years of experience from an academic institution.
Blood Cancer J 2022; 12: 135. https://doi.org/10.1038/s41408-022-00730-5 PMid:36114167 PMCid:PMC9481598
- Gao
S, Li Q, Dong F, Yang P, Chen Y, Wang J; Wang Y, Jing H. Clinical
characteristics, and survival outcomes of newly diagnosed multiple
myeloma patients presenting with extramedullary disease: a
retrospective study. Leuk Res 2022; 115: 106793 https://doi.org/10.1016/j.leukres.2022.106793 PMid:35248783
- Goldman-Mazur
S, Visram A, Rajkumar SV, Kapoor P, Dispenzieri A, Lacy MQ, Gertz MA;
Buadi FK, Hayman SR, Dingli D, et al. Predictors and impact of timing
of disease progression following primary herapy in multiple myeloma.
Clin Lymphoma Myeloma Leuk 2023; 27: S2152-2160
- Zanwar
S, Ho M, Lin Y, et al: Natural history, predictors of development of
extramedullary disease, and treatment outcomes for patients with
extramedullary multiple myeloma. Am J Hematol 98: 1540-1549, 2023 https://doi.org/10.1002/ajh.27023 PMid:37421603
- Lee
H, Neri P, Bahlis NJ. Current use of bispecific antibodies to treat
multiple myeloma. 2023; Hematology, ASH Education Program: 332-339. https://doi.org/10.1182/hematology.2023000433 PMid:38066842 PMCid:PMC10727080
- Moreau
P, Garfall AL, van de Donk N, Nahi H, San-Miguel J, Oriol A, Nooka AK,
Martin T, Rosinol L, Chari A, et al. Teclistamab in relapsed or
refractory multiple myeloma. N Engl J Med. 2022 Aug 11;387(6):495-505.
doi: 10.1056/NEJMoa2203478. https://doi.org/10.1056/NEJMoa2203478 PMid:35661166 PMCid:PMC10587778
- Moreau
P, Mateos MV, Goldschmidt H, Garcia EG, Besemer B, Perez MSG, Mohty M,
Lindsey-Hill J, Kirkpatrick S, Delforge M, et al. Outcomes of patients
with extramedullary disease in triple-class exposed relapsed/refractory
multiple myeloma from the LocoMMotion + MoMMent studies. Blood 2023;
142 (suppl.1): 4698. https://doi.org/10.1182/blood-2023-181235
- Riedhammer
C, Bassermann F, Besemer B, Bewarder M, Brunner F, Carpinteiro A,
Einsele H, Faltin J, Frenking J, Gezer D, Goldman-Mazur S, Hänel M,
Hoegner M, Kortuem KM, Krönke J, Kull M, Leitner T, Mann C,
Mecklenbrauck R, Merz M, Morgner A, Nogai A, Raab MS, Teipel R, Wäsch
R, Rasche L. Real-world analysis of teclistamab in 123 RRMM patients
from Germany. Leukemia. 2024 Feb;38(2):365-371. doi:
10.1038/s41375-024-02154-5. https://doi.org/10.1038/s41375-024-02154-5 PMid:38245601 PMCid:PMC10844072
- Chari
A, Minnema MC, Berdeja BC, Oriol A, van de Donk N, Rodriguez-Otero P,
Askari E, Mateos MV, Costa LJ, Caers J, et al. Talquetamab, a
T-cell-redirecting GPRC5D bispecific antibody for multiple myeloma. N
Engl J Med 2022; 387: 2232-2244. https://doi.org/10.1056/NEJMoa2204591 PMid:36507686
- Einsele
H, Moreau P, Bahlis N, Bhutani M, Vincent L, Karlin L, Perrot A,
Goldschmidt H, van de Donk NWCJ, Ocio EM, Martinez-Lopez J,
Rodríguez-Otero P, Dytfeld D, Diels J, Strulev V, Haddad I, Renaud T,
Ammann E, Cabrieto J, Perualila N, Gan R, Zhang Y, Parekh T, Albrecht
C, Weisel K, Mateos MV. Comparative Efficacy of Talquetamab vs. Current
Treatments in the LocoMMotion and MoMMent Studies in Patients with
Triple-Class-Exposed Relapsed/Refractory Multiple Myeloma. Adv Ther.
2024 Apr;41(4):1576-1593. doi: 10.1007/s12325-024-02797-x. https://doi.org/10.1007/s12325-024-02797-x PMid:38402374 PMCid:PMC10960754
- Mateos
MV 1, Morillo D 2, Gatt M3, Sebag M 4, Kim K5, Min CK 6 et Al. 1First
results fron RedirecTT-1 study with teclistamab (tec) + taquetamab
(tal) simultaneously targeting BCMA and GPRC5D in patients (pts) with
relapsed/refractory multiple myeloma (RRMM). Hemasphere 2023 Aug
8;7(Suppl): e15362d7. doi: 10.1097/01.HS9.0000967672.15362.d7 https://doi.org/10.1097/01.HS9.0000967672.15362.d7 PMCid:PMC10428427
- Lesokhin
AM, Tomasson MH, Arnulf B, Bahlis NJ, Prince HM, Niesvizky R,
Rodriguez- Otero P, Martinez-Lopez J, Kohne G, Touzeau C, et al.
