Although multiple myeloma remains incurable, its treatment continues to evolve rapidly. Approved therapies include immunomodulatory agents (IMiDs, such as thalidomide, lenalidomide, and pomalidomide), proteasome inhibitors (bortezomib, carfilzomib, and ixazomib), and monoclonal antibodies (mAb) targeting CD38 (especially daratumumab and isatuximab) and SLAMF7. New therapeutic avenues include bispecific antibodies and chimeric antigen receptor T-cell (CAR-T) therapy.[4-5]
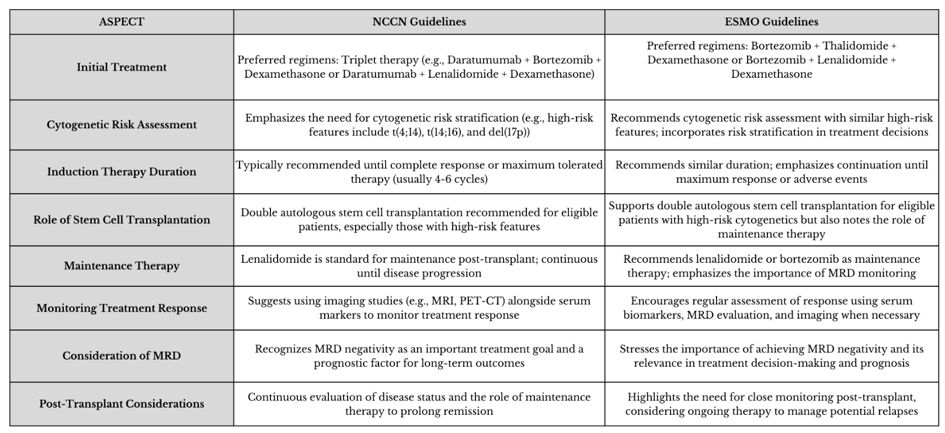
The latest ESMO (European Society for Medical Oncology)[6] and NCCN (National Comprehensive Cancer Network) guidelines[7] have set the standard of care for patients with newly diagnosed multiple myeloma (NDMM) eligible for transplantation, particularly those in good general condition and < 70 years old. This approach is divided into four phases: induction therapy, hematopoietic stem cell collection, and autologous transplant, consolidation, and maintenance. The most significant differences between the guidelines occur during the induction phase, influenced by regulatory approvals in the United States and Europe.
This article will focus on the changing landscape of therapies for newly diagnosed multiple myeloma (NDMM) in transplant-eligible (TE) patients and how the trend is increasingly focusing on therapies targeting minimal residual disease (MRD) and baseline risk stratification.
Induction Therapy
The goal of induction therapy is to reduce the disease burden before transplantation, achieving rapid and deep control of the disease. The NCCN guidelines[7] recommend a quadruplet treatment regimen known as DaraVRD (daratumumab, bortezomib, lenalidomide, and dexamethasone), which has demonstrated better progression-free survival (PFS) compared to the triplet VRD, particularly in the randomised Phase II GRIFFIN study.[8] However, these regimens have not yet been approved by the European Medicines Agency (EMA).The ESMO guidelines recommend the quadruplet DaraVTD, which combines daratumumab with bortezomib, thalidomide, and dexamethasone,[6] over the triplet VTD (without daratumumab) following the results of the Phase III CASSIOPEIA trial, which demonstrated a significant improvement in deep response and PFS.[9]
CASSIOPEIA Trial – Part 1
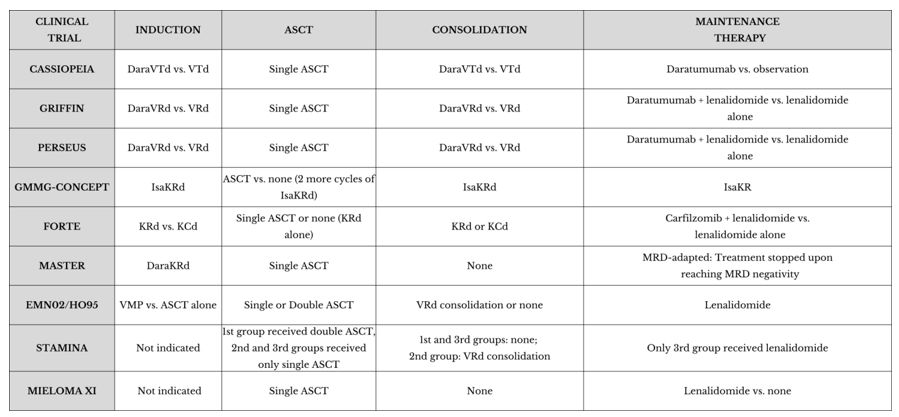
The CASSIOPEIA trial is a phase III, randomised, double-blind study that compared the quadruplet DaraVTD to the triplet VTD in patients eligible for autologous stem cell transplant (ASCT).[10] A total of 1,085 patients were enrolled. DaraVTD showed improved response depth, leading to better PFS with an acceptable safety profile. By day 100 post-transplant, the stringent complete response (sCR) rate was 29% in the DaraVTD group, compared to 20% in the VTD group. Furthermore, the overall complete response (CR) or better rate was 39% in the DaraVTD group compared to 26% in the VTD group. Due to this depth of response, the DaraVTD group achieved a PFS rate of 93% versus 85%.Even though the median PFS had not been reached in either arm, the DaraVTD group had a 53% reduction in the risk of progression or death (HR 0.47).[10] Despite the median follow-up of only 18.8 months, an incremental trend toward better overall survival (OS) was observed, with rates of 97% versus 93% for DaraVTD vs. VTD, respectively (HR 0.43). A longer follow-up is needed to confirm this benefit.[10]
The trial demonstrated the superiority of the quadruplet with daratumumab, even in subgroups with high cytogenetic risk or ISS stage III disease. Based on these results, DaraVTD became the standard of care for NDMM patients eligible for transplant in Europe following its approval by the European Commission in January 2020.[11]
GRIFFIN Trial
The Griffin trial is a phase II randomised study with the aim of evaluating the CR rates and the effect on MRD, PFS, and OS after the addition of daratumumab to the standard regimen of bortezomib, lenalidomide, and dexamethasone (VRD) in NDMM TE-patients.[8] The study included 207 patients randomised between an experimental group treated with daratumumab + VRD (DaraVRD) and a control group treated with VRD alone. Both groups received 4 cycles of induction followed by ASCT, consolidation with 2 other cycles of the assigned regimen, and maintenance with lenalidomide in the control group and lenalidomide + daratumumab in the experimental group. After induction therapy, the DaraVRD group showed a sCR rate of 63.6% compared to 47.4% in the VRD group, at a median follow-up of 27.4 months.