News in Newly Diagnosed MM Patients
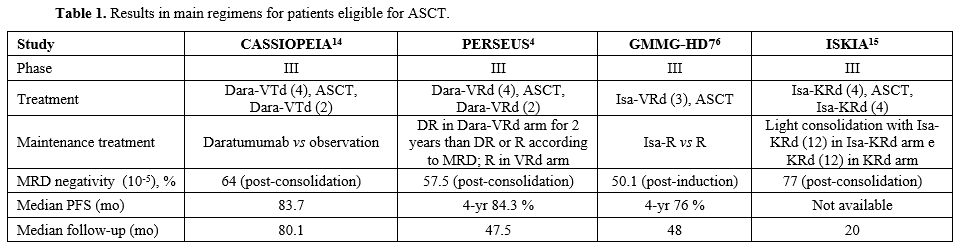
Transplant eligible patients. In the years just passed, several phase III clinical trials have confirmed already acquired data regarding the superiority of quadruplets on triplets as initial therapy in patients with Multiple Myeloma (MM) who are eligible (TE) for autologous stem cell transplant (ASCT). At the beginning of the year 2024, the phase III PERSEUS trial demonstrated a significantly improved PFS, the main endpoint of the study, in patients receiving daratumumab, bortezomib, lenalidomide, dexamethasone (Dara-VRd) induction and consolidation followed by daratumumab plus lenalidomide as maintenance vs those treated with VRd as induction and consolidation followed by lenalidomide maintenance (HR=0.42, p<0.001).[4] At the last ASH Meeting, Goldschmidt et al. reported an update of the phase III GMMG-HD7 trial comparing VRd with a quadruplet including isatuximab as an anti-CD38 monoclonal antibody (mAb) (Isa-VRd) instead of daratumumab.[5,6] TE patients were randomised to receive three 6-week cycles of VRd or Isa-VRd, and after ASCT, they were further randomly assigned for maintenance with lenalidomide alone or lenalidomide plus isatuximab. Previously, the German group reported a significantly higher MRD negativity rate (by NGF at a level of 10-5) after induction with Isa-VRd vs VRd (50.1% vs 35.6%; OR=1.83; p<0.001).[7] Notably, a significantly greater deepening in MRD response after ASCT was observed in the Isa-VRd vs VRd arm (66.2% vs 47.7%; OR=2.13; p<0.0001), although no consolidation was used after ASCT.[8] In the most recent analysis,[3,5] Authors evaluated PFS from the first randomisation, showing a 30% reduction in risk of progression or death in patients receiving Isa-VRd vs VRd after a median follow-up of 48 months (median PFS not reached in either arm). GMMG-HD7 represents a relevant study since it shows the possibility of achieving a high rate of MRD negativity with only 3 induction cycles and without consolidation after ASCT. The achievement of MRD negativity, as well as its loss, were primary topics at the last ASH Meeting. In a large dataset including 216 patients enrolled in the phase II MASTER study (receiving Dara-KRd as induction and consolidation) and those receiving Dara-VRd as standard care, Costa et al.[9] showed that, among patients who obtained MRD negativity (78%) at 10-5, 19 (11%) experienced MRD progression (MRD-P, defined as at least 1 x log10 increment of MRD burden from nadir) and 30 (18%) progression according to IMWG criteria not preceded by MRD-P. Although most patients (63%) started regimens including mAbs at MRD-P, the median time from MRD-P to progression disease was 10.1 months, and 2-year OS from MRD-P was 78% vs 56% for patients with progression disease not preceded by MRD-P, suggesting that MRD-P is driven by a plasma cell population difficult to control with anti-CD38 mAbs needing agents with new mechanisms of action. MRD assessment has also been evaluated for treatment cessation since the achievement of high rates of MRD negativity has renewed interest in fixed-duration therapy. In a dataset similar to that previously described, Giri et al.[10] aimed to define the optimal MRD-based endpoint for therapy cessation. Patients underwent MRD assessment after induction, ASCT and consolidation and yearly thereafter, with treatment cessation if MRD < 10-5 in two consecutive data points. Sustained (two assessments at least 1 year apart) MRD < 10-5 (S-MRD) was found to outperform single-point MRD and to yield the best fitting model for PFS and for progression/MRD resurgence-free survival in patients who underwent MRD-guided treatment cessation. Remarkably, among patients obtaining S-MRD and without high risk chromosomal abnormalities (HRCA), the risk of MRD resurgence or progression 2.5 years after therapy cessation was 6.2% vs 28.5% in patients with 1 HRCA and 76.3% in patients with at least 2 HRCA, showing that there is a very low-risk population in which therapy can be discontinued. Even in the post-ASCT setting maintenance, sustained MRD negativity seems to be a significant marker to guide lenalidomide discontinuation. In a prospective Greek study,[11] among 194 patients who received triplets or quadruplets as induction followed by ASCT and lenalidomide maintenance, 52 patients who had received at least 36 months of maintenance therapy had at least 3 consecutive MRD negative results and a PET/CT negative discontinued lenalidomide that was restated only in case of MRD positivity. After a median follow-up of 36 months from lenalidomide discontinuation, 12 patients (23%) converted to MRD positivity and restarted lenalidomide, and only 4 of them (7.6%) had progressive disease undergoing second-line therapy. However, a longer follow-up is awaited to better evaluate the risk of progression after MRD resurgence and to understand the role of lenalidomide retreatment in these patients. The loss of MRD negativity is conditioned by cytogenetic abnormalities, as reported by the Giri et al. study[11] in which patients with even 1 HRCA had a risk of MRD resurgence or progression of nearly 30% after therapy cessation. Among patients with even 1 HRCA enrolled in the PERSEUS trial, it is possible to identify a group of patients with high circulating tumour cells (> 0.175%) at baseline whose outcome is dismal and not improved by adding daratumumab to VRd. While in patients with HRCA and low circulating tumour cells (≤ 0.175%), 4-year PFS was 82% (vs 57% in those receiving VRd), in patients with HRCA and high circulating plasma cells, 4-year PFS was 29%, regardless of the therapy received.[12] Ultimately, phase II MASTER study[13] and phase III CASSIOPEIA,[14] PERSEUS,[4] GMMG-HD7[6] and ISKIA[15] trials demonstrated that adding mAbs as daratumumab or isatuximab to triplets as VRd or KRd induces a significant higher MRD and sustained MRD negativity rates (Table 1).Since several meta-analyses, MRD status has emerged as a significant prognostic factor for PFS and OS[16,17,18,19] in April 2024; the FDA accepted MRD-negative complete response as an early endpoint reasonably likely to predict clinical benefit in MM to be used for accelerated drug approvals.[20] MRD status, as described above, could be used to adapt therapy, optimise maintenance duration after ASCT, and, notably, use a new therapeutic strategy when an early MRD negativity loss occurs. However, quadruplet combination did not found to significantly ameliorate the outcome of some high risk groups of patients, as those with 2 or more HRCA, showing a 3-year PFS and OS of 50% and 75% vs 88% and 94%, respectively, in patients with 0 HRCA,[13] or those with HRCA and high circulating plasma cells as reported by Bertamini et al. in PERSEUS trial.