Commercially available assays measuring FXa inhibition by DOACs and heparins are all based on the same principle. However, they use different calibration curves to convert activity into concentration, meaning they are drug-specific. Therefore, the development of a standardized assay for all anti-FXa drugs is warranted.[1]
There are many recommendations regarding the laboratory assessment of DOACs,[2-6] but there is no consensus on the optimal timing for such evaluations.
We conducted a retrospective analysis of anti-factor Xa activity assays conducted at peak and trough plasma levels of DOACs at our center between Jan 1, 2023, and Aug 31, 2024, with the aim of evaluating the clinical utility of these assessments.
According to Italian consensus guidelines, the anti-factor Xa activity assays were performed with an anti-FXa chromogenic assay using Siemens reagents and certified calibrators.[5]
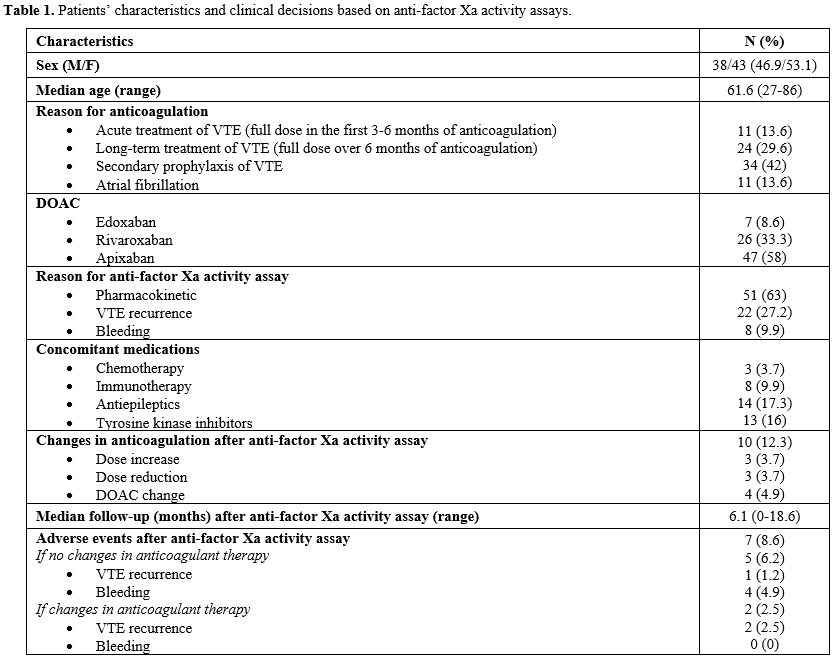
Eighty-one patients were evaluated: the reason for anticoagulation was secondary prophylaxis of VTE with low dose DOACs in 34 (42%) cases, long-term treatment of VTE over six months with full dose DOACs in 24 (29.6%) cases, acute treatment of VTE in 11 (13.6%) cases and Atrial Fibrillation in 11 (13.6%) cases. Forty-seven patients (58%) were in treatment with Apixaban, 27 (33.3%) with Rivaroxaban and 7 (8.6%) with Edoxaban. No patient resulted positive for anti-phospholipids antibodies. None of the patients required a dose adjustment based on creatinine clearance or weight.
Forty-nine (60.5%) assays were performed for a suspect of pharmacological interactions, 22 (27.2%) for VTE recurrence during anticoagulation, 8 (9.9%) for clinical relevant non-major bleeding events (CRNMBs) during DOACs therapy, 2 (2.5%) for suspect of malabsorption. As concomitant medications, we have considered the following classes of drugs: chemotherapy, immunotherapy, antiepileptics, tyrosine kinase inhibitors (TKI), and antifungals. Fourteen (17.3%) patients were in treatment with antiepileptics, 13 (16%) with TKI, 8 (9.9%) with immunotherapy and 3 (3.7%) with chemotherapy.
The anti-Xa factor activity was within the therapeutic range in 71 patients (87.7%), while it was out of range in 10 patients (12.3%). Of these 10 patients, 6 (7.4%) had values above the therapeutic range, and 4 (4.9%) had values below the range. Among the 10 patients with out-of-range results, 6 (7.4%) underwent the assay due to suspected pharmacokinetic alterations without thrombotic or bleeding adverse events, 2 (2.5%) due to bleeding events during DOAC therapy, and 2 (2.5%) due to thrombotic events during DOAC therapy.
The anticoagulation regimen was modified based on the assay results in these 10 cases. The assay was repeated after the switch in 7 patients (8.6%), and it resulted in a range.
During a median follow-up of 6.1 months (range 0-18.6 months) after the anti-Xa factor activity assay, we observed 4 (4.9%) bleeding adverse events (B-AEs) and 3 (3.7%) thrombotic (T-AEs).
Three B-AEs occurred in patients who underwent the assay for suspected pharmacological interactions and 1 in a patient who underwent the assay for a CRNMB. All four B-AEs were classified as minor according to ISTH guidelines.[7] They occurred in three patients receiving Apixaban 2.5 mg twice daily for secondary VTE prophylaxis and in one patient treated with Edoxaban 60 mg daily for acute-phase VTE. Anti-Xa activity levels were within the therapeutic range, and no adjustments were made to their treatment regimen.
The three T-AEs occurred during long-term treatment of VTE in patients who underwent the anti-Xa activity assay for VTE recurrence. One T-AE was observed in a patient receiving Apixaban 5 mg twice daily with anti-Xa factor activity in the range: he was switched to Enoxaparin 100 UI/Kg twice daily for one month and then to Rivaroxaban 20 mg daily. Two cases of T-AEs were reported in patients in therapy with Apixaban 5 mg twice daily and anti-Xa factor activity within the therapeutic range. These patients were switched to Fondaparinux 7.5 mg/day and did not resume treatment with DOACs.