[8]Preliminary data showed a reduced risk of progression or death in the experimental group even if, after a median follow-up of approximately 27 months, the difference was not yet statistically significant due to the short observation period. The DaraVRD regimen was well-tolerated; in fact, the most common adverse events included neutropenia (54.3% vs 37.8% in the VRD group), infusion-related reactions to daratumumab (mostly mild to moderate and manageable with premedication), infections (generally mild to moderate), and haematological toxicity (such as anaemia and thrombocytopenia, which were handleable with supportive interventions). Despite the increase in adverse events, the overall safety profile of the DaraVRD regimen was considered acceptable.
The GRIFFIN trial demonstrated that adding daratumumab to the standard VRD regimen (DaraVRD) significantly improves CR rates and leads to higher MRD negativity rates, indicating deeper disease control. These results supported the use of daratumumab in combination with VRD as a new induction and maintenance therapeutic option according to the latest NCCN guidelines in TE patients, redefining the standard of care for NDMM.[11]
PERSEUS Trial
A Phase III clinical study that studied the efficacy and safety of adding daratumumab to the standard VRD compared to VRD alone in NDMM TE-patients.[12] The enrolled patients (709) were randomised into two groups (DaraVRD vs VRD) to receive the assigned regimen both in induction and consolidation, with lenalidomide maintenance for both and monthly daratumumab in the DaraVRD group until progression or toxicity. The primary endpoint was PFS, while secondary objectives included CR or better and MRD negativity. At 48 months, the PFS rates were 84.3% in the DaraVRD group and 67.7% in the VRD group (HR 0.42). The rate of CR or better was higher in the experimental group (87.9% vs 70.1%), and the same happened with MRD-negativity rates (75.2% vs 47.5%). The observed adverse events (AE) were largely in line with expectations; specifically, the most common grade 3 or 4 adverse events were neutropenia (62.1% with DaraVRD and 51.0% with VRD) and thrombocytopenia (29.1% and 17.3%, respectively).[12] Serious adverse events (SAE) occurred with rates of 57% in the DaraVRD group and 49.3% in the VRD group.This study showed that adding daratumumab to the VRD regimen in induction and consolidation, along with lenalidomide maintenance, leads to better PFS among TE patients with NDMM.[11]
GMMG-CONCEPT Trial
The GMMG-CONCEPT Trial is a phase II clinical study exploring a targeted approach for the treatment of NDMM with high-risk cytogenetic abnormalities,[13] such as t(4;14), t(14;16), or del(17p), which are known to be associated with poor prognosis. The trial enrolled patients with mandatory International Staging System (ISS) stage II/III combined with del17p, t(4;14), t(14;16), or more than three copies of 1q21 as high-risk cytogenetic aberrations (HRCAs).[13] Patients were divided into two treatment arms based on transplant eligibility: TE patients received 6 cycles of Isa-KRD induction (isatuximab, carfilzomib, lenalidomide, and dexamethasone) with stem-cell collection after cycle 3, followed by high-dose therapy (HDT) and autologous stem-cell transplantation (ASCT). Consolidation consisted of 4 cycles of Isa-KRD, with following maintenance with 26 cycles of Isa-KR. Patients not transplant eligible (TNE) received the same induction, consolidation, and maintenance but had two additional cycles of Isa-KRD instead. The population for Interim Analysis (IA) included 99 TE and 26 TNE patients.[13] The trial met its primary endpoint, with MRD negativity rates after consolidation of 67.7% in TE patients and 54.2% in TNE patients. 81.8% of TE patients achieved MRD negativity at any point, with a sustained MRD negativity for ≥1 year in 62.6% of patients.[13] After a median follow-up of 44 months (TE) and 33 months (TNE), median PFS was not reached in either arm. This study was one of the first to include only high-risk multiple myeloma (HRMM) patients, without limitations based on age or transplant eligibility, and to report the use of the quadruplet Isa-KRD in extended induction and consolidation and of Isa-KR maintenance, resulting in deep and durable responses in this difficult-to-treat population.FORTE Trial