[12] Alternative therapies would be needed for these patients, and one hope might come from the introduction of novel immunotherapies in the induction or maintenance therapies. Phase II MajestTEC-5 represents the first study to evaluate the combination of teclistamab, the first-in-class bispecific antibody targeting BCMA approved for advanced MM, to daratumumab-lenalidomide (DR) or VRd in TE newly diagnosed MM patients. At the last ASH Meeting, Raab[21] presented initial data from 3 cohorts, including 49 patients who received 6 induction cycles with teclistamab QW plus DR (10 patients, arm A), teclistamab Q4W plus DR (20 patients, arm A1) or teclistamab Q4W plus VRd (19 patients, arm B). All patients enrolled in arm A1 completed induction therapy, and 100% of them achieved at least CR and 100% MRD negativity, which was maintained after cycle 6. Overall, stem cell mobilisation for ASCT, planned after induction therapy, was feasible in all patients and 65.3% developed cytokine release syndrome (CRS) in all grades 1-2. The most common grade 3-4 side effects were neutropenia occurring in 57% of patients and infections in 34.7% despite no grade 5 events being reported. In the same Meeting, Zamagni[22] reported initial safety run-in results (SRI) from the phase III EMN30/MajesTEC-4 study that is evaluating maintenance therapy with teclistamab plus lenalidomide vs teclistamab vs lenalidomide after induction followed by ASCT and ± consolidation in TE newly diagnosed MM patients. Ninety-four patients in SRI were enrolled in 3 cohorts at different teclistamab dose frequencies after the same inpatient step-up schedule; in 2 cohorts, teclistamb was combined with lenalidomide and bispecific antibody was stopped after 13 cycles if patients obtained at least CR. The duration of maintenance was 2 years for all patients. Cumulative incidence of grade 3-4 neutropenia at 6 months was lower with teclistamab 3 mg/kg Q4W from cycle 2, and grade 3-4 infections occurred in nearly 30% of patients, although only 5.3% of patients discontinued treatment due to adverse events. Regarding efficacy, in cohort 1 (in which patients received teclistamab 1.5 mg/kg for QW 2 cycles, 3 mg/kg Q2W for 4 cycles and 3 mg/kg Q4W thereafter) 100% of patients achieved at least CR during maintenance (sCR = 90.6%), and 100% of evaluable patients were MRD negative at 12 months. With a median follow-up of 21.1 months, patients enrolled in this cohort had a 2-year PFS of nearly 95%, but the median PFS was not reached in all cohorts. Besides bispecific antibodies, cilta-cel, a BCMA targeting CAR-T cell therapy approved for relapsed/refractory MM, has been explored as maintenance therapy post-ASCT in the phase II CARTITUDE-2 cohort D study,[23] including TE patients with a response < CR after 4-8 cycles of initial therapy including ASCT. Patients received cilta-cel with lenalidomide (n=12) or without lenalidomide (n=5), and their median age was 54 years (37-69). Among evaluable patients, the MRD negativity rate, the primary goal of the study, was 80% at 10-5, and ORR was 94.1%, with 88.2% of patients obtaining sCR. As per outcome measures, 18-month PFS and OS were 93.8% and 93.8%, respectively. No patients developed grade ≥ 3 CRS or immune effector cell-associated neurotoxicity syndrome (ICANS). Finally, at the EHA 2024, Du et al.[24] presented the results of a phase I study in which HR NDMM patients received an autologous BCMA and CD19 dual-targeting CAR-T therapy developed using the novel FasTCAR-T platform allowing to reduce manufacturing time significantly. Twenty-two patients received 2 cycles with VRd as induction, followed by a single CAR-T cell infusion at 3 different doses after a standard lymphodepletion. Overall, 100% of patients achieved ORR (95% sCR) and 100% MRD negativity at 10-6 with a favourable safety profile.
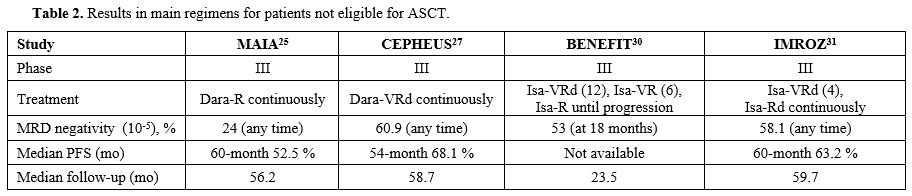
Transplant, not eligible patients. Triplet daratumumab, lenalidomide and dexamethasone (DRd) has become one of the most used initial therapy in transplant not eligible patients (TNE) after the global phase III MAIA study[25] demonstrated a significantly improved PFS with daratumumab, lenalidomide, dexamethasone (DRd) compared to lenalidomide plus dexamethasone (Rd). At the EHA Meeting, the final survival analysis of the MAIA study showed a 33% reduction in the risk of death with DRd vs Rd, with median OS being 90.3 months vs 64.1 months in the DRd and Rd arm, respectively, after a median follow-up of 89.3 months.[26] Survival benefit with daratumumab regimen was documented across all subgroups considered, and median OS was 79.6 months in patients younger than 75 years and 54.8 months in older ones. Although triplet therapy is the current standard in TNE patients, several studies also evaluated quadruplets in this setting. In the international phase III CEPHEUS trial, whose results have been reported at the IMS Meeting,[27] TNE patients were randomised to receive 8 cycles of VRd or Dara-VRd followed by Rd or DRd, respectively, until progression or unacceptable toxicity. The primary endpoint of the study was the MRD negativity rate, which is defined as the proportion of patients achieving both MRD negativity and ≥ CR. Treatment arms were well balanced, and the median age of patients treated with Dara-VRd was 70 years; 88% of them had ECOG PS 0-1 and 12.7% high risk cytogenetics. Adding daratumumab to VRd significantly increased the depth and duration of response since the overall MRD negativity rate (10-5) was 60.9% in patients receiving Dara-VRd vs 39.4% in those receiving VRd (OR = 2.37, p<0.0001). The sustained MRD negativity rate was almost double with Dara-VRd vs VRd at all prespecified time points at both 10-5 and 10-6 levels and more than 85% of patients obtaining MRD negativity at 10-6 was alive and progression-free at 54 months.[28] Median PFS was not reached vs 52.6 months, respectively, after a median follow-up of 58.7 months, with a 43% reduction in the risk of disease progression or death in the Dara-VRd arm. Unfortunately, the trial was conducted during the COVID-19 pandemia, and this infection caused the death of 6.1% and 3.1% of patients enrolled in the Dara-VRd and VRd arm, respectively. Therefore, although no significant differences in terms of OS were reported, OS trended favourably for Dara-VRd when censored for COVID-19 deaths (HR = 0.69).[27] Quadruplet Isa-VRd has been compared with triplets in phase III BENEFIT and IMROZ trials, presented at the last ASCO and ASH Meetings. In the first study by the IFM group,[29,30] NTE patients aged 65-79 years with ECOG PS ≤ 2 were randomly assigned to receive Isa-VRd (weekly bortezomib) for 12 cycles followed by 6 cycles with Isa-VR and Isa-R as maintenance vs 12 cycles of Isa-Rd followed by Isa-R maintenance. The primary endpoint (MRD negativity at 10-5 level by NGS at 18 months from randomisation) was met since it was 53% vs 26% in Isa-VRd and VRd arm, respectively (OR = 3.16, p<0.0001). After a median follow-up of 23.5 months, 2-year PFS and OS were 85.2% and 91.1% for Isa-VRd and 80% and 91.5% for Isa-Rd, respectively. The most common any-grade side effects were neutropenia occurring in 57% of patients in Isa-VRd vs 61% in Isa-Rd arm, diarrhoea occurring in 49% vs 48%, infections in 45% vs 36%, peripheral neuropathy in 52% vs 28%, respectively. The global IMROZ trial[31] enrolled 446 patients aged ≤ 80 years who received 4 Isa-VRd cycles followed by Isa-Rd until progression or 4 VRd cycles followed by Rd. Median PFS, the primary endpoint, was significantly longer with Isa-VRd than with VRd (not reached vs 54.4 months, HR = 0.60, p< 0.001) with a 5-year PFS of 63.2% vs 45.2%, respectively, after a median follow-up of 59.7 months. At least CR was achieved by 74.7% of patients treated with Isa-VRd vs 64.1% with VRd (p=0.01), and a sustained MRD negativity rate (≥ 12 months) was 46.8% vs 24.3%, respectively (OR = 2.73), and it was 2-to 3-fold higher with quadruplet at all considered time-points.[32] However, despite a significant efficacy, the toxicity of the Isa-VRd regimen was not negligible since 44% of patients developed grade ≥ 3 infections, mainly pneumonia (20%) and 11% of patients died from infectious complications.[31] Although in all phase III above-mentioned trials, the control arm is not DRd, the study suggests that quadruplet combinations containing anti-CD38 mAbs could become a standard therapy also in NTE patients since they seem to induce long-lasting responses.