In our experience, the anti-Xa factor activity assay was performed mainly for pharmacokinetic reasons (63%), mostly because of the large number of onco-hematologic patients in our center. In particular, in our cohort, two patients in treatment with TKI, one undergoing chemoimmunotherapy for onco-hematologic diseases, and three treated with immunotherapy for autoimmune diseases had out-of-range anti-Xa factor activity: none of these patients experienced T-AEs or B-AEs after the adjustment of their anticoagulation regimen.
In conclusion, more data is available regarding the utility of DOACs laboratory monitoring: one example is the recently published experience of the MAS study group[8-9] in the setting of AF. However, poor data are available regarding pharmacological interactions, and multicentric prospective clinical trials are needed.
References
- van Pelt L.J., Lukens M.V., Testa S., Chatelain B.,
Douxfils J., Mullier F. "The DaXa-inhibition assay: A concept for a
readily available, universal aXa assay that measures the direct
inhibitory effect of all anti-Xa drugs." Thromb Res. 2018
Aug;168:63-66. doi: 10.1016/j.thromres.2018.04.024. Epub 2018 Apr 30. https://doi.org/10.1016/j.thromres.2018.04.024 PMid:29909093
- Gosselin
R.C., Adcock D.M., Bates S.M., Douxfils J., Favaloro E.J.,
Gouin-Thibault I., Guillermo C., Kawai Y., Lindhoff-Last E., Kitchen S.
"International Council for Thieme E-Journals - Thrombosis and
Haemostasis / Full Text
- Tripodi
A., Ageno W., Ciaccio M., Legnani C., Lippi G., Manotti C., Marcucci
R., Moia M., Morelli B., Poli D., Steffan A., Testa S. " Position Paper
on laboratory testing for patients on direct oral anticoagulants. A
Consensus Document from the SISET, FCSA, SIBioC and SIPMeL." Blood
Transfus. 2018 Sep;16(5):462-470. doi: 10.2450/2017.0124-17. Epub 2017
Sep 13. PMID: 29106357; PMCID: PMC6125231
- Patel
J.P., Byrne R.A., Patel R.K., Arya R. "Progress in the monitoring of
direct oral anticoagulant therapy." Br J Haematol. 2019
Mar;184(6):912-924. doi: 10.1111/bjh.15756. Epub 2019 Jan 29. https://doi.org/10.1111/bjh.15756 PMid:30697708
- Paoletti
O., Legnani C., Martini G., Bertini M., Agostini P., Bondanini F.,
Cozzi M., Demicheli M., Felice G., Novembrino C., Pedrini S., Ruocco
L., Steffan A., Terzuoli L., Testa S. "Consenso sulla diagnostica di
laboratorio per i pazienti in trattamento con farmaci anticoagulanti ad
azione diretta. Sottoscritto dalla Società Italiana per lo Studio
dell'Emostasi e della Trombosi (SISET), dalla Federazione Centri per la
diagnosi della trombosi e la Sorveglianza delle terapie Antitrombotiche
(FCSA), dalla Società Italiana di Biochimica Clinica e Biologia
Molecolare Clinica (SIBioC) e dalla Società Italiana di Patologia
Clinica e Medicina di Laboratorio (SIPMeL)." La Rivista Italiana della
Medicina di Laboratorio. 15. 10.23736/S1825-859X.19.00009-4. https://doi.org/10.23736/S1825-859X.19.00009-4
- Tripodi
A., Clerici M., Scalambrino E., Agosti P., Bucciarelli P., Peyvandi F.
"How the hemostasis laboratory can help clinicians to manage patients
on oral anticoagulants." Mediterr J Hematol Infect Dis 2024, 16(1):
e2024027. https://doi.org/10.4084/MJHID.2024.027 PMid:38468834 PMCid:PMC10927209
- Kaatz
S., Ahmad D., Spyropoulos A.C., Schulman S.; Subcommittee on Control of
Anticoagulation. "Definition of clinically relevant non-major bleeding
in studies of anticoagulants in atrial fibrillation and venous
thromboembolic disease in non-surgical patients: communication from the
SSC of the ISTH." J Thromb Haemost. 2015 Nov;13(11):2119-26. doi:
10.1111/jth.13140. https://doi.org/10.1111/jth.13140 PMid:26764429
- Testa
S., Palareti G., Legnani C., Dellanoce C., Cini M., Paoletti O., Ciampa
A., Antonucci E., Poli D., Morandini R., Tala M., Chiarugi P., Santoro
R.C., Iannone A.M., De Candia E., Pignatelli P., Faioni E.M.,
Chistolini A., Esteban M.D.P., Marietta M., Tripodi A., Tosetto A.
"Thrombotic events associated with low baseline direct oral
anticoagulant levels in atrial fibrillation: the MAS study." Blood Adv.
2024 Apr 23;8(8):1846-1856. doi: 10.1182/bloodadvances.2023012408. https://doi.org/10.1182/bloodadvances.2023012408 PMid:38394387 PMCid:PMC11007438
- Palareti G., Testa S., Legnani C., Dellanoce C., Cini M., Paoletti O., Ciampa A., Antonucci E., Poli D., Morandini R., Tala M., Chiarugi P., Santoro R.C., Iannone A.M., De Candia E., Pignatelli P., Faioni E.M., Chistolini A., Esteban M.D.P., Marietta M., Tripodi A., Tosetto A. "More early bleeds associated with high baseline direct oral anticoagulant levels in atrial fibrillation: the MAS study." Blood Adv. 2024 Sep 24;8(18):4913-4923. doi: 10.1182/bloodadvances.2024013126. https://doi.org/10.1182/bloodadvances.2024013126 PMid:38842448 PMCid:PMC11421315