FORTE was a randomised phase II trial that enrolled TE NDMM patients aged 18–65 years.[14] Patients were randomly assigned to KRD plus ASCT followed by 4 KRD consolidation cycles, 12 KRD cycles, or KCD plus ASCT and four KCD consolidation cycles. After the consolidation phase, patients were stratified according to induction–consolidation treatment and randomised to maintenance treatment with carfilzomib plus lenalidomide (KR) or lenalidomide alone, until progression or intolerance in both groups. The primary endpoints were the proportion of patients with at least a very good partial response (VGPR) after induction with KRD versus KCD and PFS in the maintenance treatment with carfilzomib plus lenalidomide versus lenalidomide alone. A total of 477 patients were enrolled, of whom 396 (83%) had complete cytogenetic data on del(17p), t(4;14), t(14;16), del(1p), gain(1q) (3 copies), and amp(1q) (≥4 copies) assessed by fluorescence in situ hybridisation (FISH) analysis on CD138-positive sorted cells.[15] The median follow-up from the first randomisation was 50.9 months, and the 4-year PFS rates for KRD-ASCT, KRD alone, and KCD-ASCT were 69%, 56%, and 51%, respectively, resulting in significantly better PFS for KRD-ASCT compared to KCD-ASCT (HR 0.54; p = 0.0008) and KRD alone (HR 0.61; p = 0.008). No significant difference was noted between KRD and KCD-ASCT (HR 0.82; p = 0.3). KRD plus ASCT showed superiority in terms of higher 1-year sustained MRD-negativity compared to KRD alone (47% vs 35%) but also in terms of improved responses compared to the other two treatment approaches.[11] Furthermore, the pre-planned analysis with stratification based on cytogenetic risk showed that carfilzomib-based regimens are effective strategies in patients with standard risk (zero high-risk cytogenetic aberrations) and HRMM, resulting in similar rates of PFS and 1-year sustained MRD negativity.[15] Despite promising PFS rates, patients with ultra-high-risk disease (those with two or more high-risk cytogenetic aberrations) still have an increased risk of progression and death and, therefore, represent an unmet medical need.MASTER trial
Phase II study designed to modulate therapy based on response.[16] Specifically, in 123 NDMM patients treated with 4 cycles of daratumumab, carfilzomib, lenalidomide, and dexamethasone (DaraKRD), MRD was evaluated after induction therapy, after ASCT, and every 4 cycles during consolidation (up to a maximum of 8 cycles). 71% of patients achieved MRD negativity twice consecutively and stopped treatment, starting an MRD monitoring (MRD-SURE). The 2-year PFS was 87%, and among those who achieved MRD-SURE, the 24-month cumulative incidence of progression was 9% for individuals with no HRCAs, 9% for those with one HRCA, and 47% for those with two or more HRCAs. Importantly, there was no strong association between achieving MRD negativity after induction therapy or post-ASCT and PFS, even when using a threshold of 10⁻⁶. With extended follow-up, the study also showed that MRD positivity preceded disease progression. One limitation of this study was that it led to premature cessation of therapy, with more significant effects in patients with higher cytogenetic risk. However, at least in standard-risk disease, MRD-adapted induction and consolidation demonstrated to be an effective strategy.[17]Autologous Stem Cell Transplantation (ASCT)
Both ESMO and NCCN guidelines agree on the necessity of performing autologous stem cell transplantation (ASCT) in eligible patients, initially based on many phase 2 and 3 randomised trials conducted during the 1990s that demonstrated an improvement in event-free survival (EFS), PFS and OS over conventional chemotherapy.[18]After the induction phase, which typically lasts four cycles, a stem cell mobilisation occurs, usually with G-CSF, to facilitate the collection of stem cells for ASCT, then, there is a conditioning phase with high-dose (HD) chemotherapy prior to ASCT, generally with melphalan at 200 mg/m².
For patients with suboptimal response after the first transplant and HRMM, a tandem autologous transplant may also be considered after the first one.
The role of HD conditioning and ASCT in NDMM has been primarily validated by the IFM 2009 trial, the DETERMINATION trial, the FORTE trial, the EMN02/HO95 trial, and the STaMINA trial.