Based on the high rate of serious side effects as grade ≥ 3 neutropenia (57.7% vs 45.4%), infections (41.7% vs 31.6%) and pneumonia (19.6% vs 10.2%) reported with Dara-Rd in the MAIA trial,[33] the IFM group[34] reported results from the phase III IFM2017-03 clinical study comparing daratumumab plus lenalidomide (DR) vs Rd, both continuously administered, with the aim to evaluate efficacy and safety of a dexamethasone-sparing regimen in frail aged ≥ 65 years patients. Frail patients were defined as those with a simplified frailty scale ≥ 2.[35] After a median follow-up of 46.3 months, median PFS was significantly longer in the DR arm vs Rd arm (median 53.4 months vs 22.5 months) with a 49% reduction in the risk of progression or death in patients receiving DR (HR = 0.51, p<0.0001). PFS benefit with DR vs Rd was consistent across all considered subgroups, notably in patients older than 80 years or those with a frailty score of 4/5. The rate of treatment discontinuations due to side effects was 30% in patients receiving DR and 34% in those treated with Rd, and although grade ≥ 3 neutropenia occurred in 55% and 24% of patients, respectively, no increased incidence of ≥ grade 3 infections (19% vs 21%, respectively) were documented in the DR arm. Median OS was not reached in the DR group vs median 47.2 months in the Rd patients (HR=0.52, p=0.0001).
In newly diagnosed NTE patients, phase I DREAMM-9 study evaluated a triplet VRd regimen combined with belantamab mandolin, the first BCMA-directed antibody-drug conjugate (ADC) to have been approved for relapsed/refractory multiple myeloma (RRMM).[36] The study, whose primary endpoint was safety, enrolled 108 patients in 8 cohorts differing in Belamaf doses and schedules, and all patients received ADC combined with VRd for 8 cycles, followed by Belamaf plus Rd thereafter. The most frequent non-ocular grade ≥ 3 side effects were thrombocytopenia (30%), neutropenia (26%) and COVID-pneumonia (19%), whereas grade ≥ 3 ocular events occurred in 55% of patients, being lower in cohorts with longer dosing intervals. Overall ORR ranged from 71% to 100%, and MRD negativity rate from 50% to 100% in patients treated with a starting dose of 1 mg/kg and 1.9 mg/kg, respectively. Phase III DREAMM-10 trial (NCT06679101) will compare Belamaf plus Rd vs DRd in NTE patients. In Table 2, we summarised the results of the most recent regimens for not eligible newly diagnosed MM patients.
News in Relapsed/Refractory MM Patients
Bispecific antibodies. Recently, the approval of new immunotherapies such as bispecific antibodies and CAR-T cell therapies cells have represented a turning point for heavily pretreated patients, exposed to triple classes as proteasome inhibitors (PIs), immunomodulatory agents (IMiDs) and anti-CD38 monoclonal antibodies (mAbs). Therefore, in 2024, the topics of the various international conferences have just reported new data or updated results obtained with these new therapeutic strategies. As mentioned above, teclistamab represents the first BCMAxCD3 bispecific antibody approved for triple-class exposed MM and at ASH 2024, D'Souza et al. presented results of the combination teclistamab, daratumumab and pomalidomide from the MajesTEC-2 Cohort A and TRIMM-2 studies.[37] The first study enrolled patients who had received 1-3 prior lines of therapy (n=17), while the latter included patients treated with at least 3 prior lines of therapy (n=10). Regarding the safety of the combination, the key objective of the study was that no patients developed grade ≥ 3 CRS, and 77.8% and 22.2% of patients had grade ≥ 3 neutropenia and lymphopenia, respectively. Sixty-three per cent of patients developed grade ≥ 3 infections, mainly consisting of pneumonia (18.5%) and COVID-19 (18.5%). Six patients (22%) died due to infections, but no fatal events occurred after the intensification of infection prophylaxis, including Ig replacement, was planned. ORR was 94% (≥ CR 64.7%) in less pretreated patients vs 70% (≥ CR 50%) in those more heavily pretreated, and 2-year PFS was 59.8% and 46.7%, respectively. Notably, a subgroup analysis of patients receiving talquetamab (targeting GPRC5D) plus daratumumab and pomalidomide in the TRIMM-2 study showed, in patients previously exposed to bispecific antibodies, CD8 T-cell expansion, NK recovery, CD38+ T-cell activation and reduction of CD38+ Tregs greater than those observed in patients without prior bispecific antibodies exposure.[38]Elranatamab, approved for triple-class exposed MM patients, represents another BCMA-CD3 bispecific antibody evaluated in triplet combination with carfilzomib and dexamethasone in early relapse[39] in the Phase I MagnetisMM-20 trial. Very preliminary data referring to 12 patients with a median of 2 prior lines of therapy showed an ORR of 100%, with 75% of patients achieving at least CR. Data from MajesTEC-2 and MagnetisMM-20 studies suggest that combinations containing bispecific antibodies are extremely effective in early relapse and could become a substantial strategy in the future landscape of MM.
In advanced MM, ongoing studies are exploring other bispecific antibodies.
High efficacy has been found with linvoseltamab, a fully human BCMAxCD3 antibody, evaluated in the international phase I/II study whose results have been recently published.[40] At the EHA 2024 Meeting, Lentzsch[41] reported data of 117 patients with a median of 5 prior lines of therapy, 82% at least triple-refractory, who were treated with a 200 mg dose administered weekly through week 14 and then every other week. Patients achieving at least a VGPR with a minimum of 24 weeks of treatment transitioned to one administration every 4 weeks until progression. ORR was 71%, with 49.6% of patients achieving at least CR, and the MRD negativity rate was 90.5% among evaluable patients with a response ≥ CR. After a median follow-up of 14.3 months, estimated 1-year PFS and OS were 70% and 75.3%, respectively. High response rates were documented in subgroups of patients difficult to treat as those with HR cytogenetics (ORR = 67.4%), extramedullary plasmacytomas (ORR = 52.6%) and penta-refractory patients (ORR = 66.7%). The safety profile was similar to that of other bispecific antibodies, and grade ≥ 3 infections occurred in 34% of patients, although their frequency and severity decreased over time and was 4%-8% between 6 and 15 months. Results of another BCMA-CD3 bispecific antibody (ABBV-383, Etentamig) characterised by 2 high-affinity BCMA-binding domains, a low-affinity CD3-binding domain to reduce CRS incidence and an extended half-life allowing for every 4 weeks dosing have been presented at the same conference.[42] Overall, 220 patients who had received ≥ 3 prior lines of therapy (median 4 lines, 100% triple-class exposed) were treated with ABBV-383 and the dose of 60 mg every 4 weeks, administered to 21 patients, was found to be associated with better efficacy and lower side effects. No patients developed grade ≥ 3 CRS or ICANS, grade ≥ 3 neutropenia and infections occurred in 29% and 19% of patients, respectively. ORR was 65%, 55% of patients obtained at least VGPR and 1-year PFS was 54.8%. In the phase I Kilimanjaro study, Etentamig was explored in combination with pomalidomide, lenalidomide, or daratumumab. Promising data of ABBV-383 plus daratumumab and dexamethasone have been reported at the last ASH Meeting.[43] Eighty-six patients with a median of 4 prior lines of therapy, 70% refractory to anti-CD38 mAbs, received this combination, and ORR was 83% at 40-60 mg dose levels with no increase in toxicities compared to etentamig monotherapy. Finally, ISB 2001, a tri-specific antibody targeting BCMAxCD38xCD3, proved to be very active in heavily pretreated patients since, in a phase I dose-escalation study, ORR was 83% overall, 86% in anti-CD38 refractory patients and 75% in those with prior bispecific antibodies and/or CAR-T cell therapy.[44]
CAR-T cell therapies. After BCMA-directed CAR-T cell therapies approval, such as ide-cel and cilta-cel in highly pretreated MM patients, interest is increasingly focusing on these approaches in the early relapse setting. Leleu et al. reported, at EHA 2024, safety and efficacy data of KarMMa-2 cohort 2b, including patients who experienced disease progression < 18 months post first-line therapy without ASCT and received ide-cel.[45] The median age of 31 patients was 60 years, and a significant proportion of them had HR (38.7%) or ultra HR (16%) cytogenetics. ORR after ide-cel was 93.5%, and 71% obtained at least CR, the primary endpoint of the study. A high rate of MRD negativity was observed, 75% in patients with ≥ CR. After a median follow-up of 30.1 months, 2-year PFS and OS were 63.3% and 78.9%, respectively, with no patients developing grade ≥ 3 CRS or neurotoxicity and more severe infections occurring in 19% of patients. Very high MRD negativity rates were also obtained with cilta-cel in patients who had received 1-3 prior lines of therapy in the phase III CARTITUDE-4 trial, showing that, after a median follow-up of 33.6 months, patients treated with cilta-cel had a significantly improved PFS (HR=0.29, p<0.0001) and OS (HR=0.55, p=0.0009) vs standard of care.[46] Among evaluable patients, 69% achieved MRD negativity at 10-5 by day 56, and this rate increased to 86% after 6 months of cilta-cel infusion. The benefit was observed across subgroups, and remarkably, the odds ratio for MRD negativity in patients refractory to anti-CD38 mAbs and IMiDs treated with cilta-cel was 13.23. Moreover, in 51.7% vs 9.7% of patients in ≥ CR receiving cilta-cel and standard therapy, respectively, MRD negativity was sustained (≥12 months), translating in higher rates of PFS (93.2%) and OS (97.3%) at 30 months in patients treated with CAR-T cells (vs 46.8% and 64.6%, respectively, in patients receiving standard therapy).[47] Recently, cilta-cel has been approved by FDA and EMA for the treatment of adult patients with RRMM who have received one or more prior lines of therapy, including an IMiD and a PI, and who are refractory to lenalidomide. As well as for bispecific antibodies, results from trials demonstrated that the infusion of CAR-T cells in early relapse, when the burden of disease is lower and refractoriness is limited, improves efficacy with a manageable toxicity profile.
