Here we report on an 82-year-old man admitted to the surgery division of our Hospital on Aug 15, 2024. The patient had signs and symptoms of bowel obstruction, including nausea, vomiting, abdominal distension, and obstipation without fever. A computed tomography (CT) scan and X-rays of the abdomen excluded the suspicion of sub-occlusive accident events. Initial laboratory results included a leukocyte count of 9,600 with 89% neutrophils. Hemoglobin was 11.3 g/dl. Electrolytes were normal. Glucose was 146 mg/dl. Transaminases were mildly elevated, and coagulation tests were normal, as well as C-reactive protein. Empirical therapy with Piperacillin tazobactam was started at a dose of 4,5 g three times a day. The patient's medical history showed diabetes and peripheral vasculopathy. He also referred a contact with mosquitos two weeks before, and a bright red fleck from an insect bite was visible on his right thigh at the clinical evaluation. High fever began within a few hours of the hospital admission. He remained alert and oriented but appeared to have improvised psychomotor agitation, combative, and stiffness of upper limbs. An urgent brain CT scan was performed, and the result was negative. He was then transferred to the Infectious Diseases Unit, where a lumbar puncture was performed. CSF measurements were readily available compared to blood measurements for glucose, protein, and white and red blood cells. Despite normal intracranial pressure, elevated white blood cells (367 x 106/L), glucose (6,11 mmol/L), and hyperprotidorrachia (12.89 mmol/L) were found. The multiplex PCR system (filmarray™ Meningitis/Encephalitis Panel tests CSF) and also fungal and bacterial blood cultures were analyzed and returned negative. All the other virological tests performed on the serum were negative. Initial brain MRI demonstrated nonspecific white matter changes, and EEG showed generalized slowing, which was more prominent over the posterior regions. Therefore, in the suspicion of acute encephalitis, treatment with acyclovir (250 mg three times daily) and dexamethasone (8 mg every 6 hours) was begun. Antibiotic therapy with Vancocin (1 gr daily) in combination with Meropenem (2 g three times daily) was continued until the cultures were negative. The fever was over in three days, but his neurological condition worsened with weakness of the respiratory muscles and transient paradoxical movement of the abdominal compartment. He was rushed to the emergency room. (ICU). The patient required mechanical ventilation due to severe respiratory failure. After about four days, there was a resumption of high fever, and the patient presented signs and symptoms such as aggravation of the general condition and modification of bronchial secretion. High-resolution CT was performed, and the diagnosis of ventilator-associated pneumonia (VAP) was made according to prospectively defined criteria. A bronchoscopic specimen (PSB) was collected, and hemocultures were processed in the Bactec system. Strain identification was performed through classical biochemical methods and the Vitek 2. Acinetobacter baumannii complex was isolated from hemocolture and bronchial aspirate. Data obtained after analyzing resistance to A. baumannii strains isolated showed that antibiotic resistance was high. Cefiderocol E-test was performed and resulted in active with MIC 0,094 mg/L. The patient started Cefiderocol therapy 6 gr /die c.i.and Caspofungin for the detection of Candida Parapsilosis in the blood. One-week follow-up CSF analysis demonstrated elevated white blood cell count with lymphocytic pleocytosis. The WBC count showed 54 x 106/mmc white blood cells with an increase in the absolute number of neutrophils and lymphocytes (12.930 x 106/mmc) as well. Flow cytometry showed that 98% of lymphocytes were B cells with clonal restriction (CD19+CD20+CD23+CD5+CD43+kappa+), and the diagnosis of early-stage CLL was made. Polymerase chain reaction (PCR) for WNV returned negative from serum, CSF, and urine. ELISA first screened specific IgG and IgM. WNV serology ultimately returned positive with elevated IgM antibodies. Detectable specific IgM –antibody WNV were found in serum with index 68,90 (ELISA index WNV positivity > 5,66) and CSF (ELISA index WNV:29,63) Then, positive samples were confirmed by plaque reduction neutralization test (PRNT). A magnetic resonance imaging (MRI) of the brain was again negative and excluded the possibility of deep cerebral venous thrombosis. Hematology advice was required for the persistence of high lymphocyte count, despite the fever had disappeared. The inflammatory indices improved while his lymphocyte count remained high. The pneumonia worsened, and he was tracheotomized. The neurological condition remained serious; he did not react to the stimuli and went into a coma. Finally, he died thirty days after admission to the Hospital.