IFM 2009 trial
This study compared treatment in NDMM with 8 cycles of VRD vs 3 cycles of VRD plus HD melphalan with ASCT plus 2 consolidation VRD cycles. Both groups of patients received lenalidomide for 1 year as maintenance therapy. Median PFS resulted significantly longer in the transplant arm (50 months vs. 36 months; adjusted hazard ratio for disease progression or death, 0.65; P<0.001). An extended median follow-up (FU) at 93 months did not reveal any differences in OS or PFS2 (progression on the next line of treatment-free survival) or OS.[19,20] MRD appeared to be a strong predictor outcome, with a longer PFS, PFS2, and OS in those who achieved MRD negativity after induction, possibly useful for identifying patients who probably do not require a transplant.[20]DETERMINATION trial
In this trial, patients were randomised in a group that received 3 cycles of VRD followed by ASCT plus 2 other cycles of VRD and lenalidomide as maintenance therapy and another receiving 8 cycles of VRD and then started lenalidomide maintenance therapy. The results showed a significantly longer PFS in the group that received ASCT compared to the other (67.5 months vs 46.2 months), with no difference in OS rates after a median follow-up of more than 6 years.FORTE trial
This study[15] confronted KRD plus ASCT, KRD alone and KCD plus ASCT, demonstrating the advantage of adding ASCT to KRD induction treatment in terms of both response and progression-free survival. Moreover, the response and MRD-negativity rates were similar after consolidation in both KRD alone and KRD plus ASCT arms but sustained MRD-negativity rates, and median PFS were superior in the second one.[15,18]EMN02/HO95 Trial
The EMN02/HO95 was a randomised phase 3 trial that included 1503 NDMM patients who, initially, were randomly assigned to intensification therapy with ASCT (single or double) or 4 cycles of bortezomib-melphalan-prednisone (VMP).[21] Then, a second randomisation happened between 2 VRD consolidation cycles and no consolidation. All groups received lenalidomide maintenance therapy. The rate of very good partial response (VGPR) or better was 84% in the ASCT group versus 77% in the VMP group (p = 0.0021), with a benefit in terms of median PFS (56.7 months for ASCT compared to 41.9 months for VMP, HR 0.73; p = 0.0001) and, after a follow-up of 75 months, of OS (69% versus 63%; HR 0.80; p = 0.03), demonstrating the superiority of ASCT to intensification with VMP alone in terms of PFS, response rates, and OS.STaMINA Trial
The STaMINA trial (Stem Cell Transplantation in Multiple Myeloma Incorporating Novel Agents)[22] investigated the effectiveness in improving PFS and OS of three different post-transplant approaches for TE NDMM. After ASCT, a group of patients received a second transplant followed by lenalidomide; the second one was treated with 4 cycles of consolidation with VRD plus lenalidomide maintenance therapy, and the third group received only lenalidomide maintenance therapy after the first transplant.[22] No significant differences emerged between the three groups in terms of PFS (53%, 57%, and 52%, respectively at 38 months) and OS (a 3-year survival rate of 82-84%). However, a greater incidence of adverse events and complications was associated respectively with VRD consolidation therapy compared to lenalidomide maintenance therapy alone and with the second transplant, highlighting that a less intensive approach with maintenance therapy alone may be sufficient to achieve good outcomes while reducing the risks associated with more intensive treatments.Single Vs Tandem Transplant
The Bologna 96 trial[23] showed an advantage in terms of PFS with a second ASCT after the first one (tandem transplantation) compared to a single one, but no improvement in OS rates. Also, the GMMG HD2 trial failed to demonstrate superiority in tandem ASCT, possibly because of the high dropout rate.[24] In the previously mentioned EMN02/HO95 study,[21] there was a significantly longer 5-year-PFS rate (53.5% vs 44.9%) and better 5-year OS rates (80.3% vs 726%) with tandem ASCT. However, the STAMINA trial resulted in no difference between the two approaches in terms of both PFS and OS, maybe because of some biases such as higher dropout rates before the second ASCT and prolonged induction therapy.Consequently, double intensification is still used for patients with high-risk cytogenetics. GMMG-CONCEPT and IFM2018-04 phase 2 trials demonstrated a 3-year PFS rate of 68.9% and a 30-month PFS rate of 80%, respectively, after an induction with quadruplet regimens followed by tandem transplant in these high-risk patients.[13,25]
Consolidation Therapy
The goal of this third phase is to improve the response achieved with the transplant further, especially when MRD negativity is not reached. Often, the therapeutic regimen used in the induction phase is resumed or adapted after ASCT. However, various studies evaluated different consolidation therapy regimens, including notably the CASSIOPEIA study, the GRIFFIN study, and the PERSEUS trial.CASSIOPEIA trial – part 1
In the first part of this study, following 4 induction cycles and 2 consolidation cycles of therapy with the assigned regimens, the rates of MRD negativity were significantly higher in the DaraVTD group with rates of 9.2% vs 5.4% and 33.7% vs 19.9% respectively.[9] Sustained MRD negativity rates were also higher in the DaraVTD group at 1 year (50.1% vs 30.1) and at 2 years (35.5% vs 18.8%). Moreover, achieving MRD negativity was associated with improved PFS in both treatment groups but specifically in the DaraVTD group, with 1-year and 2-year sustained MRD negativity associated with HRs of 0.20 (p < 0.0001) and 0.04 (p < 0.0001), respectively, demonstrating that adding daratumumab to VTD in induction and consolidation significantly improves the deep response and PFS compared to the triplet without mAb.GRIFFIN trial
The data from the study following consolidation (DaraVRD vs VRD) showed a benefit in terms of MRD negativity rates (threshold of 10⁻⁵) in patients treated with DaraVRD compared to VRS (51% vs 20%). A subgroup analysis favoured DaraVRD in all prognostic subgroups, but it was not statistically significant for patients with ISS stage 3 or high-risk cytogenetic abnormalities (HRCAs), which might be due to the small number of high-risk patients. [8,26]PERSEUS trial