Anito-cel is another BCMA-targeting CAR-T cell therapy with a synthetic novel D-domain binder that was evaluated in a phase I study,[48,49] including 38 patients with a median of 4 prior lines of therapy. Sixty-eight percent of patients had high risk prognostic features, 100% were triple refractory and 68% penta-refractory. At least CR was achieved by 79% of patients, and after a median follow-up of 38.1 months, median PFS was 30.2 months, and median OS was not achieved. It is worth mentioning that this approach was found to be very effective in all risk subgroups, including extramedullary disease. The ongoing phase 2 iMMagine-1 trial[48] is a registrational study of anito-cel enrolling patients after ≥ 3 lines of therapy, whose preliminary results were presented at the 2024 ASH Meeting. After a median follow-up of 9.5 months, among evaluable patients, ORR was 97% and sCR/CR 62% with a safety manageable profile since only one of 98 patients developed grade 3 ICANS and grade ≥ 3 infections occurred in 10% of patients. In addition to BCMA, GPRC5D was also explored as a target for CAR-T cell therapy, and Arlo-cel was evaluated in a dose-escalation phase I study enrolling 86 patients with a median of 5 prior lines of therapy and 49% previously treated with anti-BCMA therapy.[50] Safety findings, the primary endpoint, showed grade ≥ 3 CRS and ICANS in 4% and 2% of patients, respectively; no grade skin, nail, oral events occurred and low grade on-target/off-tumour side effects resolved without intervention in 79% of cases; ≥ grade 3 infections developed in 19% of patients. Overall, ORR was 87%, and the median PFS was 18.3 months.
Belantaman Mafodotin. Belantamab Mafodotin (Belamaf) is the first in class ADC targeting BCMA; it, after internalisation into the plasma cell, undergoes a process of proteolytic cleavage, releasing Mafodotin with disruption of microtubular cell network leading to cell cycle arrest and apoptosis.[51] In the plenary session of the ASCO 2024 meeting, Mateos[52,53] reported results of the global phase III DREAMM-7 trial in which patients with ≥ 1 prior line of therapy were randomised to Belamaf plus bortezomib and dexamethasone (BVd) or daratumumab, bortezomib, dexamethasone (DVd). Very few patients had previously received daratumumab, and 33% vs 35% were lenalidomide-refractory in the BVd and DVd arm, respectively. The study met the primary endpoint since the median PFS was 36.6 months vs 13.4 months, respectively (HR=0.41, p<0.001). An update of the DREAMM-7 study was displayed at the ASH 2024,[54] and after a median follow-up of 39.4 months, the benefit with BVd was maintained after subsequent therapies with an HR of 0.59. Moreover, BVd was found to improve OS with modelling significantly predicting a median OS of 84 months with BVd vs 51 with DVd (HR=0.58, p=0.00023). Response rates with BVd were higher than with DVd (ORR 83.1% vs 71.3%, respectively), and patients with ≥ CR and MRD negativity were 25.1% vs 10.4%, respectively (p<0.00001). Blurred vision, the most frequent ocular event in the BVd arm, occurred in 68% of patients (≥ grade 3 = 24%) but resolved in all patients and discontinuation of therapy due to ocular toxicity was 10%. Again in patients who had received at least one prior therapy, belamaf in combination with pomalidomide and dexamethasone (BPd) significantly prolonged PFS vs pomalidomide, bortezomib, dexamethasone (PVd)[55,56] (median not reached vs 12.7 months after a median follow-up of 21.8 months; HR=0.52; p<0.001) in the DREAMM-8 trial. However, if compared with the DREAMM-7 MM population, patients enrolled in the DREAMM-8 study showed greater refractoriness since 81% vs 76% in the BPd and PVd arm were lenalidomide refractory, respectively, and 23% vs 24% were anti-CD38 refractory. At least CR was achieved by 40% of patients in the BPd arm and 16% in the PVd group, and the MRD negativity rate among patients with ≥ CR was 25% vs 5%, respectively. Grade ≥ 3 ocular events occurred in 43% of patients, but they improved or resolved in 92% and 84% of them, respectively. Therefore, triplets BVd and BPd could aim at becoming a new standard therapy in early relapse and beyond.
Another agent that was found to exert a potent antimyeloma activity is Mezigdomide, an oral cereblon E3 ligase modulatory drug (CELMoD) that, in the phase I/II CC-92480-MM-002, was evaluated in combination with bortezomib and dexamethasone in the dose-escalation cohort A (2-4 prior lines of therapy, 82.1% lenalidomide-refractory) and dose-expansion cohort D (1-3 prior lines of therapy, 63.3% lenalidomide-refractory) or in combination with carfilzomib and dexamethasone in the dose-escalation cohort C (2-4 prior lines of therapy, 77.8% lenalidomide-refractory). Updated results of this trial were reported at the ASH 2024.[57] ORR was at least 75% across all cohorts, and median PFS was 17.5 months in patients with fewer prior lines of therapy (cohort D) versus 12.3 and 13.5 months in cohorts A and C, respectively. Neutropenia and infections resulted to be the most common ≥ grade 3 side effects. In the same meeting, Costa[58] reported data from a phase I/II study with all-oral triplets, including Mezigdomide in combination with dexamethasone and novel targeted therapies as tazemetostat (EZH2 inhibitor), BMS-986158 (BET inhibitor) and trametinib (MEK inhibitor). Overall, 56 patients with a median of 5 prior lines of therapy were enrolled, 82% were triple-class refractory, and 57% had received T-cell-redirecting therapy. The highest activity was documented with mezigdomide combined with trametinib, with an ORR of 75% and a median PFS of 8.7 months.