Erlanatamab in relapsed or refractory multiple myeloma: phase 2
ManetisMM-3 trial results. Nat Med 2023; 29: 2259-2267 https://doi.org/10.1038/s41591-023-02528-9 PMid:37582952 PMCid:PMC10504075
- Zanwar
S, Sidana S, Shune L, Puglianini OC, Pasvolsky O, Gonzalez R, Dima D,
Afrough A, Kaur G, Davis JA, Herr M, Hashmi H, Forsberg P, Sborov D,
Anderson LD Jr, McGuirk JP, Wagner C, Lieberman-Cribbin A, Rossi A,
Freeman CL, Locke FL, Richard S, Khouri J, Lin Y, Patel KK, Kumar SK,
Hansen DK. Impact of extramedullary multiple myeloma on outcomes with
idecabtagene vicleucel. J Hematol Oncol. 2024 Jun 6;17(1):42. doi:
10.1186/s13045-024-01555-4. https://doi.org/10.1186/s13045-024-01555-4 PMid:38845015 PMCid:PMC11157748
- Hashmi
H, Hansen DK, Peres LC, Puglianini OC, Freeman C, De Avita G, Sidana S,
Shune L, Sborov DW, Davis J, et al. Factors associated with
refractoriness of early progression after idecabtagene vivleucel in
patients with relapsed/refractory multiple myeloma: U.S. myeloma
immunotherapy consortium real world experience. Haematologica 2024;
109(5):1514-1524. doi: 10.3324/haematol.2023.283888 https://doi.org/10.3324/haematol.2023.283888
- Wang
Y, Cao J, Gu W, Shi M, Lan J, Yan Z, Jin L, Xia J, Ma S, Liu Y, Li H,
Pan B, Chen W, Fei X, Wang C, Xie X, Yu L, Wang G, Li H, Jing G, Cheng
H, Zhu F, Sun H, Sang W, Li D, Li Z, Zheng J, Xu K. Long-Term Follow-Up
of Combination of B-Cell Maturation Antigen and CD19 Chimeric Antigen
Receptor T Cells in Multiple Myeloma. J Clin Oncol. 2022 Jul
10;40(20):2246-2256. doi: 10.1200/JCO.21.01676 https://doi.org/10.1200/JCO.21.01676 PMid:35333600
- Qi
Y, Li H, Qi K, Zhu F, Cheng H, Chen W, Yan Z, Li D, Sang W, Fei X, Gu
W, Miao Y, Huang H, Wang Y, Qiu T, Qiao J, Pan B, Shi M, Wang G, Li Z,
Zheng J, Xu K, Cao J. Clinical outcomes and microenvironment profiling
in relapsed/refractory multiple myeloma patients with extramedullary
disease receiving anti-BCMA CAR T-cell-based therapy. Am J Hematol.
2024 Dec;99(12):2286-2295. doi: 10.1002/ajh.27469 https://doi.org/10.1002/ajh.27469 PMid:39194355
- Pan
D, Mouhieddine TH, Fu W, Moshier E, Parekh S, Jagannathan S, Rossi AC,
Richter J, Rodriguez C, Sanchez LJ, et al. Outcomes after CAR T cells
in multiple myeloma patients with extramedullary and paramedullary
disease. Blood 2023; 142(suppl. 1): 652 https://doi.org/10.1182/blood-2023-177749
- Dima D, Abdallah AO, Davis JA, Awada H, Goel U, Rashid A, Dejarnette S, Anwer F, Shune L, Raza S, et al. Impact of extraosseous extramedullary disease on outcomes of patients with relapsed-refractory multiple myeloma receiving standard-of-care chimeric antigen receptor T-cell therapy. Blood Cancer J 2024; 14: 90. https://doi.org/10.1038/s41408-024-01068-w PMid:38821914 PMCid:PMC11143360
- Sidana S, Patel KK, Peres LC, Bansal R, Kocoglu MH, Shune L, Atrash S, Smith K, Midha S, Ferreri CJ, Dhakal B, Dima D, Costello P, Wagner C, Reshef R, Hosoya H, Mikkilineni L, Atanackovic D, Chhabra S, Parrondo RD, Nadeem O, Mann H, Kalariya N, Hovanky V, DeAvila G, Freeman CL, Locke FL, Alsina M, Wong S, Herr MM, Htut M, McGuirk JP, Sborov DW, Khouri J, Martin T, Janakiram M, Lin Y, Hansen DK. Safety and Efficacy of Standard of Care Ciltacabtagene Autoleucel for Relapsed/Refractory Multiple Myeloma. Blood. 2024 Oct 4: blood. 2024025945. https://doi.org/10.1182/blood.2024025945 PMid:39365257