The clinical benefits of daratumumab combined with VRD induction and consolidation therapy, as well as with lenalidomide maintenance therapy, observed in the PERSEUS trial reinforce those seen in the GRIFFIN study[26] and are also consistent with those observed in the CASSIOPEIA trial.[9]Maintenance Therapy
Post-transplant maintenance is recommended to prolong PFS and improve long-term disease control. The following studies have evaluated various therapeutic options for maintenance therapy.CASSIOPEIA trial – part 2
The second part of the CASSIOPEIA trial[27] studied the efficacy of maintenance therapy with daratumumab compared to observation (no maintenance therapy) after ASCT in patients who had already received induction and consolidation regimens with DaraVTD or VTD. The results showed a significant increase of MRD negativity rates in the group with daratumumab maintenance therapy compared to observation (58.6% vs 47.1%);[11] however, no significant advantage was noted in the DaraVTD group compared to patients who received VTD induction/consolidation, only; the rates of MRD-negativity and sustained negativity at 1 and 2 years were similar between daratumumab maintenance and observation alone.[9] This shows that the use of daratumumab as maintenance therapy is only advantageous in daratumumab naive patients and that its use during induction and consolidation is likely enough.FORTE trial
Carfilzomib plus lenalidomide as maintenance therapy improved PFS compared with the standard-of-care lenalidomide alone.[11]GRIFFIN trial
Maintenance therapy was lenalidomide in the control group (VRD) and lenalidomide + daratumumab in the experimental group (DaraVRD). Updated results were presented at ASH 2021 after 24 months of maintenance therapy (DR vs R, median follow-up 38.6 months),[26] and the rates of sCR significantly favored the DaraVRD group (66% vs 47.4%), as did rates of MRD-negativity (64.4% vs. 30.1% at 10-5 and 35.6% vs 14.6% at 10−6) with 1 year sustained MRD-negativity (10−5) rates of 44.2% in the DaraVRD group vs. 12.6% in VRD alone. Median PFS was not reached in either arm after 38.6 months follow-up but did favour the daratumumab group (36-month PFS rates of 88.9% vs 81.2%).PERSEUS trial
In this study, the control group had lenalidomide maintenance, while the experimental group was treated with daratumumab and lenalidomide.[12] After at least 24 months of maintenance therapy, daratumumab was discontinued in patients who had achieved a CR or better and maintained MRD negativity (10-5) for at least 12 months. These patients continued to receive lenalidomide until disease progression or AE. If patients experienced a confirmed loss of CR without disease progression (reappearance of serum or urine M-protein on immunofixation or electrophoresis or the presence of ≥5% plasma cells in the bone marrow) or a recurrence of MRD positivity (10⁻⁴ or higher), daratumumab therapy was resumed. Among patients who were MRD positive at the end of consolidation, significantly higher proportions in the DaraVRD group achieved MRD negativity during maintenance therapy compared to the VRD group (60.2% vs 40.5% at a threshold of 10⁻⁵ and 56.7% vs 25.2% at a threshold of 10⁻⁶). Additionally, 1-year sustained MRD negativity rates were significantly higher in the DaraVRD group compared to the VRD one (38.6% vs 17.4% at 10⁻⁵ and 31.3% vs 10.3% at 10⁻⁶).[12] Achieving MRD negativity at the end of consolidation and overall MRD negativity at both thresholds were associated with improved PFS.MIELOMA XI trial
Myeloma XI is a phase 3 trial in the UK that showed that MRD is a predictor of survival outcomes at 3 and 9 months post-ASCT.[28] A total of 1,248 patients were randomly assigned after three months from ASCT to lenalidomide maintenance or observation. MRD (10-5) was assessed before maintenance at ASCT + 3 months and ASCT + 9 months. At ASCT + 3, those who achieved MRD negativity had longer PFS compared to those who did not (44 vs. 24 months). Patients who passed from MRD positive to MRD negative at ASCT + 9 had similar PFS outcomes as patients who were negative at both points; moreover, lenalidomide maintenance seemed to increase the rates of conversion from MRS positivity to MRD negativity 6 months later (lenalidomide 30%, observation 17%). High-risk molecular features had an adverse effect on PFS and OS even after achieving MRD-negativity. Also, maintenance therapy and molecular risk maintained prognostic impact at both ASCT + 3 and ASCT + 9. An updated follow-up analysis showed that PFS benefits were no longer statistically significant for those patients with MRD negativity at 3 years,[29] suggesting that the magnitude of the benefit of extended maintenance in patients with deepest long-term responses may not offset medical and financial toxicities.[17]AURIGA trial
The Phase 3 AURIGA study evaluates the efficacy of daratumumab combined with lenalidomide (D-R) compared to lenalidomide (R) maintenance in NDMM patients who achieved at least a VGPR and are MRD30 (threshold 10⁻⁵) positive, as well as being anti-CD38 naïve post-transplant. A total of 200 patients were randomised to receive either D-R or R maintenance for up to 36 cycles. The primary endpoint was the 1-year MRD-negative (10⁻⁵ threshold) conversion rate, which resulted to be significantly higher in the D-R group (50.5% vs. 18.8%). A similar trend was observed for the MRD-negative conversion rate at the 10⁻⁶ threshold (23.2% vs. 5.0%). The same happened after a median follow-up of 32.3 months (60.6% vs 27.7% at 10⁻⁵), with also a greater CR rate or better in the D-R group (75.8% vs 61.4%).[30] PFS was also significantly improved with D-R, with an estimated 30-month PFS rates of 82.7% vs 66.4%. However, the incidences of grade 3/4 cytopenias (54.2% vs. 46.9%) and infections (18.8% vs. 13.3%) were slightly higher in the D-R group compared to R.[30] In conclusion, D-R maintenance therapy resulted in a higher MRD-negative conversion rate and improved PFS compared to R alone, with an acceptable safety profile.MajesTEC-4 trial