News on Supportive Care in the Era of Novel Immunotherapies
The introduction of bispecific antibodies and CAR-T cell therapies into clinical practice brought out new toxicities such as CRS and ICANS but also led to assaying strategies for preventing or mitigating these adverse events. At the ASH 2024, Kowalski[59] presented data from a single US real-world study, including 72 patients who received prophylactic tocilizumab prior to bispecific antibodies (teclistamab, elranatamab and talquetamab) administered outside the context of a clinical trial. The median age was 67 years; 86% of patients were triple-class refractory and 26% were penta-drug refractory. Overall, CRS occurred in 14% of patients, and it was grade 1 in 12.5% and grade 2 in 1.5% of them, whereas ICANS developed in 8% of patients, being grade 3 in 3% of them. Although prospective randomised trials are needed to confirm these findings, this study shows that tocilizumab can be effective as a preventive measure without impacting response, which was 66%. Another explored approach to improve the tolerability of bispecific antibodies has been the administration of less frequent dosing.[60] Among 86 patients treated with teclistamab at Memorial Sloan Kettering Cancer Center, 32 patients transitioned from QW dosing to Q2W dosing after a median of 3.3 months from teclistamab initiation and main reasons for changing schedules were achievement of at least PR and/or safety management. The median age of this group was 70 years, 34% had extramedullary disease, a median number of prior lines of therapy was 6 and 31% of patients had previously received BCMA therapy. ORR was 94%, and after a median follow-up of 6.4 months since the switch, the 6-month PFS was 90%.Infections represent a common complication in patients who are treated with novel immunotherapies, and the risk of infection ranges from 3.5 to 10.7 per 100 patients per month in patients receiving bispecific antibodies. Notably, comparing infection rates between the first 8 months and the subsequent 8 months, no significant differences were found.[61] Considering that profound hypogammaglobulinemia is universal in patients who respond to bispecific antibodies, a multi-institutional study evaluated the effect of intravenous immunoglobulin supplementation (IVIG) on infectious complications in patients with RRMM treated with teclistamab or other BCMA directed bispecific therapy.[62] Among patients treated with at least one dose of bispecific antibodies, 92 received IVIG primary prophylaxis, and 133 did not. Primary IVIG prophylaxis was associated with a significantly better outcome in any grade (median 7.7 months vs 3 months, p=0.021) and ≥ grade 3 (14 months vs 7.5 months, p=0.022) infection-free survival if compared with no prophylaxis. Moreover, multivariate analysis selected baseline lymphopenia, prior infection, and more prior lines of therapy as factors affecting all grade and ≥ grade 3 infections.
In a multicenter observational study, Rejeski et al. explored the association between pre-apheresis risk factors and survival in patients with RRMM receiving BCMA CAR-T cell therapy.[63] Among 530 patients with a median age of 65 years and a median of 5 prior lines of therapy, 184 were treated with cilta-cel and 346 with ide-cel. Based on the CAR-HEMATOTOX (HT) score,[64] 6.6 % of patients shifted from high risk pre-apheresis to low-risk pre-lymphodepletion, whereas in 7.4% of patients, the shift was from low to high risk. Patients with high risk HT (score ≥ 3) pre-apheresis had a significantly higher rate of ≥ grade 3 early (25% vs 8% in low-risk, p<0.001) and late cytopenias (21% vs 7%, p<0.001); a higher rate of severe infections (27% vs 14%, p=0.007); more frequent ICU admission (12% vs 6%, p=0.05) and longer hospitalisation (15 days vs 12 days, p=0.009). After a median follow-up of 12.4 months, 1-year PFS (29% vs 63%, p<0.0001) and OS (49% vs 83%, p<0.0001) were significantly inferior in high HT risk score pre-apheresis patients. Multivariate analysis was selected as factors independently associated with PFS, a high HT pre-apheresis score, prior anti-BCMA therapy, and a history of extramedullary disease and penta-refractoriness. The PFS was 15% at one year in patients with at least 3 risk factors.
This model could help to select better patients who likely benefit from CAR-T cell therapies and could be used to explore strategies for mitigating toxicities.
Conclusions
In the year 2024, new quadruplet regimens, investigated in several global phase III trials, confirmed their key role in both TE and TIE for ASCT newly diagnosed MM patients. Combinations, including anti-CD38 monoclonal antibodies, have been shown to increase the rate and improve the depth of response compared to triplets, translating into a significantly longer PFS. It should be emphasised that all quadruplets such as Dara-VRd, Isa-VRd or Isa-KRd were found to be superior to triplets regardless of which anti-CD38 mAb was used in the combinations or which PI (bortezomib or carlfizomib) was included in the triplet. Therefore, for TE patients, other induction regimens, in addition to Dara-VTd, the current standard of therapy, at least in Europe, may soon be available. However, even for the newest and most effective quadruplet combinations, there is an Achilles heel represented by patients with 2 or more HRCA or with high circulating plasma cells who continue to show unsatisfactory outcomes. The introduction of new immunotherapies in upfront therapy could, in the future, overcome those prognostic factors that currently seem to be insurmountable. Based on the results of phase III, CEPHEUS[27] and IMROZ[31] quadruplets will likely also be the new therapies for TIE patients. However, considering that all these trials enrolled patients up to 80 years of age and that, as described above, toxicities, especially cytopenias and infections, are not all negligible with quadruplets, it will be necessary to identify predictive markers of response and toxicity as to tailor initial therapy as much as possible.MRD assessment will become a critical factor in clinical practice because, as studies are showing, sustained MRD negativity will allow discontinuing therapy in low-risk patients, such as those without HRCA, avoiding overtreatment; on the other hand, serial MRD assessments will allow detection early MRD progression likely to be treated with a preemptive therapy.
In relapsed/refractory settings, many oral communications at the 2024 Meetings involved bispecific antibodies and CAR-T cell therapies. Regarding the former, promising data are those of bispecific antibodies in combination with other classes of agents (PIs, IMiDs and anti-CD38 mAbs) evaluated in early relapse. Moreover, considering that their use in advanced MM will be increasing, all approaches to reduce toxicity and risk of hospitalisation, such as prophylaxis with tocilizumab, less frequent dosing, or immunoglobulin supplementation, were particularly interesting. CAR-T cell therapies confirmed their efficacy also in functional HR patients and in those with early relapses, and novel products such as anito-cel seem to exert a potent antimyeloma activity also in very difficult relapses. Again, since not all relapsed/refractory MM patients will be able to receive bispecific antibodies or CAR-T cells, it will be crucial to develop risk models to identify patients best suited to undergo and tolerate these treatments. Among novel immunotherapies, it is necessary to mention belantaab mafodotin that, in combination with bortezomib and dexamethasone or pomalidomide and dexamethasone, could become a viable alternative in the future.
References
- Ebraheem MS, Chakraborty R, Rochwerg B, et
al. Quadruplet regimens for patients with newly diagnosed multiple
myeloma: a systematic review and meta-analysis. Blood Adv.
2024;8(23):5993-6002. https://doi.org/10.1182/bloodadvances.2024014139 PMid:39348665 PMCid:PMC11629212
- Martino
EA, Mele G, Vigna E, Morabito F, Gentile M. Refining High-Risk Multiple
Myeloma: Advancements in Genomic, Clinical, and Prognostic Criteria.
Mediterr J Hematol Infect Dis. 2025;17(1):e2025006. https://doi.org/10.4084/MJHID.2025.006 PMid:39830800 PMCid:PMC11740893
- Mohty
M, Avet-Loiseau H, Malard F, Harousseau JL. Potential future direction
of measurable residual disease evaluation in multiple myeloma. Blood.
2023;142(18):1509-1517. https://doi.org/10.1182/blood.2023020284 PMid:37471603
- Sonneveld
P, Dimopoulos MA, Boccadoro M, et al. Daratumumab, Bortezomib,
Lenalidomide, and Dexamethasone for Multiple Myeloma. N Engl J Med.
2024;390(4):301-313. https://doi.org/10.1056/NEJMoa2312054 PMid:38084760
- Goldschmidt
H, Bertsch U, Pozek E, et al. Isatuximab, Lenalidomide, Bortezomib and
Dexamethasone Induction Therapy for Transplant-Eligible Patients with
Newly Diagnosed Multiple Myeloma: Final Progression-Free Survival
Analysis of Part 1 of an Open-Label, Multicenter, Randomized, Phase 3
Trial (GMMG-HD7). Blood. 2024;144(Supplement 1):769. https://doi.org/10.1182/blood-2024-193308
- Mai EK, Bertsch U, Pozek E, et al. Isatuximab, Lenalidomide, Bortezomib, and Dexamethasone Induction Therapy for Transplant-Eligible Newly Diagnosed Multiple Myeloma: Final Part 1 Analysis of the GMMG-HD7 Trial. J Clin Oncol. 2024;0(0):JCO-24-02266.
- Goldschmidt
H, Mai EK, Bertsch U, et al. Addition of isatuximab to lenalidomide,
bortezomib, and dexamethasone as induction therapy for newly diagnosed,
transplantation-eligible patients with multiple myeloma (GMMG-HD7):
part 1 of an open-label, multicentre, randomised, active-controlled,
phase. Lancet Haematol. 2022;9(11):e810-e821.