- Ferment B, Lambert J, Caillot D, Lafon I, Karlin L, Lazareth A, Touzeau C, Leleu X, Moya N, Harel S, Perrot A, Bories P, Vincent L, Lamure S, Mohty M, Malard F, Manier S, Yakoub-Agha I, Schiano De Colella JM, Brisou G, Talbot A, Decaux O, Houot R, Le Gouill S, Bigot N, Facon T, Corre J, Moreau P, Arnulf B; Intergroupe Francophone du Myélome. French early nationwide idecabtagene vicleucel chimeric antigen receptor T-cell therapy experience in patients with relapsed/refractory multiple myeloma (FENIX): A real-world IFM study from the DESCAR-T registry. Br J Haematol. 2024 Sep;205(3):990-998. https://doi.org/10.1111/bjh.19505 PMid:38747092
- Dowling,
M. R., Scott, H. W., Connor, T. L., Kelly, H., Lee, I. Y., Domingues,
M., ... & Harrison, S. J. (2024). CAR-T Cell Therapy in Advanced
Myeloma with Extramedullary Disease-an In Vivo Imaging and Molecular
Monitoring Study (CARAMEL): First Results of Cu-64 Radiolabelled
Nanoparticle PET-CT and PET-MRI. Blood, 144, 1029. https://doi.org/10.1182/blood-2024-210017
- Xia
J, Li H, Yan Z, Zhou D, Wang Y, Qi Y, Cao J, Li D, Cheng H, Sang W, et
al. Anti-G protein- coupled receptor, class C group 5 member D chimeric
antigen receptor T cells in patients with relapsed or refractory
multiple myeloma: a single-arm, phase II trial. J Clin Oncol 2023; 41:
2583-2593 https://doi.org/10.1200/JCO.22.01824 PMid:36881785 PMCid:PMC10414745
- Vegivinti
CT, Santhi J, Liu L, Keesari PR, Thakur R, Hammami MB, Kapu V,
Pericherla S, Gopireddy MM, Poojary N, et al. Efficacy of bispecific
antibodies cs CAR-T in the treatment of extramedullary disease and
high-risk cytogenetics in relapsed multiple myeloma: a systematic
review and meta-analysis. Blood 2023; 142 (suppl.1): 1994 https://doi.org/10.1182/blood-2023-190019
- Touzeau
C, Krishnan AY, Moreau P, Perrot A, Usmani SZ, Manier S, Cavo M,
Martinez Chamorro C, Nooka AK, Martin TG, Karlin L, Leleu X, Bahlis NJ,
Besemer B, Pei L, Stein S, Wang Lin SX, Trancucci D, Verona RI, Girgis
S, Miao X, Uhlar CM, Chastain K, Garfall AL. Efficacy and safety of
teclistamab in patients with relapsed/refractory multiple myeloma after
BCMA-targeting therapies. Blood. 2024 Dec 5;144(23):2375-2388. doi:
10.1182/blood.2023023616. https://doi.org/10.1182/blood.2023023616 PMid:39172760
- Merz
M, Dima D, Hashmi H, Ahmed N, Stölzel F, Holderried TAW, Fenk R, Müller
F, Tovar N, Oliver-Cáldes A, Rathje K, Davis JA, Fandrei D, Vucinic V,
Kharboutli S, Baermann BN, Ayuk F, Platzbecker U, Albici AM, Schub N,
Schmitz F, Shune L, Khouri J, Anwer F, Raza S, McGuirk J, Mahmoudjafari
Z, Green K, Khandanpour C, Teichert M, Jeker B, Hoffmann M, Kröger N,
von Tresckow B, de Larrea CF, Pabst T, Abdallah AO, Gagelmann N.
Bispecific antibodies targeting BCMA or GPRC5D are highly effective in
relapsed myeloma after CAR T-cell therapy. Blood Cancer J. 2024 Dec
5;14(1):214. doi: 10.1038/s41408-024-01197-2. https://doi.org/10.1038/s41408-024-01197-2 PMid:39632797 PMCid:PMC11618392
- Jelinek
T, Sevcikova T, Zihala D, Popkova T, Kapustova V, Broskevicova L,
Capkova L, Rihova L, Bezdekova R, Sevcikova S, et al. Limited efficacy
of daratumumab in multiple myeloma with extramedullary disease.
Leukemia 2022; 36: 288-291 https://doi.org/10.1038/s41375-021-01343-w PMid:34247197
- Notarfranchi
L, Accardi F, Mancini C, Martella E, Bonomini S, Segreto R, Vescovini
R, Dalla Palma AB, Sammarelli G, Todaro G, et al. CD38 expression by
plasma cells in extramedullary multiple myeloma. Haematologica 2024;
109(4):1297-1300. doi: 10.3324/haematol.2023.284169 https://doi.org/10.3324/haematol.2023.284169 PMid:37941401