The MAJESTEC-4 trial (also known as MajesTEC-4)[31] is an ongoing phase III clinical trial designed to evaluate the efficacy and safety of teclistamab, a bispecific antibody targeting B-cell maturation antigen (BCMA) and CD3, in combination with subcutaneous daratumumab and lenalidomide, compared to a control arm with daratumumab and lenalidomide (D-R) for post-transplant maintenance therapy. The secondary objectives of the trial include assessing the rate of MRD negativity with teclistamab compared to the control regimen, monitoring the safety of the experimental combination (particularly for AE such as cytokine release syndromes – CRS – and haematological toxicities), and evaluating the impact of the treatments on patients' quality of life, a crucial factor in long-term maintenance regimens. The results of this study may lead to a significant change in the standard of care for maintenance therapy since the introduction of a bispecific antibody-like teclistamab may represent a further advancement in improving PFS and prolonging remission.EXCALIBER maintenance (EMN26 study)
The EMN26 study[32] is a phase II trial that includes patients who have achieved at least a partial response (PR) after induction therapy containing a proteasome inhibitor (PI) and an immunomodulatory drug (IMiD), followed by single or double ASCT with or without consolidation. Participants were enrolled in one of three different cohorts receiving iberdomide at doses of 0.75, 1.0, or 1.3 mg; treatment continued until disease progression or unacceptable toxicity, with 40 patients in each cohort. The primary outcome is the improvement in response, while secondary outcomes include safety and PFS. The response was evaluated at screening and after each treatment cycle. After 6 treatment cycles, both 1.0 mg and 1.3 mg cohorts showed comparable deepening of response, with an improvement in response in 48% of patients treated in the 1.0 mg cohort and 45% in the 1.3 mg one. The PFS at 6 months was 97% vs 94%. These results show that iberdomide represents an effective post-ASCT maintenance strategy with a favourable safety profile and superior response improvement at 6 months compared to lenalidomide maintenance (26% at 6 months in the EMN02 study). Additional follow-up is needed to define the recommended maintenance dose that will be used in the next phase 3 EXCALIBER maintenance study.Future Perspectives With CAR-T
CAR-T therapy has shown its first positive outcomes in relapsed/refractory multiple myeloma (RRMM), with results that may lead to a potential use in first-line treatment for NDMM. Specifically, the phase I/II KarMMa study with idecabtagene vicleucel (ide-cel) demonstrated an overall response rate (ORR) of 73% in RRMM patients, with a sCR of 33%. These patients had a median PFS of 8.8 months and an OS of 19.4 months.[33] Currently, various clinical trials, such as the CARTITUDE-2 and KarMMa-4 studies, are evaluating CAR-T therapy in first-line settings, especially in high-risk populations.In the ongoing CARTITUDE-2 study,[34] which enrolled NDMM patients who were ineligible for transplant or had HRCAs, cilta-cel demonstrated an MRD negativity rate (10-5) of 93%. Among high-risk patients, the projected 1-year PFS rate was 95%, significantly better than the PFS observed with standard regimens like VRD in these populations (generally around 65-70%). Given the depth of response and high MRD negativity rates, OS in these patients is expected to be significantly higher, although long-term follow-up is required to confirm this trend.
The integration of CAR-T therapy into a first-line treatment for NDMM is incredibly promising, particularly for high-risk patients who have historically had poor outcomes with conventional therapies. However, its associated toxicities remain significant, most commonly including CRS and neurotoxicity. In the CARTITUDE-2 trial,[34] 88% of patients experienced CRS, though most cases were grade 1 or 2. Additionally, the high cost and complexity of the manufacture of CAR-T therapy are important obstacles to the diffusion of this approach.
Conclusions
In conclusion, the treatment landscape for NDMM TE patients is significantly turning, even if current strategies remain predominantly non-risk-oriented. While the double ASCT approach remains important and diffused, particularly in high-risk cytogenetic populations, the evidence supporting its superiority in the context of novel therapies, such as quadruplet regimens, is still evolving and remains to be fully validated.It is increasingly crucial to have a comprehensive risk-adapted treatment paradigm because current therapies, although effective in many cases, are not sufficient for patients with high-risk molecular features. The tandem ASCT is the only treatment option explicitly tailored to the high-risk cytogenetic profile.
Moreover, as novel therapeutic combinations, including quadruplet regimens, become more widely adopted, it is critical to assess their impact on patients with varying cytogenetic risks. An essential component of treatment evaluation should include both stratifying patients based on high-risk cytogenetic features and considering MRD status. MRD negativity has emerged as an important predictor of long-term outcomes, providing data about the effectiveness of therapy and helping with subsequent treatment decisions. In fact, the integration of MRD assessment into routine clinical practice is imperative for optimising treatment strategies.
By evaluating therapy based on both MRD status and baseline cytogenetic risk, clinicians can implement a more personalised approach to patient management, moving towards precision medicine in multiple myeloma and an improvement in patient outcomes, as it allows for adjustments in therapy based on individual responses and disease characteristics.
Looking ahead, further research is essential to establish and validate risk-adapted strategies that can improve the effectiveness of current therapies. The focus in this phase must be on developing individualised approaches that are developed over the understanding of multiple myeloma biology, the implications of cytogenetic abnormalities, and the potential of novel therapeutic agents. The only way to improve survival rates and quality of life for patients battling this complex disease is through a comprehensive and informed approach.