- Mai
EK, Salwender H, Hundemer M, et al. Impact of Minimal Residual Disease
on Progression-Free Survival in Patients with Newly Diagnosed Multiple
Myeloma Treated with Isatuximab, Lenalidomide, Bortezomib and
Dexamethasone Induction Therapy in the Phase 3 GMMG-HD7 Trial. Blood.
2024;144(Supplement 1):364. https://doi.org/10.1182/blood-2024-194768
- Costa
LJ, Medvedova E, Dhakal B, et al. Implications of MRD Progression in
Newly Diagnosed Multiple Myeloma (NDMM) Treated with Quadruplet Therapy
and Autologous Stem Cell Transplantation. Blood. 2024;144(Supplement
1):363. https://doi.org/10.1182/blood-2024-201914
- Giri
S, Dhakal B, Callander N, et al. Optimal MRD-Based Endpoint in the
Setting of Upfront Quadruplets (QUADs) to Support Response-Adapted
Treatment Cessation in Newly Diagnosed Multiple Myeloma (NDMM). Blood.
2024;144(Supplement 1):257. https://doi.org/10.1182/blood-2024-205611
- Malandrakis
P, Ntanasis-Stathopoulos I, Kostopoulos I V, et al. Sustained MRD
Negativity for Three Years Can Guide Discontinuation of Lenalidomide
Maintenance after ASCT in Multiple Myeloma: Results from a Prospective
Cohort Study. Blood. 2024;144(Supplement 1):361. https://doi.org/10.1182/blood-2024-209826
- Bertamini
L, Fokkema C, Rodríguez-Otero P, et al. Circulating Tumor Cells As a
Biomarker to Identify High-Risk Transplant Eligible Myeloma Patients
Treated with Bortezomib, Lenalidomide and Dexamethasone with or without
Daratumumab during Induction/Consolidation, and Lenalidomide with or
without Daratumumab during Maintenance: Results from the Perseus Study.
Blood. 2024;144(Supplement 1):487 https://doi.org/10.1182/blood-2024-199550
- Costa
LJ, Chhabra S, Medvedova E, et al. Minimal residual disease
response-adapted therapy in newly diagnosed multiple myeloma (MASTER):
final report of the multicentre, single-arm, phase 2 trial. Lancet
Haematol. Published online September 2023. https://doi.org/10.1016/S2352-3026(23)00236-3 PMid:37776872
- Moreau
P, Hulin C, Perrot A, et al. Bortezomib, thalidomide, and dexamethasone
with or without daratumumab and followed by daratumumab maintenance or
observation in transplant-eligible newly diagnosed multiple myeloma:
long-term follow-up of the CASSIOPEIA randomised controlled phase 3
trial. Lancet Oncol. 2024;25(8):1003-1014. https://doi.org/10.1016/S1470-2045(24)00282-1 PMid:38889735
- Gay
F, Roeloffzen W, Dimopoulos MA, et al. Results of the Phase III
Randomized Iskia Trial:
Isatuximab-Carfilzomib-Lenalidomide-Dexamethasone Vs
Carfilzomib-Lenalidomide-Dexamethasone As Pre-Transplant Induction and
Post-Transplant Consolidation in Newly Diagnosed Multiple Myeloma
Patients. Blood. 2023;142(Supplement 1):4. https://doi.org/10.1182/blood-2023-177546
- Munshi
NC, Avet-Loiseau H, Anderson KC, et al. A large meta-analysis
establishes the role of MRD negativity in long-term survival outcomes
in patients with multiple myeloma. Blood Adv. 2020;4(23):5988-5999. https://doi.org/10.1182/bloodadvances.2020002827 PMid:33284948 PMCid:PMC7724898
- Paiva
B, Zherniakova A, Nuñez-Córdoba JM, et al. Impact of treatment effect
on MRD and PFS: an aggregate data analysis from randomised clinical
trials in multiple myeloma. Blood Adv. 2024;8(1):219-223. https://doi.org/10.1182/bloodadvances.2023010821 PMid:37639322 PMCid:PMC10805640
- Landgren
O, Prior TJ, Masterson T, et al. EVIDENCE meta-analysis: evaluating
minimal residual disease as an intermediate clinical endpoint for
multiple myeloma. Blood. 2024;144(4):359-367. https://doi.org/10.1182/blood.2024024371 PMid:38768337
- Cavo
M, San-Miguel J, Usmani SZ, et al. Prognostic value of minimal residual
disease negativity in myeloma: combined analysis of POLLUX, CASTOR,
ALCYONE, and MAIA. Blood. 2022;139(6):835-844. https://doi.org/10.1182/blood.2021011101 PMid:34289038 PMCid:PMC8832474
- Landgren
O, Devlin SM. Minimal Residual Disease as an Early Endpoint for
Accelerated Drug Approval in Myeloma: A Roadmap. Blood Cancer Discov.
2025;6(1):13-22. https://doi.org/10.1158/2643-3230.BCD-24-0292 PMid:39630969 PMCid:PMC11707509
- Raab
MS, Weinhold N, Kortüm KM, et al. Phase 2 Study of Teclistamab-Based
Induction Regimens in Patients with Transplant-Eligible (TE) Newly
Diagnosed Multiple Myeloma (NDMM): Results from the GMMG-HD10/DSMM-XX
(MajesTEC-5) Trial. Blood. 2024;144(Supplement 1):493. https://doi.org/10.1182/blood-2024-206003
- Zamagni
E, Silzle T, Špička I, et al. Phase 3 Study of Teclistamab (Tec) in
Combination with Lenalidomide (Len) and Tec Alone Versus Len Alone in
Newly Diagnosed Multiple Myeloma (NDMM) As Maintenance Therapy
Following Autologous Stem Cell Transplantation (ASCT): Safety Run-in
(SRI) Results from the Majestec-4/EMN30 Trial. Blood.
2024;144(Supplement 1):494. https://doi.org/10.1182/blood-2024-205608
- Arnulf
B, Kerre T, Agha M, et al. Efficacy and Safety of Ciltacabtagene
Autoleucel ± Lenalidomide Maintenance in Newly Diagnosed Multiple
Myeloma With Suboptimal Response to Frontline Autologous Stem Cell
Transplant: CARTITUDE-2 Cohort D. Clin Lymphoma Myeloma Leuk.
2024;24:S241. https://doi.org/10.1016/S2152-2650(24)00980-7
- Juan
Du, Wanting Qiang, Jing Lu, Yanchun Jia, Haiyan He, Jin Liu, Pei Guo,
Ying Yang, Zhongyuan Feng, Lina Jin, Xiaoqiang Fan, Jia Liu, Qi Zhang,
Lihong Weng, Lianjun Shen, Wenling Li WC. A PHASE I OPEN-LABEL
SINGLE-ARM STUDY OF DUAL TARGETING BCMA AND CD19 FASTCAR-T (GC012F) AS
FIRST-LINE THERAPY FOR TRANSPLANT-ELIGIBLE NEWLY DIAGNOSED HIGH-RISK
MULTIPLE MYELOMA. Hemasphere. 2024;422304(S200).
- Facon
T, Kumar SK, Plesner T, et al. Daratumumab, lenalidomide, and
dexamethasone versus lenalidomide and dexamethasone alone in newly
diagnosed multiple myeloma (MAIA): overall survival results from a
randomised, open-label, phase 3 trial. Lancet Oncol.
2021;22(11):1582-1596. https://doi.org/10.1016/S1470-2045(21)00466-6 PMid:34655533
- Thierry
Facon, Shaji Kumar, Robert Z Orlowski, Nizar J Bahlis, Philippe Moreau,
Hartmut Goldschmidt, supratik basu, Cyrille Hulin, Sonja Zweegman,
Katja Weisel, Aurore Perrot, Caroline Jacquet, Noopur Raje, Salomon
Manier, Ajai Chari, Markus Hansson, Moha SZU. FINAL SURVIVAL ANALYSIS
OF DARATUMUMAB PLUS LENALIDOMIDE AND DEXAMETHASONE VERSUS LENALIDOMIDE
AND DEXAMETHASONE IN TRANSPLANT-INELIGIBLE PATIENTS WITH NEWLY
DIAGNOSED MULTIPLE MYELOMA: MAIA STUDY. Hemasphere. 2024;8:(S1).