So far, the knowledge of the clinical course, the rate of central nervous system (CNS) involvement, and the outcome of WNV infection in patients with hematological malignancies is scanty, with only a few reports. Recently, Visentin and coworkers reported an Italian multicentre study on clinical features and outcomes of WNV infection in patients with lymphoid malignancies.[8] Most of the patients described were affected by CLL (13/21), and most of them had a poor outcome due to the high frequency of SNC involvement. Of relevance, at the variance of other cases, our patient was at the onset of the disease.[8] Furthermore, immunodeficiency is a hallmark of CLL, causing morbidity and mortality.[9] In fact, CLL is characterized by abnormalities in the number, phenotype, and function of immune cells.[10] Both adaptive and innate immunity are involved. Antibody production, the complement system and neutrophil, natural killer cell, and macrophage function are demonstrated to be abnormal in CLL.[9] Moreover, the T cell compartment is immunosuppressive and tumor-supportive.[9-11] As a matter of fact, infections and second primary malignancies are the clinical manifestations.[12] Finally, it has been recently demonstrated that immune deregulation in CLL may arise early in the disease development, as in our cases in which an early-stage CLL was not diagnosed until the onset of WNV infection.[13,14]
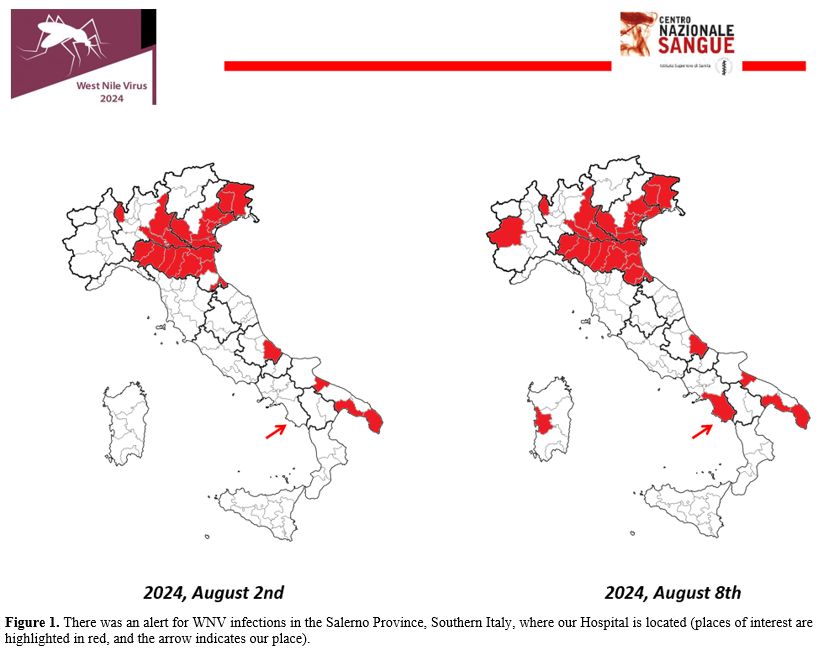
The Italian National Blood Center, belonging to the National Institute of Health, periodically sent to all Transfusional Centers of Italy a press release with an alert for the Provinces where cases of WNV infection have been identified. A temporary suspension (28 days) for blood donors who have stayed even for just one night in the places indicated is requested. Alternatively, acid nucleic testing for WNV is mandatory. Figure 1 shows that there was an alert for WNV infection in the Province of Salerno in Southern Italy, where our Hospital is located.
 |
|
This case illustrates the importance of including WNV in differential diagnosis in case of fever of unknown origin (FUO) onset in immunocompromised patients living in endemic and/or affected areas with a positive history of mosquito bites in order to start supportive care rapidly. Furthermore, despite case reports on WNV encephalitis in hematological settings being limited in number, as also demonstrated in our case, the diagnostic workup of clinicians has to take into account WNV when immunocompromised patients present with fever and neurological symptoms in endemic areas, especially in summer.[15,16] No specific prophylaxis or treatment is available against WNV infection so far. Measures to protect against mosquito bites can be used: mosquito bed nets, sleeping or resting in screened or air-conditioned rooms, wearing of cloche covering most of the body, and mosquito repellents.
In conclusion, this case emphasizes the relevance of testing for WNV infection when immunocompromised patients present with fever and neurological symptoms in endemic areas, especially in summer. Furthermore, clinicians in Italy can benefit from the National Blood Center's alerts that are issued from time to time, although they aim to indicate the local incidence of viral infections for safe donor selection.
Aknowledgements
This work was supported by a grant from the Associazione Donatori Volontari del Cilento (DVC), Italy.References
- Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med 2005; 352: 804-815. https://doi.org/10.1056/NEJMra041720 PMid:15728813
- Morrison
VA. Infectious complications of chronic lymphocytic leukaemia:
pathogenesis, spectrum of infection, preventive approaches. Best Pract
Res Clin Haematol 2010; 23: 145-153. https://doi.org/10.1016/j.beha.2009.12.004 PMid:20620978
- Fattizzo
B, Barcellini W. Autoimmune cytopenias in chronic lymphocytic leukemia:
focus on molecular aspects. Front Oncol. 2020 Jan 10;9:1435. https://doi.org/10.3389/fonc.2019.01435 PMid:31998632 PMCid:PMC6967408
- Petersen LR, Brault AC, Nasci RS. West Nile virus: review of the literature. JAMA. 2013 Jul 17;310(3):308-15. https://doi.org/10.1001/jama.2013.8042 PMid:23860989 PMCid:PMC4563989
- Zeller
H, Schuffenecker I. West Nile virus: an overview of its spread in
Europe and the Mediterranean basin in contrast to its spread in the
Americas. Eur J Clin Microbiol Infect Dis. 2004 Mar;23(3):147-56. https://doi.org/10.1007/s10096-003-1085-1 PMid:14986160
- O'Leary
DR, Kuhn S, Kniss KL, Hinckley AF, Rasmussen SA, Pape WJ, Kightlinger
LK, Beecham BD, Miller TK, Neitzel DF, Michaels SR, Campbell GL,
Lanciotti RS, Hayes EB. Birth outcomes following West Nile virus
infection of pregnant women in the United States; 2003-2004.