References
- Siegel, R. L., Giaquinto, A. N. & Jemal, A. Cancer statistics, 2024. CA Cancer J Clin 74, 12-49 (2024). https://doi.org/10.3322/caac.21820 PMid:38230766
- Roodman, G. D. Pathogenesis of myeloma bone disease. Leukemia 23, 435-441 (2009). https://doi.org/10.1038/leu.2008.336 PMid:19039321
- Short,
K. D. et al. Incidence of extramedullary disease in patients with
multiple myeloma in the era of novel therapy, and the activity of
pomalidomide on extramedullary myeloma. Leukemia 25, 906-908 (2011). https://doi.org/10.1038/leu.2011.29 PMid:21350560 PMCid:PMC3736849
- Barlogie, B. et al. Treatment of multiple myeloma. Blood 103, 20-32 (2004). https://doi.org/10.1182/blood-2003-04-1045 PMid:12969978
- Verma,
M., Obergfell, K., Topp, S., Panier, V. & Wu, J. The
next-generation CAR-T therapy landscape. Nat Rev Drug Discov 22,
776-777 (2023). https://doi.org/10.1038/d41573-023-00140-7 PMid:37673977
- Dimopoulos,
M. A. et al. Multiple myeloma: EHA-ESMO Clinical Practice Guidelines
for diagnosis, treatment and follow-up†. Annals of Oncology 32, 309-322
(2021). https://doi.org/10.1016/j.annonc.2020.11.014 PMid:33549387
- Callander,
N. S. et al. NCCN Guidelines® Insights: Multiple Myeloma, Version
3.2022. Journal of the National Comprehensive Cancer Network 20, 8-19
(2022). https://doi.org/10.6004/jnccn.2022.0002 PMid:34991075
- Voorhees,
P. M. et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone
for transplant-eligible newly diagnosed multiple myeloma: the GRIFFIN
trial. Blood 136, 936-945 (2020). https://doi.org/10.1182/blood.2020005288 PMid:32325490 PMCid:PMC7441167
- Avet
Loiseau, H. et al. Daratumumab (DARA) with Bortezomib, Thalidomide, and
Dexamethasone (VTd) in Transplant-Eligible Patients (Pts) with Newly
Diagnosed Multiple Myeloma (NDMM): Analysis of Minimal Residual Disease
(MRD) Negativity in Cassiopeia Part 1 and Part 2. Blood 138, 82-82
(2021). https://doi.org/10.1182/blood-2021-147897
- Moreau,
P. et al. Bortezomib, thalidomide, and dexamethasone with or without
daratumumab before and after autologous stem-cell transplantation for
newly diagnosed multiple myeloma (CASSIOPEIA): a randomisedrandomised,
open-label, phase 3 study. The Lancet 394, 29-38 (2019). https://doi.org/10.1016/S0140-6736(19)31240-1 PMid:31171419
- Bazarbachi,
A. H. et al. Induction therapy prior to autologous stem cell
transplantation (ASCT) in newly diagnosed multiple myeloma: an update.
Blood Cancer J 12, 47 (2022). https://doi.org/10.1038/s41408-022-00645-1 PMid:35347107 PMCid:PMC8960754
- Rodríguez-Otero,
P. et al. Daratumumab (DARA) + bortezomib/lenalidomide/dexamethasone
(VRd) in transplant-eligible (TE) patients (pts) with newly diagnosed
multiple myeloma (NDMM): Analysis of minimal residual disease (MRD) in
the PERSEUS trial. Journal of Clinical Oncology 42, 7502-7502 (2024). https://doi.org/10.1200/JCO.2024.42.16_suppl.7502
- Leypoldt,
L. B. et al. Isatuximab, Carfilzomib, Lenalidomide, and Dexamethasone
for the Treatment of High-Risk Newly Diagnosed Multiple Myeloma.
Journal of Clinical Oncology 42, 26-37 (2024). https://doi.org/10.1200/JCO.23.01696 PMid:37753960 PMCid:PMC10730063
- Gay,
F. et al. Carfilzomib with cyclophosphamide and dexamethasone or
lenalidomide and dexamethasone plus autologous transplantation or
carfilzomib plus lenalidomide and dexamethasone, followed by
maintenance with carfilzomib plus lenalidomide or lenalidomide alone
for patients with newly diagnosed multiple myeloma (FORTE): a
randomisedrandomised, open-label, phase 2 trial. Lancet Oncol 22,
1705-1720 (2021). https://doi.org/10.1016/S1470-2045(21)00535-0 PMid:34774221
- Mina,
R. et al. Carfilzomib induction, consolidation, and maintenance with or
without autologous stem-cell transplantation in patients with newly
diagnosed multiple myeloma: pre-planned cytogenetic subgroup analysis
of the randomisedrandomised, phase 2 FORTE trial. Lancet Oncol 24,
64-76 (2023). https://doi.org/10.1016/S1470-2045(22)00693-3 PMid:36528035
- Costa,
L. J. et al. Minimal residual disease response-adapted therapy in newly
diagnosed multiple myeloma (MASTER): final report of the multicentre,
single-arm, phase 2 trial. Lancet Haematol 10, e890-e901 (2023). https://doi.org/10.1016/S2352-3026(23)00236-3 PMid:37776872
- Meseha,
M., Hoffman, J., Kazandjian, D., Landgren, O. & Diamond, B. Minimal
Residual Disease-Adapted Therapy in Multiple Myeloma: Current Evidence
and Opinions. Curr Oncol Rep 26, 679-690 (2024). https://doi.org/10.1007/s11912-024-01537-2 PMid:38676789 PMCid:PMC11169024