- Usmani
SZ, Facon T, Hungria V, et al. OA-63 Daratumumab +
Bortezomib/Lenalidomide/Dexamethasone in Patients With
Transplant-ineligible or Transplant-deferred Newly Diagnosed Multiple
Myeloma: Results of the Phase 3 CEPHEUS Study. Clin Lymphoma Myeloma
Leuk. 2024;24:S288-S289. https://doi.org/10.1016/S2152-2650(24)02344-9
- Zweegman
S, Facon T, Hungria V, et al. Phase 3 Randomized Study of Daratumumab
(DARA) + Bortezomib, Lenalidomide and Dexamethasone (VRd) Versus Alone
in Patients with Transplant-Ineligible Newly Diagnosed Multiple Myeloma
or for Whom Transplant Is Not Planned As Initial Therapy: Analysis of
Minimal Residual Disease in the Cepheus Trial. Blood.
2024;144(Supplement 1):362. https://doi.org/10.1182/blood-2024-200871
- Leleu
XP, Hulin C, Lambert J, et al. Phase 3 randomised study of isatuximab
(Isa) plus lenalidomide and dexamethasone (Rd) with bortezomib versus
isard in patients with newly diagnosed transplant ineligible multiple
myeloma (NDMM TI). J Clin Oncol. 2024;42(16_suppl):7501. https://doi.org/10.1200/JCO.2024.42.16_suppl.7501
- Leleu
X, Hulin C, Lambert J, et al. Isatuximab, lenalidomide, dexamethasone
and bortezomib in transplant-ineligible multiple myeloma: the
randomised phase 3 BENEFIT trial. Nat Med. 2024;30(8):2235-2241. https://doi.org/10.1038/s41591-024-03050-2 PMid:38830994 PMCid:PMC11333283
- Facon
T, Dimopoulos MA, Leleu XP, et al. Phase 3 study results of isatuximab,
bortezomib, lenalidomide, and dexamethasone (Isa-VRd) versus VRd for
transplant-ineligible patients with newly diagnosed multiple myeloma
(IMROZ). J Clin Oncol. 2024;42(16_suppl):7500. https://doi.org/10.1200/JCO.2024.42.16_suppl.7500
- Orlowski
RZ, Dimopoulos MA, Leleu X, et al. Isatuximab, Bortezomib,
Lenalidomide, and Dexamethasone (Isa-VRd) in Patients with Newly
Diagnosed Multiple Myeloma (NDMM): Analyses of Minimal Residual Disease
(MRD) Negativity Dynamics in the Phase 3 Imroz Study. Blood.
2024;144(Supplement 1):770. https://doi.org/10.1182/blood-2024-203818
- Facon
T, Cook G, Usmani SZ, et al. Daratumumab plus lenalidomide and
dexamethasone in transplant-ineligible newly diagnosed multiple
myeloma: frailty subgroup analysis of MAIA. Leukemia.
2022;36(4):1066-1077. https://doi.org/10.1038/s41375-021-01488-8 PMid:34974527 PMCid:PMC8979809
- Manier
S, Lambert J, Hulin C, et al. The IFM2017-03 Phase 3 Trial: A
Dexamethasone Sparing-Regimen with Daratumumab and Lenalidomide for
Frail Patients with Newly-Diagnosed Multiple Myeloma. Blood.
2024;144(Supplement 1):774. https://doi.org/10.1182/blood-2024-203045
- Facon
T, Dimopoulos MA, Meuleman N, et al. A simplified frailty scale
predicts outcomes in transplant-ineligible patients with newly
diagnosed multiple myeloma treated in the FIRST (MM-020) trial.
Leukemia. 2020;34(1):224-233. https://doi.org/10.1038/s41375-019-0539-0 PMid:31427722 PMCid:PMC7214253
- Usmani
SZ, Mielnik M, Garg M, et al. Phase I Study of Belantamab Mafodotin in
Combination with Standard of Care in Transplant-Ineligible Newly
Diagnosed Multiple Myeloma: Dreamm-9 Updated Interim Analysis. Blood.
2024;144(Supplement 1):497. https://doi.org/10.1182/blood-2024-198669
- D'Souza
A, Costa LJ, San-Miguel JF, et al. Teclistamab, Daratumumab, and
Pomalidomide in Patients with Relapsed/Refractory Multiple Myeloma:
Results from the Majestec-2 Cohort a and Trimm-2 Studies. Blood.
2024;144(Supplement 1):495. https://doi.org/10.1182/blood-2024-200181
- Vishwamitra
D, Skerget S, Cortes D, et al. Pharmacodynamic Signatures and
Correlatives of Response in Patients with Relapsed/Refractory Multiple
Myeloma (RRMM) Treated with Talquetamab or Teclistamab Plus Daratumumab
and Pomalidomide. Blood. 2024;144(Supplement 1):594. https://doi.org/10.1182/blood-2024-200300
- Tomasson
MH, Gabayan E, Ali SA, et al. Efficacy of Elranatamab (ELRA) in
Combination with Carfilzomib (CFZ) and Dexamethasone (DEX) in the Phase
1b MagnetisMM-20 Trial in Relapsed or Refractory Multiple Myeloma
(RRMM). Blood. 2024;144(Supplement 1):1024. https://doi.org/10.1182/blood-2024-210520
- Bumma
N, Richter J, Jagannath S, et al. Linvoseltamab for Treatment of
Relapsed/Refractory Multiple Myeloma. J Clin Oncol.
2024;42(22):2702-2712. https://doi.org/10.1200/JCO.24.01008 PMid:38879802 PMCid:PMC11272139
- Suzanne
Lentzsch, Naresh Bumma, Hans Lee, Attaya Suvannasankha, James E.
Hoffman, Joshua Richter, Madhav Dhodapkar, Joseph J. Maly, Rebecca
Silbermann, Chang-Ki Min, Matthew J. Pianko, Marie-Christiane Vekemans,
Michelle DeVeaux, Dhruti Chokshi, Anita Boy SJ. LINVOSELTAMAB IN
PATIENTS WITH RELAPSED/REFRACTORY MULTIPLE MYELOMA IN THE LINKER-MM1
STUDY: DEPTH AND DURABILITY OF RESPONSE AT 14-MONTH MEDIAN FOLLOW-UP.
HemaSphere. 2024;422316(S212).
- Katja
Weisel, Cesar Rodriguez-Valdes, Peter Voorhees, Anita D'Souza, Alfred
Chung, Sascha Tuchman, Hana Safah, John Mckay, Raphael Teipel, Neha
Korde, Ravi Vij, Orlando Bueno, Tanya Rosenberg, Rajvineeth Kumar
Pothacamury, Akshanth Polepally, Aarif Ahsan, SK. EFFICACY, SAFETY, AND
DETERMINATION OF RP2D OF ABBV-383, A BCMA BISPECIFIC ANTIBODY, IN
PATIENTS WITH RELAPSED/REFRACTORY MULTIPLE MYELOMA (RRMM). HemaSphere.
2024;422315(S211). https://doi.org/10.1200/JCO.2024.42.16_suppl.7531
- Rodriguez
C, Mielnik M, Kazandjian D, et al. ABBV-383 Plus
Daratumumab-Dexamethasone in Relapsed or Refractory Multiple Myeloma: A
Phase 1b Dose-Escalation and Safety Expansion Study. Blood.
2024;144(Supplement 1):496. https://doi.org/10.1182/blood-2024-205083
- Quach
H, Augustson B, Sia H, et al. First Results of a Phase 1,
First-in-Human, Dose Escalation Study of ISB 2001, a BCMAxCD38xCD3
Targeting Trispecific Antibody in Patients with Relapsed/Refractory
Multiple Myeloma (RRMM). Blood. 2024;144(Supplement 1):1026. https://doi.org/10.1182/blood-2024-208189
- Xavier
Leleu, Alfred Chung, Noopur Raje, Meera Mohan, Reuben Benjamin, Adam
Sperling, Larry D. Anderson, Madhav Dhodapkar, Shambavi Richard,
Violetta Kivovich, Sarah Johnston, Fan Wu, Debashree Basudhar, Ethan
Thompson, Devender Dhanda, Laurie Eliason, Si MA. IDECABTAGENE
VICLEUCEL (IDE-CEL) IN PATIENTS (PTS) WITH CLINICAL HIGH-RISK EARLY
RELAPSE MULTIPLE MYELOMA (MM) WITHOUT FRONTLINE (1L) AUTOLOGOUS STEM
CELL TRANSPLANTATION (ASCT): KARMMA-2 COHORT 2B. HemaSphere.