Pediatrics. 2006 Mar;117(3):e537-45. https://doi.org/10.1542/peds.2005-2024 PMid:16510632
- Popescu
CP, Florescu SA, Hasbun R, Harxhi A, Evendar R, Kahraman H, Neuberger
A, Codreanu D, Zaharia MF, Tosun S, Ceausu E, Ruta SM, Dragovac G,
Pshenichnaya N, Gopatsa G, Shmaylenko O, Nagy É, Malbasa JD, Strbac M,
Pandak N, Pullukcu H, Lakatos B, Cag Y, Cascio A, Coledan I, Oncu S,
Erdem H. Prediction of unfavourable outcomes in West Nile virus
neuroinvasive infection - results of a multinational ID-IRI study. J
Clin Virol. 2020 Jan;122:104213. https://doi.org/10.1016/j.jcv.2019.104213 PMid:31778945
- Visentin
A, Nasillo V, Marchetti M, Ferrarini I, Paolini R, Sancetta R, Rigolin
GM, Cibien F, Riva M, Briani C, Marinello S, Piazza F, Gherlinzoni F,
Krampera M, Bassan R, Cuneo A, Luppi M, Semenzato G, Marasca R, Trentin
L. Clinical Characteristics and Outcome of West Nile Virus Infection in
Patients with Lymphoid Neoplasms: An Italian Multicentre Study.
Hemasphere. 2020 Jun 8;4(3):e395. https://doi.org/10.1097/HS9.0000000000000395 PMid:32647801 PMCid:PMC7306307
- Forconi F, Moss P. Perturbation of the normal immune system in patients with CLL. lood. 2015 Jul 30;126(5):573-81. https://doi.org/10.1182/blood-2015-03-567388 PMid:26084672
- D'Arena
G, Rossi G, Vannata B, Deaglio S, Mansueto G, D'Auria F, Statuto T,
Simeon V, De Martino L, Marandino A, Del Poeta G, De Feo V, Musto P.
Regulatory T-cells in chronic lymphocytic leukemia and autoimmune
diseases. Mediterr J Hematol Infect Dis 2012; 4; e2012053
2012;4(1):e2012053. https://doi.org/10.4084/mjhid.2012.053 PMid:22973497 PMCid:PMC3435126
- D'Arena
G, Guariglia R, La Rocca F. Tirino S, Condelli V, De Martino L, De Feo
V, Musto P. Autoimmune cytopenias in chronic lymphocytic leukemia. Clin
Dev Immunol. 2013;2013:730131. https://doi.org/10.1155/2013/730131 PMid:23690826 PMCid:PMC3652131
- Arruga
F, Baffour Gyau B, Iannello A, Vitale N, Vaisitti T, Deaglio S. Immune
response dysfunction in chronic lymphocytic leukemia: dissecting
molecular mechanisms and microenvironmental conditions. Int J Mol Sci.
2020 Mar 6;21(5):1825. https://doi.org/10.3390/ijms21051825 PMid:32155826 PMCid:PMC7084946
- Purroy
N, Tong YE, Lemvigh CK, Cieri N, S Li, Parry EM, Zhang W, Rassenti LZ,
Kipps TJ, Slager SL, Kay NE, Lesnick C, Shanafelt TD, Ghia P, Scarfò L,
Livak K, Kharchenko PV, Neuberg DS, Olsen LR, Jan J, Gohil SH, Wu CJ.
Single-cell analysis reveals immune dysfunction from the earliest
stages of CLL that can be reversed by ibrutinib. Blood 2022; 139:
2252-2256. https://doi.org/10.1182/blood.2021013926 PMid:35020831 PMCid:PMC8990375
- Danilov AV. Immunity in CLL: corrupt at inception? Blood 2022; 139: 2104-2105. https://doi.org/10.1182/blood.2022015413 PMid:35389445
- Hollander
H, Schaefer PW, Hedley-Whyte T. Case 22-2005: An 81-year-old man with
cough, fever, and altered mental status. N Engl J Med 2005; 353:
287-95. https://doi.org/10.1056/NEJMcpc059017 PMid:16034015
- Ferrarini
I, Rigo A, Gandini A, Vinante F. West Nile encephalitis in
haematological setting: report of two cases and a brief review of
literature. Mediterr J Hematol Infect Dis 2019; 11; e2019033
10.4084/MJHID.2019.033 https://doi.org/10.4084/mjhid.2019.033 PMid:31205637 PMCid:PMC6548214