- Perrot, A. Transplant in myeloma: who, when, and why? Hematology 2024, 561-568 (2024). https://doi.org/10.1182/hematology.2024000580 PMid:39643987 PMCid:PMC11665519
- Attal,
M. et al. Lenalidomide, Bortezomib, and Dexamethasone with
Transplantation for Myeloma. New England Journal of Medicine 376,
1311-1320 (2017). https://doi.org/10.1056/NEJMoa1611750 PMid:28379796 PMCid:PMC6201242
- Perrot,
A. et al. Early Versus Late Autologous Stem Cell Transplant in Newly
Diagnosed Multiple Myeloma: Long-Term Follow-up Analysis of the IFM
2009 Trial. Blood 136, 39-39 (2020). https://doi.org/10.1182/blood-2020-134538
- Cavo,
M. et al. Autologous haematopoietic stem-cell transplantation versus
bortezomib-melphalan-prednisone, with or without
bortezomib-lenalidomide-dexamethasone consolidation therapy, and
lenalidomide maintenance for newly diagnosed multiple myeloma
(EMN02/HO95): a multicentre, randomisedrandomised, open-label, phase 3
study. Lancet Haematol 7, e456-e468 (2020). https://doi.org/10.1016/S2352-3026(20)30099-5 PMid:32359506
- Dispenzieri,
A. et al. Mass-Fix better predicts for PFS and OS than standard methods
among multiple myeloma patients participating on the STAMINA trial (BMT
CTN 0702 /07LT). Blood Cancer J 12, 27 (2022). https://doi.org/10.1038/s41408-022-00624-6 PMid:35145071 PMCid:PMC8831597
- Cavo,
M. et al. Prospective, RandomisedRandomised Study of Single Compared
With Double Autologous Stem-Cell Transplantation for Multiple Myeloma:
Bologna 96 Clinical Study. Journal of Clinical Oncology 25, 2434-2441
(2007). https://doi.org/10.1200/JCO.2006.10.2509 PMid:17485707
- Mai,
E. K. et al. Single versus tandem high-dose melphalan followed by
autologous blood stem cell transplantation in multiple myeloma:
long-term results from the phase III GMMG-HD2 trial. Br J Haematol 173,
731-741 (2016). https://doi.org/10.1111/bjh.13994 PMid:26990892
- Touzeau,
C. et al. Daratumumab, carfilzomib, lenalidomide, and dexamethasone
with tandem transplant for high-risk newly diagnosed myeloma. Blood
143, 2029-2036 (2024). https://doi.org/10.1182/blood.2023023597 PMid:38394666
- Laubach,
J. P. et al. Daratumumab (DARA) Plus Lenalidomide, Bortezomib, and
Dexamethasone (RVd) in Patients (Pts) with Transplant-Eligible Newly
Diagnosed Multiple Myeloma (NDMM): Updated Analysis of Griffin after 24
Months of Maintenance. Blood 138, 79-79 (2021). https://doi.org/10.1182/blood-2021-149024
- Moreau,
P. et al. Bortezomib, thalidomide, and dexamethasone with or without
daratumumab and followed by daratumumab maintenance or observation in
transplant-eligible newly diagnosed multiple myeloma: long-term
follow-up of the CASSIOPEIA randomisedrandomised controlled phase 3
trial. Lancet Oncol 25, 1003-1014 (2024). https://doi.org/10.1016/S1470-2045(24)00282-1 PMid:38889735
- de
Tute, R. M. et al. Minimal Residual Disease After Autologous Stem-Cell
Transplant for Patients With Myeloma: Prognostic Significance and the
Impact of Lenalidomide Maintenance and Molecular Risk. Journal of
Clinical Oncology 40, 2889-2900 (2022). https://doi.org/10.1200/JCO.21.02228 PMid:35377708
- Pawlyn,
C. et al. Defining the Optimal Duration of Lenalidomide Maintenance
after Autologous Stem Cell Transplant - Data from the Myeloma XI Trial.
Blood 140, 1371-1372 (2022). https://doi.org/10.1182/blood-2022-165376
- Badros,
A. Z. et al. Daratumumab with lenalidomide as maintenance after
transplant in newly diagnosed multiple myeloma: the AURIGA study. Blood
Journal (2024) doi:10.1182/blood.2024025746. https://doi.org/10.1182/blood.2024025746 PMid:39331724 PMCid:PMC11775507
- Zamagni,
E. et al. MajesTEC-4 (EMN30): A Phase 3 Trial of Teclistamab +
Lenalidomide Versus Lenalidomide Alone As Maintenance Therapy Following
Autologous Stem Cell Transplantation in Patients with Newly Diagnosed
Multiple Myeloma. Blood 140, 7289-7291 (2022). https://doi.org/10.1182/blood-2022-159756
- Niels
W.C.J. van de Donk, Cyrille Touzeau, Evangelos Terpos & Aurore
Perrot. Iberdomide Maintenance after Autologous Stem-Cell
Transplantation in Newly Diagnosed MM: First Results of the Phase 2
EMN26 Study.
- Munshi,
N. C. et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple
Myeloma. New England Journal of Medicine 384, 705-716 (2021). https://doi.org/10.1056/NEJMoa2024850 PMid:33626253
- Arnulf,
B. et al. Efficacy and safety of ciltacabtagene autoleucel ±
lenalidomide maintenance in newly diagnosed multiple myeloma with
suboptimal response to frontline autologous stem cell transplant:
CARTITUDE-2 cohort D. Journal of Clinical Oncology 42, 7505-7505
(2024). https://doi.org/10.1200/JCO.2024.42.16_suppl.7505