2024;422312(S208). https://doi.org/10.1016/S2152-2650(24)01948-7
- Mateos
MV, San-Miguel J, Dhakal B, et al. OA-65 Overall Survival (OS) With
Ciltacabtagene Autoleucel (Cilta-cel) Versus Standard of Care (SoC) in
Lenalidomide (Len)-Refractory Multiple Myeloma (MM): Phase 3
CARTITUDE-4 Study Update. Clin Lymphoma, Myeloma Leuk. 2024;24:S290. https://doi.org/10.1016/S2152-2650(24)02346-2
- Popat
R, Oriol A, Cavo M, et al. Ciltacabtagene Autoleucel (Cilta-cel) Vs
Standard of Care (SoC) in Patients with Lenalidomide (Len)-Refractory
Multiple Myeloma (MM) after 1-3 Lines of Therapy: Minimal Residual
Disease (MRD) Negativity in the Phase 3 Cartitude-4 Trial. Blood.
2024;144:1032. https://doi.org/10.1182/blood-2024-201533
- Freeman
CL, Dhakal B, Kaur G, et al. Phase 2 Registrational Study of
Anitocabtagene Autoleucel for the Treatment of Patients with Relapsed
and/or Refractory Multiple Myeloma: Preliminary Results from the
IMMagine-1 Trial. Blood. 2024;144(Supplement 1):1031. https://doi.org/10.1182/blood-2024-198499
- Matthew
Frigault, Jacalyn Rosenblatt, Binod Dhakal, Noopur Raje, Daniella Cook,
Mahmoud Gaballa, Estelle Emmanuel-Alejandro, Danielle Nissen, Kamalika
Bannerjee, Anand Rotte, Christopher Heery, David Avigan, Andrzej
Jakubowiak MB. PHASE 1 STUDY OF ANITOCABTAGENE AUTOLEUCEL FOR THE
TREATMENT OF PATIENTS WITH RELAPSED AND/OR REFRACTORY MULTIPLE MYELOMA:
RESULTS FROM AT LEAST 1-YEAR FOLLOW-UP IN ALL PATIENTS. HemaSphere.
2024;422311(S207). https://doi.org/10.1016/S2152-2650(24)00960-1
- Bal
S, Anderson Jr. LD, Nadeem O, et al. Efficacy and Safety with Extended
Follow-up in a Phase 1 Study of BMS-986393, a G Protein-Coupled
Receptor Class C Group 5 Member D (GPRC5D)-Targeted CAR T Cell Therapy,
in Patients (pts) with Heavily Pretreated Relapsed/Refractory (RR)
Multiple Myeloma (MM). Blood. 2024;144(Supplement 1):922. https://doi.org/10.1182/blood-2024-201356
- Lonial
S, Lee HC, Badros A, et al. Belantamab mafodotin for relapsed or
refractory multiple myeloma (DREAMM-2): a two-arm, randomised,
open-label, phase 2 study. Lancet Oncol. 2020;21(2):207-221. https://doi.org/10.1016/S1470-2045(19)30788-0 PMid:31859245
- Mateos
MV, Robak P, Hus M, et al. Results from the randomised phase III
DREAMM-7 study of belantamab mafodotin (belamaf) + bortezomib, and
dexamethasone (BVd) vs daratumumab, bortezomib, and dexamethasone (DVd)
in relapsed/refractory multiple myeloma (RRMM). J Clin Oncol.
2024;42(36_suppl):439572. https://doi.org/10.1200/JCO.2024.42.36_suppl.439572
- Vania
H, Pawel R, Marek H, et al. Belantamab Mafodotin, Bortezomib, and
Dexamethasone for Multiple Myeloma. N Engl J Med. 2024;391(5):393-407. https://doi.org/10.1056/NEJMoa2405090 PMid:38828933
- Hungria
V, Robak P, Hus M, et al. Belantamab Mafodotin, Bortezomib, and
Dexamethasone Vs Daratumumab, Bortezomib, and Dexamethasone in
Relapsed/Refractory Multiple Myeloma: Overall Survival Analysis and
Updated Efficacy Outcomes of the Phase 3 Dreamm-7 Trial. Blood.
2024;144(Supplement 1):772. https://doi.org/10.1182/blood-2024-200336
- Trudel
S, Beksac M, Pour L, et al. Results from the randomised phase 3
DREAMM-8 study of belantamab mafodotin plus pomalidomide and
dexamethasone (BPd) vs pomalidomide plus bortezomib and dexamethasone
(PVd) in relapsed/refractory multiple myeloma (RRMM). J Clin Oncol.
2024;42(17_suppl):LBA105-LBA105. https://doi.org/10.1200/JCO.2024.42.17_suppl.LBA105
- Dimopoulos
MA, Beksac M, Pour L, et al. Belantamab Mafodotin, Pomalidomide, and
Dexamethasone in Multiple Myeloma. N Engl J Med. 2024;391(5):408-421. https://doi.org/10.1056/NEJMoa2403407 PMid:38828951
- Sandhu
I, Richardson PG, Oriol A, et al. Mezigdomide (MEZI) Plus Dexamethasone
(DEX) and Bortezomib (BORT) or Carfilzomib (CFZ) in Patients (pts) with
Relapsed/Refractory Multiple Myeloma (RRMM): Updated Results from the
CC-92480-MM-002 Trial. Blood. 2024;144(Supplement 1):1025. https://doi.org/10.1182/blood-2024-198408
- Costa
LJ, Schjesvold F, Popat R, et al. Mezigdomide (MEZI) in Novel-Novel
Combinations for Relapsed or Refractory Multiple Myeloma (RRMM):
Preliminary Results from the CA057-003 Trial. Blood.
2024;144(Supplement 1):677. https://doi.org/10.1182/blood-2024-198403
- Kowalski
A, Lykon J, Diamond B, et al. Tocilizumab Prophylaxis for Patients with
Relapsed or Refractory Multiple Myeloma Treated with Teclistamab,
Elranatamab or Talquetamab. Blood. 2024;144(Supplement 1):932. https://doi.org/10.1182/blood-2024-203426
- Tan
CRC, Derkach A, Maclachlan K, et al. Real-world schedule de-escalation
of teclistamab in patients with relapsed/refractory multiple myeloma. J
Clin Oncol. 2024;42(16_suppl):7536. https://doi.org/10.1200/JCO.2024.42.16_suppl.7536
- Ludwig
H, Munshi NC, Terpos E, Raje N, Moreau P, Nooka A. Improving reporting
of infection events in clinical trials. Blood Adv.
2024;8(22):5927-5928. https://doi.org/10.1182/bloodadvances.2024014102 PMid:39110989 PMCid:PMC11662264
- Cheruvalath
H, Clennon A, Shrestha A, et al. Effects of Intravenous Immunoglobulin
Supplementation (IVIG) on Infections in Recipients of Teclistamab
Therapy for Multiple Myeloma (MM): A Multi-Institutional Study. Blood.
2024;144(Supplement 1):256. https://doi.org/10.1182/blood-2024-199374
- Rejeski
K, Hansen DK, Cordas Dos Santos DM, et al. Pre-Apheresis Prediction of
Toxicity and Response in Patients Receiving BCMA-Directed CAR-T for
Relapsed/Refractory Multiple Myeloma. Blood. 2024;144(Supplement
1):895. https://doi.org/10.1182/blood-2024-208376
- Rejeski
K, Perez A, Sesques P, et al. CAR-HEMATOTOX: a model for CAR
T-cell-related hematologic toxicity in relapsed/refractory large B-cell
lymphoma. Blood. 2021;138(24):2499-2513. https://doi.org/10.1182/blood.2020010543 PMid:34166502 PMCid:PMC8893508